Abstract
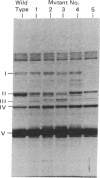
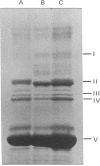
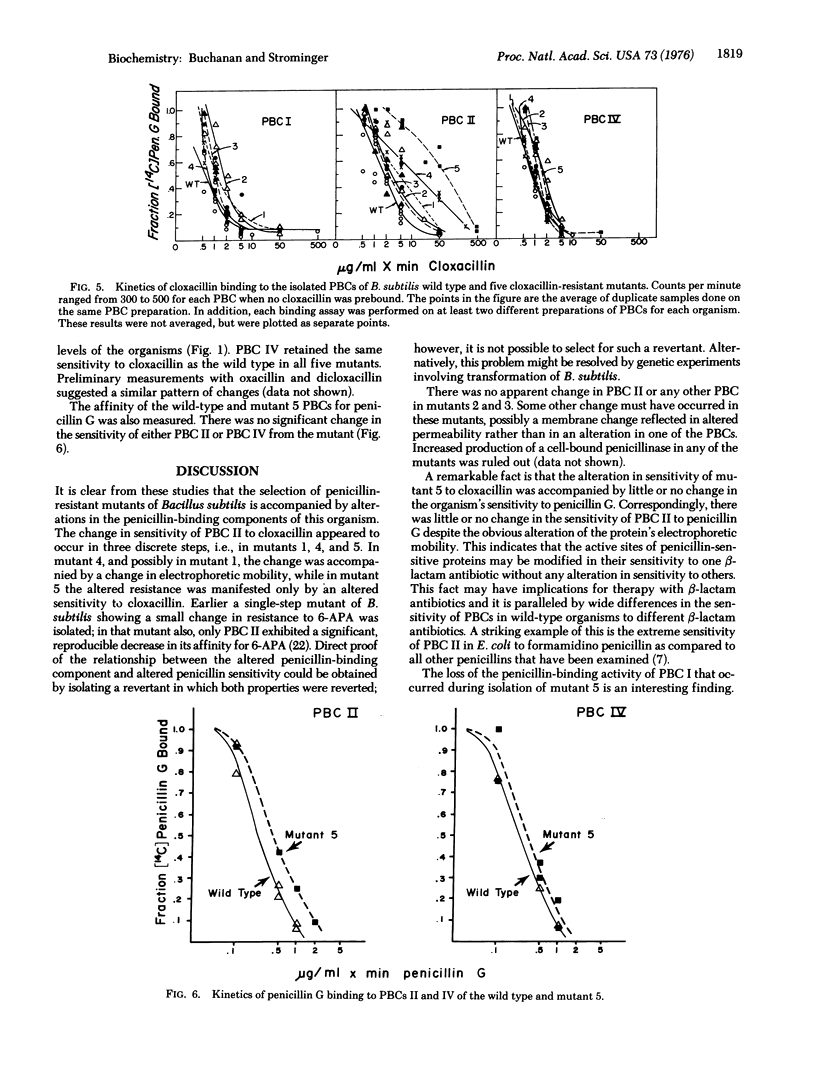
Penicillin- (cloxacillin-) resistant mutants of Bacillus subtilis were isolated in a stepwise fashion and the five penicillin-binding components (PBCs) in each were examined to determine which of the proteins, if any, corresponds to the penicillin killing site. PBCs II and V were previously eliminated as the likely penicillin targett. In the present work, PBC IV showed no change in sensitivity to cloxacillin in any of the resistant mutants isolated. PBC I did not change until the fifth-step mutant, in which it could not be detected by penicillin binding. Since PBC I did not bind penicillins that are lethal for this mutant, it also cannot be the lethal target. PBC II showed increased resistance to cloxacillin in three discrete steps, i.e., in mutants 1, 4, and 5, accompanied by changes in its electrophoretic mobility. However, the sensitivity of PBC II to penicillin G changed very little. Correspondingly, the cloxacillin-resistant mutants were unaltered in their sensitivity to penicillin G in vivo. Thus, of the five PBCs found in B. subtilis, PBC II is the most likely target for killing by penicillins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M., Strominger J. L. Five penicillin-binding components occur in Bacillus subtilis membranes. J Biol Chem. 1972 Dec 25;247(24):8107–8113. [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Inactivation of D-alanine carboxypeptidase by penicillins and cephalosporins is not lethal in Bacillus subtilis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2814–2817. doi: 10.1073/pnas.68.11.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Isolation by covalent affinity chromatography of the penicillin-binding components from membranes of Bacillus subtilis. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3751–3755. doi: 10.1073/pnas.69.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Demerec M. Origin of Bacterial Resistance to Antibiotics. J Bacteriol. 1948 Jul;56(1):63–74. doi: 10.1128/jb.56.1.63-74.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. The binding of penicillin in relation to its cytotoxic action. I. Correlation between the penicillin sensitivity and combining activity of intact bacteria and cell-free extracts. J Exp Med. 1954 Mar;99(3):207–226. doi: 10.1084/jem.99.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. R., Park J. T. Correlation between growth inhibition and the binding of various penicillins and cephalosporins to Staphylococcus aureus. J Bacteriol. 1969 Aug;99(2):459–462. doi: 10.1128/jb.99.2.459-462.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. E., Lawrence P. J. The formation of functional penicillin-binding proteins. J Biol Chem. 1975 Aug 25;250(16):6578–6585. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linn T., Losick R., Sonenshein A. L. Rifampin resistance mutation of Bacillus subtilis altering the electrophoretic mobility of the beta subunit of ribonucleic acid polymerase. J Bacteriol. 1975 Jun;122(3):1387–1390. doi: 10.1128/jb.122.3.1387-1390.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D., Cooper P. D., Roberts P. W. The site of action of penicillin. 1. Uptake of penicillin on bacteria. Biochem J. 1950 Feb;46(2):157–161. doi: 10.1042/bj0460157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A., Blumberg P. M., Strominger J. L. D-alanine carboxypeptidase and cell wall cross-linking in Bacillus subtilis. J Bacteriol. 1974 Feb;117(2):926–927. doi: 10.1128/jb.117.2.926-927.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Suginaka H., Blumberg P. M., Strominger J. L. Multiple penicillin-binding components in Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, and Escherichia coli. J Biol Chem. 1972 Sep 10;247(17):5279–5288. [PubMed] [Google Scholar]
- Swaney J. B., Vande Woude G. F., Bachrach H. L. Sodium dodecylsulfate-dependent anomalies in gel electrophoresis: alterations in the banding patterns of foot-and-mouth disease virus polypeptides. Anal Biochem. 1974 Apr;58(2):337–346. doi: 10.1016/0003-2697(74)90201-2. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterberg O. Staining of protein zones after isoelectric focusing in polyacrylamide gels. Biochim Biophys Acta. 1971 Aug 27;243(2):345–348. doi: 10.1016/0005-2795(71)90094-8. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]