Abstract
Over-expression of the dystrophin-related gene utrophin represents a promising therapeutic strategy for Duchenne muscular dystrophy (DMD). The strategy is based on the ability of utrophin to functionally replace defective dystrophin. We developed the artificial zinc finger transcription factor “Jazz” that up-regulates both the human and mouse utrophin promoter. We observed a significant recovery of muscle strength in dystrophic Jazz-transgenic mdx mice. Here we demonstrate the efficacy of an experimental gene therapy based on the systemic delivery of Jazz gene in mdx mice by adeno-associated virus (AAV). AAV serotype 8 was chosen on the basis of its high affinity for skeletal muscle. Muscle-specific expression of the therapeutic Jazz gene was enhanced by adding the muscle α-actin promoter to the AAV vector (mAAV). Injection of mAAV8-Jazz viral preparations into mdx mice resulted in muscle-specific Jazz expression coupled with up-regulation of the utrophin gene. We show a significant recovery from the dystrophic phenotype in mAAV8-Jazz-treated mdx mice. Histological and physiological analysis revealed a reduction of fiber necrosis and inflammatory cell infiltration associated with functional recovery in muscle contractile force. The combination of ZF-ATF technology with the AAV delivery can open a new avenue to obtain a therapeutic strategy for treatment of DMD. J. Cell. Physiol. 229: 1283–1291, 2014. © 2014 The Authors. Journal of Cellular Physiology Published by Wiley Periodicals, Inc.
Duchenne muscular dystrophy (DMD) is a severe X-linked muscle degenerative disease caused by the absence of the cytoskeletal protein dystrophin (McNally and Pytel, 2007). The dystrophin protein provides stability to the sarcolemma by linking the intracellular cytoskeletal network to the extracellular matrix. In the absence of dystrophin, muscle contraction mechanically stresses the plasma membrane, inducing progressive damage to the myofibers. Currently no curative treatments for this disease exist, but different therapeutic approaches are being studied (Goyenvalle et al., 2011; Fairclough et al., 2013; Konieczny et al., 2013). Our approach is to up-regulate the expression level of the dystrophin-related gene “utrophin” in DMD to complement the lack of dystrophin functions. Utrophin is considered the autosomal homologue of dystrophin because dystrophin and utrophin share structural and functional motifs through the entire molecule (Blake et al., 2002). Utrophin up-regulation is well established as a promising strategy to cure DMD (Miura and Jasmin, 2006; Tinsley et al., 2011; Amenta et al., 2012; Moorwood and Khurana, 2013; Gordon et al., 2013). To achieve utrophin up-regulation, we have engineered artificial zinc finger-based transcription factors (ZF-ATFs) capable of binding and activating transcription from the promoter “A” of both human and mouse utrophin genes (Corbi et al., 2000; Onori et al., 2009, 2007; Desantis et al., 2009). We generated a transgenic mouse model that over-expresses the artificial mini-gene coding a three zinc finger-containing protein, that we named “Jazz” (Mattei et al., 2007). Jazz transgenic mice are viable, fertile, and live to adulthood without any health problem. In addition, the Jazz transgenic mice are the first example of a transgenic mouse line expressing an artificial zinc finger transcription factor mini-gene capable of specifically up-regulating the utrophin gene at the muscular level. To evaluate the therapeutic potential of the artificial Jazz gene, we generated an mdx/Jazz mouse model by crossing Jazz transgenic mice (Jazz mice) with dystrophin-deficient mdx mice (Di Certo et al., 2010; Passananti et al., 2010). The resulting mdx/Jazz mice display a strong amelioration of the dystrophic phenotype. The relevance of using ZF-ATFs in the treatment of human diseases was recently strengthened by several studies, which encourage their use in new gene therapy strategies (Sera, 2009; Klug, 2010; Beltran and Blancafort, 2010; Blancafort et al., 2013). We used the recombinant adeno-associated virus (rAAV) strategy to deliver Jazz to the muscle of dystrophic mice. Gene transfer by rAAV has gained in the last years increasing value for experimental and human gene therapy (Carter, 2004; Carter, 2005; Grieger and Samulski, 2012). AAVs are small viruses that elicit a very mild immune response and do not cause any known human diseases. Additional advantages of these viral vectors include the existence of several serotypes ensuring a wide spectrum of tissue-specific tropism and a robust, long-term transgene expression in vivo (Kwon and Schaffer, 2008). Many successful preclinical studies on animal models, paved the way for the successive use of rAAV-based gene delivery for clinical application (Mueller and Flotte, 2008; Flotte et al., 2011; Vandenberghe and Auricchio, 2012). In addition, phase I or phase II human trials for various diseases such as hemophilia, Parkinson's disease and muscular dystrophy are underway or completed and promising results have been obtained, without serious adverse events (Kaplitt et al., 2007; Mendell et al., 2010; Nathwani et al., 2011). In particular, we focused on serotype “8” (AAV8), which efficiently infects skeletal and cardiac muscle after systemic injection and can persist for months (Wang et al., 2005). Here we report on the in vivo efficacy of the Jazz mini-gene delivered to the muscle in mdx mice by an ad hoc muscle-specific rAAV vector coupled with serotype “8.”
Material and Methods
Construction of muscle specific-rAAV vector
The human DNA fragment from chromosome 1 (NT_167186.1), 1542 base pairs long containing the α-actin enhancer, promoter, and part of the first intron has been amplified (from position 23087438–23088980) using the following oligonucleotides: 5′-ACGCGTCACCAACTGGGTAACCTCTGCTGA-3′ and 5′-GCTAGCAAGCTTACCAGGTGAACCGACTGGGTTCTG-3′. The PCR conditions were the following: 30 cycles at 95°C for 15 sec, 70°C for 30 sec, 72°C for 2 min, and a final extension at 72°C for 10 min. The transcription regulative region from cytomegalovirus of pAAV vector (Stratagene, La Jolla, CA) has been deleted. The deleted pAAV-hrGFP vector has been used to insert the 1542 base pairs long DNA fragment containing the α-actin regulative region previously amplified. The novel muscle specific-pAAV vector was named “mAAV.” mAAV vector has been used to clone Jazz transcription factor.
Production and purification of recombinant mAAV8 stocks
The AAV8 packaging plasmid containing the capsid gene was obtained from Dr. James Wilson, University of Pennsylvania. Recombinant mAAV8 were generated by the triple-plasmid transfection method “AAV Helper-Free System” (Stratagene) according to the manufacturer's instructions. mAAV8 viral particles were purified from DMEM growth medium 72 h after transfection. The cells growth medium was extensively centrifuged and viral particles were concentrated in a Spectra-Por® Float-A-Lyzer® G2 Dialysis System (Sigma–Aldrich, St. Louis, MO) using Slide-A-Lyzer Concentrating Solution for Dialysis (Thermo Fisher Scientific, St. Waltham, MA). 20:1 concentrated viral suspension was dialyzed twice against physiological solution for 5–6 h.
Titration of recombinant mAAV8 preparations using quantitative real-time PCR
The amount of recombinant mAAV8 present in the cells growth medium was assessed using a quantitative real-time PCR assay. Growth medium fractions containing mAAV8 particles were pre-treated with DNase. For DNase digestion, 5 µl of the viral suspension was incubated with 35 U of DNase I (Roche Molecular Biochemicals, Mannheim, Germany) in a final volume of 90 µl of PCR buffer (50 mM KCl, 10 mM Tris–HCl pH 8, 5 mM MgCl2) (Roche Molecular Biochemicals) at 37°C for 30 min. DNase I was inactivated by incubation at 70°C for 10 min. After DNase I treatment viral suspension was incubated with 10 µg Proteinase K (Roche Molecular Biochemicals), at 50°C for 60 min. A sample of 2.5 µl was used for qPCR. Briefly in each qPCR run a standard curve was generated using serial dilution of the vector mAAV-Jazz containing one α-actin promoter per plasmid molecule (Mayginnes et al., 2006). The standard curve was generated using the plasmid mAAV-Jazz ranging from 3 × 103 to 3 × 1013 copies. Each dilution step was measured in triplicate per ABI Prism run. PCR was performed using the SYBR Green DNA Master Mix (Life Technologies Corporation, Carlsbad, CA). PCR products were subjected to melting curve analysis using the light cycler system to exclude the amplification of unspecific products. Primers were synthesized by Life Technologies Corporation.
The following primers were used: 5′-CGAGCCGAGAGTAGCAGTTGTAG-3′; 5-′GCTAGCTAGCAAGCTTACCAGGTGAACCGACTGGGTTCTG-3′. The single-stranded nature of the mAAV8 genome as well as the double-stranded plasmid standard curve values were taken into consideration.
Animal care
Dystrophin-deficient C57BL/10ScSn-DMDmdx/J mice (mdx) were housed under a 12-h light–dark schedule and were fed with fat-enriched rodent chow to ameliorate the low fertility of this strain. All experiments were carried out in accordance with the Directive 2010/63/EU of the European Community for the care and use of laboratory animals. Housing of the animals meets the behavioral needing of the specie and was supervised by the Responsible Veterinarian.
Mice treatments
Mice were injected with mAAV8-GFP or mAAV8-Jazz viruses at 5 days of age. Systemic infection was achieved by intraperitoneal (i.p.) injection of 150 μl of viral suspension (5 × 1012 v.p./ml; 75 μl on each side of the lower quadrant of the abdomen) using a 0.3-ml Accu-Fine syringe (Roche Molecular Biochemicals). Control mice were injected with the same volume of saline solution.
RNA extraction, RT-PCR, and real-time PCR
Total RNA was isolated from skeletal muscle and reverse transcribed as previously described (Di Certo et al., 2010). Real-time PCR assays were performed in a 96-well format using the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). To obtain the utrophin gene expression rate the amount of target gene was normalized to that of the housekeeping gene β2-microglobulin (β2M). Primers and probes for the target gene (UTRN) and for housekeeping gene were purchased as TaqMan Gene Expression Assays (Life Technologies Corporation). PCR reaction was performed as previously described (Mattei et al., 2007). The results were analyzed using Applied Biosystems analysis software. The data are expressed as the ratio between UTRN and β2M mRNA expression. A minimal number of three mice were analyzed for each category. The expression of Jazz-mRNA in different tissues was studied with RT-PCR using the following primers: 5′-GTCGCCCCCCCGACCGATGTCAGC-3′ and 5′-GGGCGATCCAGGATCCCCGGGAAT-3′. Primers for β2M expression were used to control amplification: 5′-TTCTGGTGCTTGTCTCACTGA-3′ and 5′-CAGTATGTTCGGCTTCCCATTC-3′. PCR products were analyzed by agarose gel electrophoresis.
Western blot analysis
Protein extracts were obtained as previously described (Di Certo et al., 2010). Fifty micrograms of protein extracts was electrophoresed through standard 6% SDS–PAGE or NuPAGE 4–12% (Life Technologies Corporation), according to the manufacturer's instructions. The following antibodies were used: anti-myc monoclonal antibody (9E10 clone, DSHB, Iowa City, IO); anti-GFP polyclonal antibody (Agilent Technologies, Santa Clara, CA); anti-utrophin monoclonal antibody (Santa Cruz, Santa Cruz, CA); anti α-tubulin monoclonal antibody (Sigma Corporation, St. Louis, Missouri), and anti-laminin polyclonal antibody (Sigma Corporation). Immunoreactive bands probed with horseradish peroxidase-conjugated antibodies were visualized by chemiluminescence (ECL; GE Healthcare, Little Chalfont, UK), according to the manufacture's instructions.
Histological analysis
Transversal sections of muscles from mdx mice injected with mAAV8-Jazz, mAAV8-EGFP or saline were obtained as previously reported (Di Certo et al., 2010). Sections were stained with hematoxylin and eosin (H&E; Roth, Karlsruhe, Germany) following the manufacturer's instructions. The entire cross-section, taken at mid-belly, was analyzed by microscope (Olympus BX51; Tokyo, Japan). Images were captured using a digital camera at 10× magnification.
Measure of cross-sectional area
Quantification of cross-sectional area (CSA) of single muscle fibers, in various skeletal muscle from 8-week-old mAAV8-Jazz, mAAV8-EGFP, or saline injected mdx mice, was expressed in μm2. Six mice per group were analyzed and at least 200 myofibers were counted in each section; data are expressed as means ± SEM.
EGFP detection
At the age of 15 and 60 days, mAAV8-EGFP-injected and control mdx mice were sacrificed and perfused with 4% paraformaldehyde in PBS. Cryostatic sections from muscles and other organs were observed for direct EGFP fluorescence by conventional epifluorescence microscope (Olympus BX51). Images were captured using a digital camera at 10× magnification and merged using the IAS2000 software.
Immunohistochemistry
Skeletal muscle cross-sections were subjected to indirect immunofluorescence as previously described (Di Certo et al., 2010). The following primary antibodies were used: anti-laminin polyclonal antibody (Sigma Corporation); anti-CD68 monoclonal antibody (Lifespan Biosciences, Seattle, WA). To visualize myc-tagged protein expression, transversal sections of 6-μm thick were treated as described (Veal and Jackson, 1998). Briefly, slides were fixed in ice-cold acetone followed by immersion in 1% H2O2 in methanol at room temperature. Sections were blocked with 10% goat serum in PBS for 30 min and dual-stained with anti-myc monoclonal antibody (9E10 clone) and anti-laminin polyclonal antibody (Sigma Corporation), in 10% goat serum in PBS at 4°C overnight. The following secondary antibodies were used: Alexa Fluor 594 and 488 conjugated IgG (Life Technologies Corporation). Slides were mounted with ProLong Gold antifade reagent with Dapi (Life Technologies Corporation). Stained specimens were analyzed by conventional epifluorescence microscope (Olympus BX51).
Mechanical response of isolated muscles and Procion orange uptake
Contractile activity of muscles from mAAV8-Jazz-treated and control mdx mice was examined in vitro by physiological assessment of the muscle force on isolated extensor digitorum longus (EDL) preparations of both hind limbs and of abdominal longitudinal strips (ABD). Muscle preparations were suspended in a 20-ml bath of oxygenated Krebs solution (120 mM NaCl, 25.1 mM NaHCO3, 2.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1.3 mM CaCl2, and 5 mM d-glucose) maintained at 37°C, stretched to a tension of 1.0 g and allowed to equilibrate for 30–60 min, changing the superfusion buffer every 15–20 min. Both EDL muscles, mounted vertically with their tendons intact and ABD muscles were exposed to direct field stimulation via platinum wire electrodes using single stimulations of rectilinear pulses of 0,5 msec duration at 0.05–0.2 Hz (Electric Stimulatore Digit 3 T, Lace Elettronica, Pisa, Italy). Muscle excitability was examined by varying the voltage from 0.5 to 7 V, until the supramaximal voltage was reached. Muscle mechanical activity was recorded isotonically by a strain-gauge transducer (7006 isotonic transducer) and displayed on a recording microdynamometer (Unirecord 7050, Basile, Milano, Italy). At the end of the tension recordings, EDL and ABD muscles were subjected to a period of repetitive stimulation. Muscle contractions were elicited by trains of stimuli at a frequency of 40 Hz for 250 msec every second for 3 min. Following this procedure, able to obtain the muscle fatigue, tissues were removed from the chamber and subjected to Procion Orange staining as previously described (Di Certo et al., 2010).
Assays of mice performances by treadmill running and Evans Blue dye
Exercise studies were performed on five-lane motorized treadmill equipped with an electronic control unit (Treadmill Model LE8710, PanLab, Cornellà, Barcelona, Spain), and an electric shock grid at one end of the treadmill. Shock intensity was set at 0.4 mA. Inclination of treadmill was set at 0°. During the first session, mice were familiarized to the treadmill apparatus for 2 min before starting the belt followed by a running with speed set at 6 m/min. Each mouse was immediately removed from treadmill after three electric shock. One day after habituation, mice were subjected to an endurance protocol, repeated once a week for four consecutive weeks, with belt running at accelerated speed. Mice were first acclimated with treadmill for 2 min, followed by a running session with belt speed initially set at 12 m/min. At 5 min after the initiation of exercise, the speed was increased by 1 m/min every 2 min and exercise continued until exhaustion defined as inability to maintain running speed despite repeated contact with the electric grid. The time for removal of mice from the treadmill was 5 sec on the shocker plate without attempting to reengage the treadmill. The time to exhaustion was automatically recorded from the beginning of the running session. After the treadmill performance some mice were injected intraperitoneal with 1% of Evans Blue dye solution in saline buffer. Muscle specimens were taken and processed as described above.
Results
Here we report on the in vivo muscle-specific expression of the ZF-ATF “Jazz” in mdx mice upon adeno-associated viral delivery. To achieve the highest degree of Jazz muscle-specific expression, we constructed a novel muscle rAAV vector and combined it with the use of AAV serotypes with muscle tropism. The Stratagene commercial pAAV vector was modified by substituting the CMV regulatory regions with the human muscle-specific enhancer-promoter of the α-actin gene. To further stabilize the transcript, part of the first intron of the human α-actin gene with the beta-Globin intron acceptor was introduced. This novel muscle-specific pAAV vector was named “mAAV.” As shown in Figure 1a, the mAAV vector has been used to produce two different constructs expressing either the test EGFP gene (mAAV-EGFP) or the ZF-ATF Jazz (mAAV-Jazz). The mAAV-EGFP construct has been used to develop virus purification protocols and to tune tissue infection conditions (Fig. 1b). The AAV tissue specificity is determined by the capsid serotype, and rAAV vectors can alter their tropism when coupled with different capsid serotypes. We focused on AAV serotype 8 (AAV8), which has been demonstrated to display high tropism for both skeletal and cardiac muscles (Wang et al., 2005; Gruntman et al., 2013). Efficiency, timing and tissue specificity of the mAAV8 infection in the mdx mice were initially assessed by the EGFP reporter gene expression. In Figure 1c, fluorescent microscopy on muscle cryosections shows that mAAV8-EGFP expression is already established in several mdx muscles 15 days after intraperitoneal injection. This expression appears to be persistent after 2 months, but it shows a decrease of intensity in the different muscles analyzed. Saline solution was injected as a control. Notably, no EGFP expression is detectable in the liver (Fig. 1c) or in other non-muscular tissues tested (data not shown). These results confirm the AAV8 efficacy in transducing muscles by intraperitoneal diffusion underlining the high muscle specificity of our mAAV vectors. In Figure 1d, the expression of mAAV8-EGFP was also evaluated by Western blot analysis of the indicated tissues. The expression of the artificial transcription factor gene Jazz, delivered in mdx mice by mAAV8 infection was monitored by different approaches. As shown in Figure 2a, the presence of Jazz transcripts was checked by RT-PCR experiments using RNA extracted from abdominal and quadriceps muscles of mdx mice intraperitoneally injected either with mAAV8-Jazz or saline solution (as a control). In Figure 2b, Western blot analysis of total proteins extracted from skeletal muscles, heart, and liver of mAAV8-Jazz-treated, mAAV8-EGFP-treated and control mice (saline injected) confirmed muscle-specific Jazz expression. Finally, immunohistochemical analysis on cryosections of quadriceps muscles, stained with anti-myc tag antibody, revealed the presence of Jazz protein in the nuclei of infected myofibers (Fig. 2c). To determine whether utrophin is up-regulated in the muscles infected by mAAV8-Jazz, specimens from skeletal and cardiac muscles were analyzed by both real-time PCR and Western blot experiments. As shown in Figure 2d, in tissues infected with mAAV8-Jazz, the utrophin mRNA expression level is clearly up-regulated (up to twofold induction). The Western blot presented in Figure 2e shows a utrophin protein up-regulation in the muscles infected with mAAV8-Jazz. These data demonstrate that the delivery of mAAV8-Jazz by intraperitoneal injection in mdx mice is effective and adequate to re-program utrophin expression in muscle.
Figure 1.
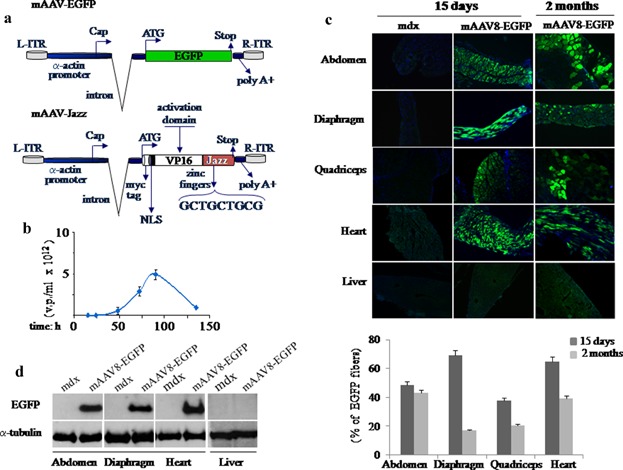
mAAV virus vectors. a: Schematic representation of the engineered mAAV-EGFP and mAAV-Jazz vectors under the control of the muscle-specific human α-actin promoter. b: Time course titration curve obtained by qPCRs performed with growth medium fractions containing viral particles from AAV-293 transfected cells. c, top: EGFP expression in skeletal and cardiac muscles and liver tissues. The mAAV-EGFP-treated and untreated mdx mice were injected intraperitoneally at 5 days of age. Injections were performed with 150 µl of mAAV8-EGFP virus suspension at the concentration of 5 × 1012 v.p./ml, or with the same volume of saline solution. At different times after injection mice were analyzed and cryostatic sections were examined for direct EGFP fluorescence. Nuclei are stained in blue with Dapi. All the images were taken at 10× magnification. c, bottom: The number of EGFP fibers for each group was obtained by normalizing to the number of total fibers per cross-sectional area. The resulting percentages at the indicate times are reported in a histogram. d: Evaluation of EGFP protein expression by Western blot analysis in skeletal and cardiac muscles and in liver tissues. Five-day-old mdx mice were injected as above and examined 15 days after injection using the polyclonal antibody against EGFP. Detection of α-tubulin was used to normalize the amount of proteins.
Figure 2.
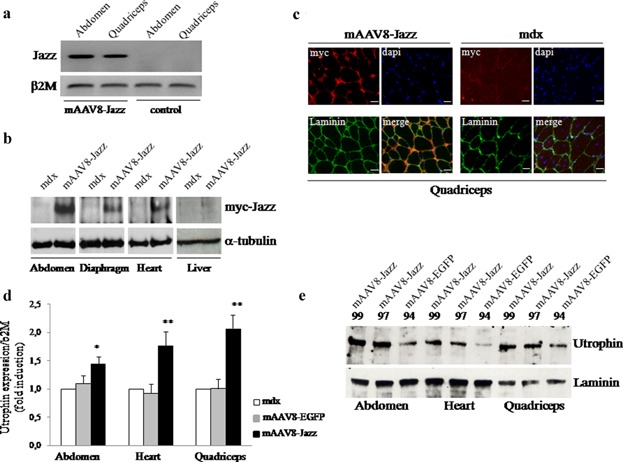
Expression of mAAV8-Jazz and utrophin up-regulation in mdx mice. a: Evaluation of Jazz-mRNA expression by RT-PCR of skeletal muscle mRNA from AAV8-Jazz-treated and untreated mdx mice. A β2M control from the same samples is shown below. b: Evaluation of Jazz protein expression by Western blot analysis in skeletal and cardiac muscles and in liver tissues. Five-day-old mdx mice were intraperitoneally injected and examined 15 days after injection using the anti-myc tag monoclonal 9E10 antibody. Detection of α-tubulin was used to normalize the amount of proteins. c: Immunohistochemistry of the quadriceps muscle derived from 2-month-old untreated and mAAV8-Jazz-treated mdx mice stained with the anti-myc tag monoclonal 9E10 antibody (red). The extracellular matrix is stained with the anti-laminin polyclonal antibody (green), and nuclei are counterstained with Dapi. Scale bar: 20 μm. d: Quantification by real-time PCR of utrophin transcripts from skeletal and heart muscles isolated from 2-month-old untreated mdx mice and mAAV8-EGFP-treated or mAAV8-Jazz-treated mdx mice. The gene expression ratio between utrophin and β2-microglobulin (β2 M) is shown as the mean ± SEM. from three independent experiments performed in triplicate. *P < 0.05 and **P < 0.01 indicate statistical significance by t-test. e: Western blot analysis of utrophin protein levels in abdominal, heart, and quadriceps muscles isolated from 2-month-old mAAV8-Jazz-treated or mAAV8-EGFP-treated mdx mice. Representative individual mice are indicated with numbers. Detection of laminin was used to normalize the amount of proteins.
The possible therapeutic effects of utrophin up-regulation in the muscle of mdx mice treated with mAAV8-Jazz were evaluated by typical DMD diagnostic parameters. Analysis of the slices derived from mAAV8-Jazz muscles shows a substantial morphology/architecture amelioration in comparison with mAAV8-EGFP and control saline-injected mdx mice (Fig. 3a). We also quantified the frequency of centrally nucleated myofibers (CNFs), the fiber CSA, and the extent of mononuclear inflammatory cell infiltration in muscle slices stained with hematoxylin and eosin (H&E), and the results further demonstrate reduced degenerative/regenerative processes in mAAV8-Jazz muscles (Fig. 3b). Next, as shown in Figure 3c, by immunostaining of the dystrophic muscle with the macrophage marker CD68, we observed a reduction of the muscular inflammatory response in mAAV8-Jazz infected mdx mice. Dystrophin-deficient muscles are characterized by a severe deficit in contractile force and marked susceptibility to contraction-induced injury (Di Certo et al., 2010). To verify whether mAAV8-Jazz expression improves the mechanical responses in dystrophic muscle, we measured the contractile activity of muscles from 2-month-old mAAV8-Jazz infected and control mdx mice. Isolated abdominal muscle strips and EDL were subjected to in vitro physiological assessment of muscle force using variable voltages until the supramaximal value was reached. As shown in Figure 4a, muscles from mAAV8-Jazz-treated mdx mice showed a significant increase in strength compared with muscle preparations from control mdx mice. At the end of the force test, we assessed contraction-induced injury of the sarcolemma by staining each muscle with Procion Orange dye. Uptake of this fluorescent dye into individual fibers is an index of membrane integrity loss. As shown in Figure 4b-left, fluorescence microscopy of both abdominal and EDL muscles cross-sections showed extensive uptake of the Procion Orange dye into non-treated mdx mice compared with mAAV8-Jazz injected mdx mice. Quantification of the percentage of dye-positive area in each section confirmed the increased ability of Jazz-expressing muscles to exclude the dye from stressed fibers (Fig. 4b, right). Altogether, these data provide physiological evidence for recovery in both contractile force and sarcolemmal integrity in mdx mice infected with mAAV8-Jazz. In addition to the in vitro testing, we assessed the effects of the experimental gene therapy with mAAV8-Jazz on the overall strength of dystrophic muscles in vivo. At 3 months of age mAAV8-Jazz mdx-treated mice and control mAAV8-EGFP mdx-treated mice or mdx non-treated mice were subjected to forced physical exercise on an accelerating treadmill. The exercise was repeated once a week for four consecutive weeks and the running time was recorded in each session. As shown in Figure 4c, mAAV8-Jazz mdx-treated mice scored, over the four performances, a cumulative running time of approximately 110 min before reaching exhaustion, compared to the 70 min mean performance of mdx untreated/mAAV8-EGFP-treated mice. Thus, the up-regulation of utrophin achieved by the mAAV8-mediated delivery of ZF-ATF Jazz effectively counteracts the symptoms of dystrophic pathology, resulting in an enhanced endurance performance. Furthermore, as already demonstrated in vitro with Procion Orange dye, Jazz-mediated utrophin over-expression preserves in vivo sarcolemmal integrity during exercise, as shown by the reduced uptake of Evan's blue dye systemically injected in mice immediately after the end of the treadmill experiments (Fig. 4d). These results demonstrate the remarkable functional recovery of overall muscle strength in mdx mice expressing the artificial transcription factor Jazz systemically administered by the mAAV8 vector and provide important insight for the possible future clinical use of ZF-ATFs to up-regulate utrophin expression for DMD gene therapy.
Figure 3.
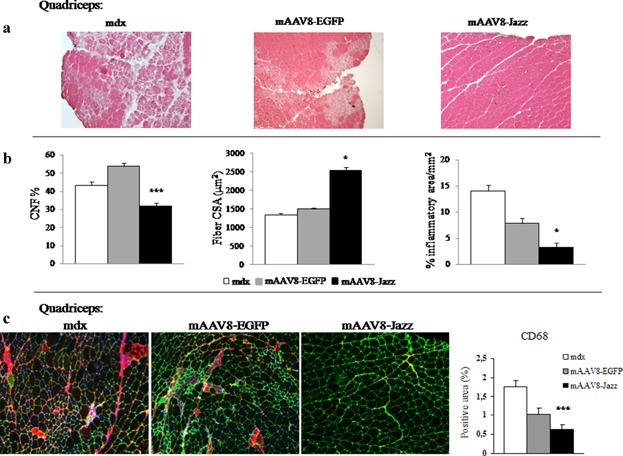
mAAV8-Jazz ameliorates mdx muscle morphology. a: H&E staining of the quadriceps muscle from 2-month-old untreated, mAAV8-EGFP-treated, and mAAV8-Jazz-treated mdx mice. Reduction in degeneration, necrotic foci, and inflammatory cells is observed in mAAV8-Jazz-treated myofibers (representative sections out of six mice examined in each group). b: Quantification of central nucleation (CNF), cross-sectional area (CSA), and inflammatory infiltrates on H&E stained sections of quadriceps muscle from 2-month-old untreated, mAAV8-EGFP-treated, and mAAV8-Jazz-treated mdx mice (six mice were analyzed in each group; the number of CNFs was obtained by normalizing to the number of total myofibers per CSA, and at least 200 myofibers per section were counted). c: Immunohistochemistry of the quadriceps muscle isolated from 2-month-old untreated, mAAV8-Jazz-treated, and mAAV-EGFP-treated mdx mice (four mice were analyzed in each group). Quantification of macrophage infiltration by staining with CD68 monoclonal antibody (red). The extracellular matrix is counterstained with the anti-laminin polyclonal antibody (green). Nuclei are stained with Dapi (blue; see Materials and Medhods section). Right: Graph shows the quantification of the CD68-positive area (four mice were analyzed in each group). All values are expressed as the mean ± SEM. *P < 0.05 and ***P < 0.001 indicate statistical significance by t-test.
Figure 4.
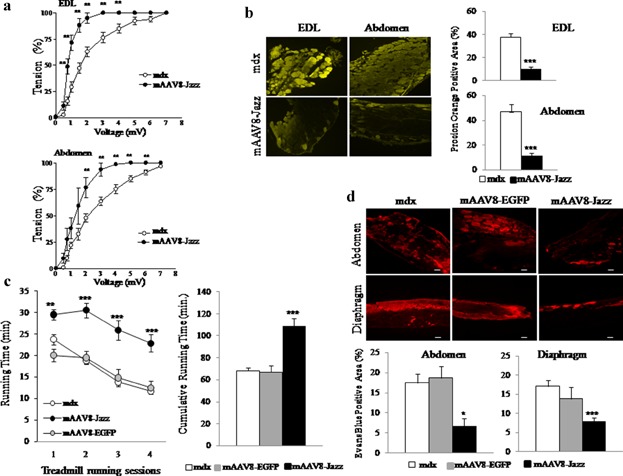
Rescue of muscle function by mAAV8-Jazz treatment. a: Mechanical response of isolated muscles. Effects of changes in voltage on isotonic contractions in EDL and abdominal muscles, from control and mAAV8-Jazz-treated mdx mice. Muscle contractile force for each voltage was determined and considered as a percentage of maximal contraction. Each point represents the mean of 10 EDL muscles (5 animals) and five abdominal muscle strips, with error bars indicating SEM and **P < 0.01 indicating the statistical significance by t-test. b: Procion Orange dye uptake in sections of abdominal and EDL muscles after force test. Left: Representative images demonstrating the increased ability of mAAV8-Jazz-treated mdx mice to exclude dye from stretched fibers. Right: graph shows the mean (±SEM) area of dye-positive fibers expressed as the percentage of the total CSA of muscle sections. c: Single session performance (left) and total running time (right) relative to four weekly treadmill trials with exhaustive exercise protocol. The mdx mice injected with saline and mAAV8-EGFP (controls) or mAAV8-Jazz at day 5 after birth were tested at 3 months of age. For each group, lines indicate the mean duration of running time during each trial (left), and columns indicate the cumulative running time over the four consecutive trials (right). Number of animals was 10 for each experimental group. The mAAV8-Jazz-treated mdx mice showed a significant improvement of exercise performance as compared to mdx and to mAAV8-EGFP-treated mdx mice. Statistical analysis with “unpaired t-test” showed a significant main effect of treatment on mice performance indicated by **P < 0.01 and ***P < 0.001. d: Evan's blue dye (EBD) uptake was used to compare skeletal muscle membrane integrity after exercise. Top: abdominal muscle and diaphragm muscles from untreated and mAAV8-EGFP-treated (controls) or mAAV8-Jazz-treated mdx mice were monitored for EBD uptake by fluorescence microscope. EBD uptake (red) was significantly higher in sections of control mdx mice as compared with mAAV8-Jazz-treated mdx mice. Bottom: Evans blue uptake was also scored as a percentage of Evans blue positive myofibers *P < 0.05 and ***P < 0.001. Scale Bar: 50 μm.
Discussion
The use of ZF-ATFs, designed ad hoc to alter the expression profile of target genes related to diseases, is practicable and offers promising prospects in medical research (Klug, 2010; Beltran and Blancafort, 2010; Blancafort et al., 2013). Two crucial steps need to be faced and analyzed in all aspects: safe delivery and absence/reduction of immunogenicity induced by both ZF-ATFs and their delivery vehicles. Gene delivery using viral vectors has been widely explored (Carter, 2004). AAVs are emerging as a powerful gene transfer vehicles in the treatment of neuromuscular disorders because of their ability to transduce the vast majority of the mouse striated musculature with a single administration (Odom et al., 2008). The additional advantages of rAAV vectors include their intrinsic capacity for persistent transgene expression in post-mitotic cells and the existence of several muscle-tropic serotypes. These features make rAAV a very attractive viral-vector for DMD gene therapy, and early clinical trials based on rAAV delivery have been already described (Flotte et al., 2011; Vandenberghe and Auricchio, 2012). The fundamental idea underlining our DMD therapeutic approach was to confine the expression of the Jazz artificial transcription factor only in muscle tissue. Therefore, we combined the systemic intraperitoneal injection with the use of AAV serotype 8 that displays high tropism for both skeletal and cardiac muscles (Wang et al., 2005; Gruntman et al., 2013). The AAV8 efficiently transduces muscle of non-human primates without inducing a cellular immune response (Boutin et al., 2010) and, among the different serotypes, elicits low immune responses in healthy humans (Rodino-Klapac et al., 2010). These features are crucial given the possible AAV8 use in clinical applications. We have further guaranteed muscle-specific expression by engineering our rAAV viral vector with the α-actin human muscle promoter. The replacement of the CMV regulatory sequence with the larger human muscle α-actin promoter in the commercial Stratagene pAAV vector was possible because of the small size of our ZF-ATFs. The human α-actin promoter ensures a strong and persistent muscle transgene expression. To enhance the transgene mRNA stability, we also substituted, in our mAAV vector, the intron acceptor of the human α-actin promoter with the more efficient beta-globin intron acceptor. The expression of the mAAV8-EGFP test gene was early, strong and persistent in both skeletal and cardiac muscle upon a single intraperitoneal injection. This observation is very promising in view of future gene therapy targeting cardiomyopathy associated with DMD (Nigro et al., 1990; Konieczny et al., 2013). Another important feature is that no off-target EGFP fluorescence/expression was measured in organs, such as the liver, that are located intraperitoneally. A mosaic EGFP fluorescent pattern is observed in the analyzed muscles. In mdx mice, starting from the third week of life, a fast myofibers turnover occurs, this event may account for the decline of EGFP expression observed at the age of two months in a portion of fibers. Importantly, we have previously reported that the Jazz artificial transcription factor in transgenic mice is well tolerated and safe, and that Jazz works accurately without significant non-specific global transcriptional effects (Mattei et al., 2007). We also demonstrated, in transgenic mdx mice, the ability of Jazz to ameliorate the dystrophic phenotype by utrophin up-regulation (Di Certo et al., 2010). Another consideration is that the Jazz protein could interfere with the epigenetic mechanisms that postnatally regulate utrophin promoter A (Dennis et al., 1996; Perkins et al., 2007; Basu et al., 2007). A large number of muscle fibers expressing the transgene in transgenic mice is not currently achievable by any possible delivery system. Nevertheless, here we show that the Jazz levels reached upon mAAV8 delivery, resulted in a significant utrophin up-regulation in both skeletal and cardiac mdx muscle. The utrophin levels attained are sufficient to provide therapeutic effects, ameliorating the dystrophic phenotype. Morphological analysis of mAAV8-Jazz-treated mdx mice showed a significant improvement in muscle fibers architecture associated with the reduction of central nucleation/inflammatory infiltrates and the increase in fiber diameter. The mAAV8-Jazz-treated mdx mice display reduced muscle infiltration of CD68-positive cells, confirming the ability of Jazz to prevent myofiber damage. This finding is consistent with our previous microarray data showing that ZF-ATF Jazz switches inflammatory pathways off in dystrophic muscle of the Jazz-mdx transgenic model (Di Certo et al., 2010). Next, we showed that treating mdx mice with mAAV8-Jazz not only improves skeletal muscle morphology but also significantly corrects muscle function. By in vitro testing on isolated muscles, we showed that treatment of mdx mice with mAAV8-Jazz improves both muscle contractile force and sarcolemmal integrity. Importantly, statistical analysis relative to treadmill exhaustive exercise demonstrated that mAAV8-Jazz-injected mdx mice exhibited better exercise performance than untreated and mAAV8-EGFP-injected mdx mice.
DMD and other muscular dystrophies are among the most difficult diseases to treat, although the underlying pathogenesis is well understood (Fairclough et al., 2013; Konieczny et al., 2013). Research into therapeutic approaches for muscular distrophy moved rapidly in recent years and several novel strategies are ready to enter clinical trials (Mendell et al., 2012). ZF-ATF genes could represent advantageous tools to develop novel therapeutic molecules for DMD treatment (Lu et al., 2008; Di Certo et al., 2010; Passananti et al., 2010). There are several clinical protocols ongoing to evaluate the effectiveness of ZF-ATFs as novel therapeutic molecules (http://www.sangamo.com). Importantly, ZF-ATFs tested in humans to date have an excellent safety profile. Adeno-associated vectors ad hoc engineered to vehicle and express ZF-ATFs exclusively in the target pathologic tissue represent an additional value in the gene therapy field. In conclusion, we show that a gene therapy approach based on the combination of ZF-ATF technology and rAAV delivery succeeded in amelioration of the dystrophic pathology in mdx mice. Our ongoing work involves minimizing the ZF-ATF host immune-response. To this end, we are developing novel ZF-ATFs engineered to appear almost identical to natural resident zinc finger transcription factors.
Acknowledgments
We thank Dr. R. G. Ruscitti for her precious constant assistance. This manuscript was edited for proper English language by American Journal Experts; Certificate Verification Key: 4C5A-2C3C-9EF4-9949-0761. This work was supported by Telethon-Italy (grant no. GGP10094), Associazione Italiana per la Ricerca sul Cancro (AIRC) Project grant no IG 9073, PRIN-2008-81213, AFM Project grant no. 15586. Dr. F.G. fellowship was supported by Regione Lazio fundings for “Sviluppo della Ricerca sul Cervello” and FARMM-Onlus.
Literature Cited
- Amenta AR, Creely HE, Mercado ML, Hagiwara H, McKechnie BA, Lechner BE, Rossi SG, Wang Q, Owens RT, Marrero E, Mei L, Hoch W, Young MF, McQuillan DJ, Rotundo RL, Fallon JR. Biglycan is an extracellular MuSK binding protein important for synapse stability. J Neurosci. 2012;32:2324–2334. doi: 10.1523/JNEUROSCI.4610-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu U, Gyrd-Hansen M, Baby SM, Lozynska O, Krag TO, Jensen CJ, Frödin M, Khurana TS. Heregulin-induced epigenetic regulation of the utrophin-A promoter. FEBS Lett. 2007;581:4153–4158. doi: 10.1016/j.febslet.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran AS, Blancafort P. Remodeling genomes with artificial transcription factors (ATFs) Methods Mol Biol. 2010;649:163–182. doi: 10.1007/978-1-60761-753-2_10. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- Blancafort P, Jin J, Frye S. Writing and rewriting the epigenetic code of cancer cells: From engineered proteins to small molecules. Mol Pharmacol. 2013;83:563–576. doi: 10.1124/mol.112.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- Carter BJ. Adeno-associated virus and the development of adeno-associated virus vectors: A historical perspective. Mol Ther. 2004;10:981–989. doi: 10.1016/j.ymthe.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Carter BJ. Adeno-associated virus vectors in clinical trials. Hum Gene Ther. 2005;16:541–550. doi: 10.1089/hum.2005.16.541. [DOI] [PubMed] [Google Scholar]
- Corbi N, Libri V, Fanciulli M, Tinsley JM, Davies KE, Passananti C. The artificial zinc finger coding gene ‘Jazz’ binds the utrophin promoter and activates transcription. Gene Ther. 2000;7:1076–1083. doi: 10.1038/sj.gt.3301204. [DOI] [PubMed] [Google Scholar]
- Dennis CL, Tinsley JM, Deconinck AE, Davies KE. Molecular and functional analysis of the utrophin promoter. Nucleic Acids Res. 1996;24:1646–1652. doi: 10.1093/nar/24.9.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desantis A, Onori A, Di Certo MG, Mattei E, Fanciulli M, Passananti C, Corbi N. Novel activation domain derived from Che-1 cofactor coupled with the artificial protein Jazz drives utrophin upregulation. Neuromuscul Disord. 2009;19:158–162. doi: 10.1016/j.nmd.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Di Certo MG, Corbi N, Strimpakos G, Onori A, Luvisetto S, Severini C, Guglielmotti A, Batassa EM, Pisani C, Floridi A, Benassi B, Fanciulli M, Magrelli A, Mattei E, Passananti C. The artificial gene Jazz, a transcriptional regulator of utrophin, corrects the dystrophic pathology in mdx mice. Hum Mol Genet. 2010;19:752–760. doi: 10.1093/hmg/ddp539. [DOI] [PubMed] [Google Scholar]
- Fairclough RJ, Wood MJ, Davies KE. Therapy for Duchenne muscular dystrophy: Renewed optimism from genetic approaches. Nat Rev Genet. 2013;14:373–378. doi: 10.1038/nrg3460. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Conlon TJ, Mueller C. Preclinical study design for rAAV. Methods Mol Biol. 2011;807:317–337. doi: 10.1007/978-1-61779-370-7_14. [DOI] [PubMed] [Google Scholar]
- Gordon BS, Delgado Díaz DC, Kostek MC. Resveratrol decreases inflammation and increases utrophin gene expression in the mdx mouse model of duchenne muscular dystrophy. Clin Nutr. 2013;32:104–111. doi: 10.1016/j.clnu.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Goyenvalle A, Seto JT, Davies KE, Chamberlain J. Therapeutic approaches to muscular dystrophy. Hum Mol Genet. 2011;20:R69–R78. doi: 10.1093/hmg/ddr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger JC, Samulski RJ. Adeno-associated virus vectorology, manufacturing, and clinical applications. Methods Enzymol. 2012;507:229–254. doi: 10.1016/B978-0-12-386509-0.00012-0. [DOI] [PubMed] [Google Scholar]
- Gruntman AM, Bish LT, Mueller C, Sweeney HL, Flotte TR, Gao G. Gene transfer in skeletal and cardiac muscle using recombinant adeno-associated virus. Curr Protoc Microbiol. 2013;14:Unit 14D.3. doi: 10.1002/9780471729259.mc14d03s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: An open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Klug A. The discovery of zinc fingers and their development for practical applications in gene regulation and genome manipulation. Q Rev Biophys. 2010;43:1–21. doi: 10.1017/S0033583510000089. [DOI] [PubMed] [Google Scholar]
- Konieczny P, Swiderski K, Chamberlain JS. Gene and cell-mediated therapies for muscular dystrophy. Muscle Nerve. 2013;47:649–663. doi: 10.1002/mus.23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon I, Schaffer DV. Designer gene delivery vectors: Molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer. Pharm Res. 2008;25:489–499. doi: 10.1007/s11095-007-9431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Tian C, Danialou G, Gilbert R, Petrof BJ, Karpati G, Nalbantoglu J. Targeting artificial transcription factors to the utrophin A promoter: Effects on dystrophic pathology and muscle function. J Biol Chem. 2008;283:34720–34727. doi: 10.1074/jbc.M804518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei E, Corbi N, Di Certo MG, Strimpakos G, Severini C, Onori A, Desantis A, Libri V, Buontempo S, Floridi A, Fanciulli M, Baban D, Davies KE, Passananti C. Utrophin up-regulation by an artificial transcription factor in transgenic mice. PLoS One. 2007;2:e774. doi: 10.1371/journal.pone.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayginnes JP, Reed SE, Berg HG, Staley EM, Pintel DJ, Tullis GE. Quantitation of encapsidated recombinant adeno-associated virus DNA in crude cell lysates and tissue culture medium by quantitative, real-time PCR. J Virol Methods. 2006;137:193–204. doi: 10.1016/j.jviromet.2006.06.011. [DOI] [PubMed] [Google Scholar]
- McNally EM, Pytel P. Muscle diseases: The muscular dystrophies. Annu Rev Pathol. 2007;2:87–109. doi: 10.1146/annurev.pathol.2.010506.091936. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, Lewis S, Malik V, Shilling C, Byrne BJ, Conlon T, Campbell KJ, Bremer WG, Taylor LE, Flanigan KM, Gastier-Foster JM, Astbury C, Kota J, Sahenk Z, Walker CM, Clark KR. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol. 2010;68:629–638. doi: 10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JR, Rodino-Klapac L, Sahenk Z, Malik V, Kaspar BK, Walker CM, Clark KR. Gene therapy for muscular dystrophy: Lessons learned and path forward. Neurosci Lett. 2012;527:90–99. doi: 10.1016/j.neulet.2012.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura P, Jasmin BJ. Utrophin upregulation for treating Duchenne or Becker muscular dystrophy: How close are we? Trends Mol Med. 2006;12:122–129. doi: 10.1016/j.molmed.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Moorwood C, Khurana TS. Duchenne muscular dystrophy drug discovery—The application of utrophin promoter activation screening. Expert Opin Drug Discov. 2013;8:569–581. doi: 10.1517/17460441.2013.777040. [DOI] [PubMed] [Google Scholar]
- Mueller C, Flotte TR. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O‘Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990;26:271–277. doi: 10.1016/0167-5273(90)90082-g. [DOI] [PubMed] [Google Scholar]
- Odom GL, Gregorevic P, Allen JM, Finn E, Chamberlain JS. Microutrophin delivery through rAAV6 increases lifespan and improves muscle function in dystrophic dystrophin/utrophin-deficient mice. Mol Ther. 2008;16:1539–1545. doi: 10.1038/mt.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onori A, Desantis A, Buontempo S, Di Certo MG, Fanciulli M, Salvatori L, Passananti C, Corbi N. The artificial 4-zinc-finger protein Bagly binds human utrophin promoter A at the endogenous chromosomal site and activates transcription. Biochem Cell Biol. 2007;85:358–365. doi: 10.1139/o07-015. [DOI] [PubMed] [Google Scholar]
- Onori A, Pisani C, Strimpakos G, Monaco L, Mattei E, Passananti C, Corbi N. UtroUp is a novel six zinc finger artificial transcription factor that recognises 18 base pairs of the utrophin promoter and efficiently drives utrophin upregulation. BMC Mol Biol. 2013;14:3. doi: 10.1186/1471-2199-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passananti C, Corbi N, Onori A, Di Certo MG, Mattei E. Transgenic mice expressing an artificial zinc finger regulator targeting an endogenous gene. Methods Mol Biol. 2010;649:183–206. doi: 10.1007/978-1-60761-753-2_11. [DOI] [PubMed] [Google Scholar]
- Perkins KJ, Basu U, Budak MT, Ketterer C, Baby SM, Lozynska O, Lunde JA, Jasmin BJ, Rubinstein NA, Khurana TS. Ets-2 repressor factor silences extrasynaptic utrophin by N-box mediated repression in skeletal muscle. Mol Biol Cell. 2007;18:2864–2872. doi: 10.1091/mbc.E06-12-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodino-Klapac LR, Montgomery CL, Bremer WG, Shontz KM, Malik V, Davis N, Sprinkle S, Campbell KJ, Sahenk Z, Clark KR, Walker CM, Mendell JR, Chicoine LG. Persistent expression of FLAG-tagged micro dystrophin in nonhuman primates following intramuscular and vascular delivery. Mol Ther. 2010;18:109–117. doi: 10.1038/mt.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sera T. Zinc-finger-based artificial transcription factors and their applications. Adv Drug Deliv Rev. 2009;61:513–526. doi: 10.1016/j.addr.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Tinsley JM, Fairclough RJ, Storer R, Wilkes FJ, Potter AC, Squire SE, Powell DS, Cozzoli A, Capogrosso RF, Lambert A, Wilson FX, Wren SP, De Luca A, Davies KE. Daily treatment with SMTC1100, a novel small molecule utrophin upregulator, dramatically reduces the dystrophic symptoms in the mdx mouse. PLoS ONE. 2011;6:e19189. doi: 10.1371/journal.pone.0019189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe LH, Auricchio A. Novel adeno-associated viral vectors for retinal gene therapy. Gene Ther. 2012;19:162–168. doi: 10.1038/gt.2011.151. [DOI] [PubMed] [Google Scholar]
- Veal EA, Jackson MJ. C-myc is expressed in mouse skeletal muscle nuclei during post-natal maturation. Int J Biochem Cell Biol. 1998;30:811–821. doi: 10.1016/s1357-2725(98)00032-6. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, Chen C, Li J, Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]


