Abstract
Jasin-hwan-gagambang (BHH10), a modified prescription of Jasin-hwan, contains Astragalus membranaceus, Cinnamomum cassia, and Phellodendron amurense, and it has been traditionally used to treat osteoporosis and other inflammatory diseases. In this study, we systematically investigated the protective effects of BHH10 in ovariectomy (OVX)-induced rats. Sprague–Dawley rats were randomly divided into sham and OVX subgroups. The rats in the OVX group were treated with vehicle, BHH10, alendronate (ALN), and 17β-estradiol (E2). BHH10 treatment significantly inhibited OVX-induced increases in body weight and uterus atrophy. In addition, it significantly increased the bone mineral density (BMD) and prevented a decrease in trabecular bone volume, connectivity density, trabecular number, thickness, and separation at the total femur and femur neck. The OVX rats showed significant decreases in the serum levels of calcium and phosphorous and significant increases in the serum levels of cholesterol, low-density lipoprotein cholesterol, alkaline phosphatase, osteocalcin, C-telopeptide type 1 collagen, and bone morphogenetic protein-2. These changes were significantly reduced to near sham levels by administration of BHH10 to OVX rats. BHH10-treated rats had a greater bone mass, a better structural architecture of the bone, and higher levels of biochemical markers of the bone than did the ALN-treated or E2-treated rats. These results suggest that BHH10 reverses osteoporosis in OVX rats by stimulating bone formation or regulating bone resorption and is not associated with toxicity. © 2014 The Authors. Phytotherapy Research published by John Wiley & Sons Ltd.
Keywords: BHH10, Astragalus membranaceus, Cinnamomum cassia, Phellodendron amurense, antiosteoporosis, bone formation
Introduction
Osteoporosis, the most widespread metabolic bone disease, is associated with a decrease in bone mass and deterioration of the trabecular architecture of the bone tissue, and patients with osteoporosis have increased skeletal fragility and susceptibility to fractures (Imai et al., 2009). Fractures are an important cause of morbidity and mortality among elderly patients with osteoporosis (Maruotti et al., 2011). Estrogen deficiency at menopause requires antiresorptive therapies. Therapies that significantly decrease the levels of bone-turnover markers and increase bone mineral density (BMD) greatly reduce the risk of nonvertebral fractures (Popp et al., 2014).
Alendronate (ALN) attenuates the increase in osteoclast-mediated bone resorption and bone loss that contributes to fracture risk in the skeleton (Black et al., 2006). Severe suppression of bone turnover with prolonged ALN therapy may inhibit normal bone repair and bone elasticity (Odvina et al., 2005). Although estrogen replacement therapy reduces the incidence of fracture and prevents bone loss, recent studies suggest that long periods of treatment with estrogen are discouraged because of the possibility of malignant effects on the reproductive tissues, including the mammary glands and endometrium (Prelevic et al., 2005; Basha et al., 2013). Thus, more effective and safer bone-protecting agents with long-term efficacy are required. Furthermore, most therapeutic agents used to treat osteoporosis are inhibitors of osteoclastic bone resorption that induce a decline in either osteoclast activity or osteoclast generation (Shoback, 2007). These concerns have prompted the discovery of osteoblast-based therapeutic agents that stimulate bone formation or increase bone mass and bone strength (Baron and Hesse, 2012).
Jasin-hwan-gagambang (BHH10) is produced by modifying Jasin-hwan, the herbal components of which have shown favorable safety profiles in the studies performed by the faculty of College of Oriental Medicine, Kyung Hee University (Seoul, Korea). According to the literature on traditional Korean and Chinese medicines (Gal-Moscovici and Sprague, 2007), Jasin-hwan has been traditionally prescribed for kidney and urinary diseases and can be used to treat osteoporosis. BHH10, a novel herbal extract, consists of the root of Astragalus membranaceus, the bark of Cinnamomum cassia, and the bark of Phellodendron amurense. We developed BHH10 on the basis of the results from our preliminary experiments and those reported previously about the beneficial in vitro and in vivo effects of these three components (Huh et al., 2009; Huh et al., 2010; Huh et al., 2011). A. membranaceus has cardiotonic, neuroprotective, antiosteoporotic, antiaging, antitumor, and anti-inflammatory effects (Li et al., 2012; Nalbantsoy et al., 2012). A recent study showed that formononetin, a bioactive phytoestrogen isolated from A. membranaceus, is a promising agent for the prevention of osteoarthritis and osteoporosis (Kaczmarczyk-Sedlak et al., 2013). Moreover, our previous studies showed that formononetin stimulates angiogenesis and osteogenesis, inhibits the expression of osteogenic markers and inflammatory cytokines, prevents osteoarthritis, and promotes growth factor activation, endothelial repair, and wound healing (Huh et al., 2011; Huh et al., 2009; Huh et al., 2010). C. cassia has a wide range of pharmacological properties, including antiosteoporotic, antiulcerogenic, anti-inflammatory, antipyretic, antimicrobial, antidiabetic, and antitumor activities (Sung et al., 2011). Recent studies show that cinnamic acid, the active compound of C. cassia, stimulates bone formation and inhibits bone-resorbing factors in vitro (Lee and Choi, 2006). P. amurense is a herbal remedy for controlling inflammation, because it alleviates lipopolysaccharide-induced acute airway inflammation and inhibits inflammation in rheumatoid arthritis (Zhang et al., 2011). Berberine, the active compound from P. amurense, inhibits osteoclast activity and promotes osteoblast differentiation and bone density accumulation (Lee et al., 2008). Although these three medicinal herbs have shown long-term therapeutic benefits with minimal risks, a detailed analysis of the therapeutic effects and mechanisms of action of BHH10 derivatives has not been performed thus far. We investigated the potential bone-protective effects of BHH10, including the preservation of bone mass and the structural properties of the entire femur and the femoral neck in an animal model of postmenopausal osteoporosis by using ovariectomized (OVX) rats. We administered comparable oral doses of ALN and 17β-estradiol (E2) and used them as reference compounds for evaluating BHH10-induced inhibition of bone turnover and estrogenic activity on the bone, respectively.
Materials and Methods
Plant materials
The root of A. membranaceus, the bark of C. cassia, and the bark of P. amurense were obtained from Kyung Hee Herb Pharm (Wonju, Korea). Identification and classification of plant materials was performed by Professor Kim Nam Jae at the Pharmacy of Oriental Medicine at Kyung Hee Medical Center, Kyung Hee University (Seoul, Korea), and the samples were deposited at the herbarium of Quality Control in the Herbal Medicine Department of Kyung Hee Medical Center (Seoul, Korea) and Hanpoong Pharm and Foods Co., Ltd. (Junju, Korea). BHH10 was prepared by extracting a mixture of the root of A. membranaceus, the bark of C. cassia, and the bark of P. amurense, at a ratio of 2:2:1 (w/w), respectively, with 30% (v/v) ethanol solution; subsequently, the solution was filtered. BHH10 was prepared by Hanpoong Pharm and Foods Co., Ltd. (Junju, Korea). The partitioned portion was vacuum-dried to yield BHH10 (yield, 16.3%).
High-performance liquid chromatography analysis of BHH10 and its standard compounds
BHH10 was standardized for quality control by using high-performance liquid chromatography (HPLC) analysis and compared with the reference standard compounds (formononetin, cinnamic acid, and berberine). Chromatographic analysis of BHH10 and the standard compounds was performed using a reverse-phase HPLC system (Waters, Milford, MA, USA) equipped with the Waters Breeze System [Alliance 2695 separation module and 2996 photodiode array detector (PDA)]. The separations were performed using a Hydrosphere C18 column (4.6 × 250 mm; particle diameter, 5 µm; YMC, Kyoto, Japan) at 30–45°C. The mobile phases of formononetin, cinnamic acid, and berberine consisted of acetonitrile (AcN), water (H2O), and acetic acid at a ratio of 15:37.5:1 and AcN, methanol (MeOH), and acetic acid at a ratio of 15:37.5:1. The concentrations of formononetin, cinnamic acid, and berberine in BHH10 were 0.8, 1.3, and 4.5%, respectively; they were detected at 260, 280, and 345 nm, respectively (Fig. 1). Analyses were performed at the laboratory for Inter-Hanpoong Pharm and Foods Co., Ltd. (Junju, Korea).
Figure 1.
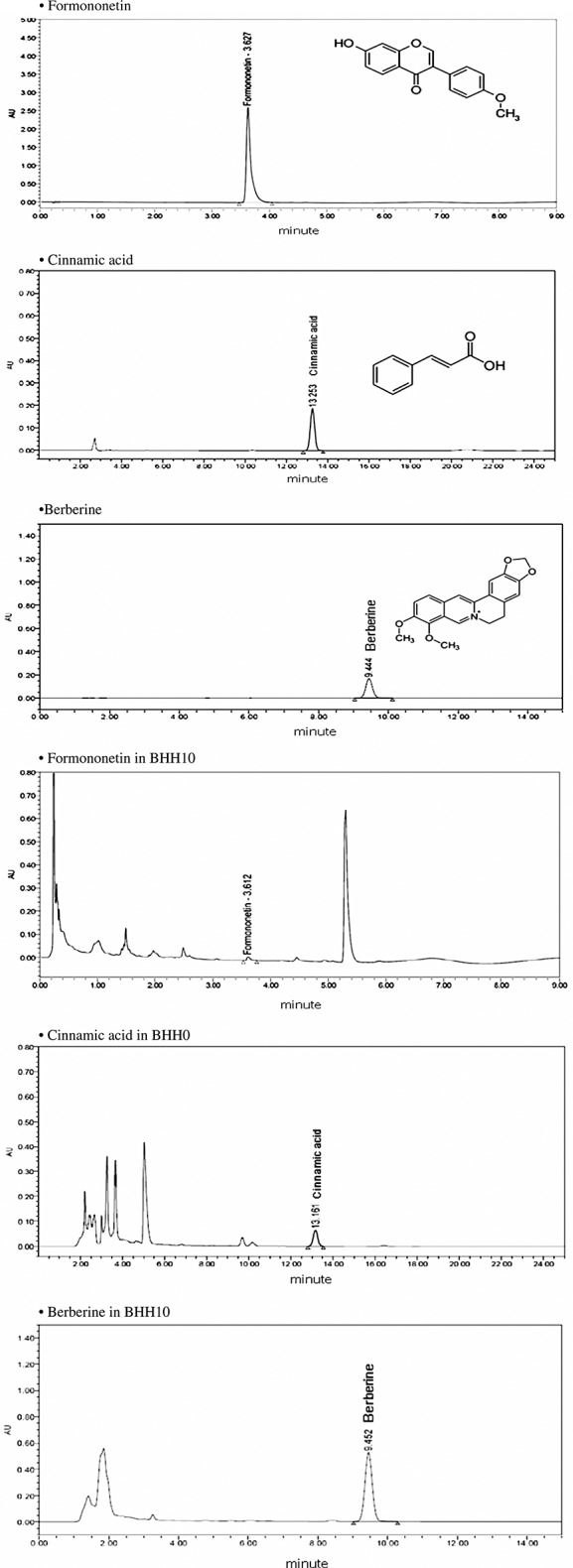
High-performance liquid chromatography profiles of BHH10 and the standard compounds formononetin, cinnamic acid, and berberine. The figures (a), (b), and (c) are profiles of standard formononetin, cinnamic acid, and berberine, respectively. The figures (d), (e), and (f) are profiles of formononetin, cinnamic acid, and berberine extracted from BHH10. These compounds were detected at 260 nm (about 3.6 min), 280 nm (about 13.2 min), and 345 nm (about 9.4 min), respectively.
Acute toxicity testing of a single dose of BHH10
We determined the toxicity of a single dose of BHH10 on four groups of rats. Each group of rats consisted of five males and five females. Each rat received a single oral dose of 0, 500, 1000, or 2000 mg/kg BHH10. All animals were continuously observed for symptoms and/or mortality for 14 days. All animals were weighed immediately before administration of the test dose and at 1, 3, 7, and 14 days after treatment. At 14 days after treatment, the rats were anesthetized with ether, exsanguinated by severing the postcaval veins and abdominal arteries, and visually examined for changes in the body surface and internal organs. This study was performed at Medvil Co., Ltd., a good laboratory practice (GLP) institute approved by the Korea Food and Drug Administration (KFDA), in compliance with the Testing Guidelines for the Safety Evaluation of Drugs (Notification No. A01-11007) issued by the KFDA and OECD Principles of GLP.
Chronic toxicity testing of BHH10 for 13 weeks
We examined the long-term toxicity of BHH10 in four groups of rats. Rats were given water or a BHH10 solution once daily for 13 weeks. The control group (15 male and 15 female rats) received water only. A group of 10 male and 10 female rats received 500 mg/kg BHH10, a group of 10 male and 10 female rats received 1000 mg/kg, and a group of 15 male and 15 female rats received 2000 mg/kg. We performed ocular exams, urine tests, blood tests, gross pathological examinations, biopsies, and autopsies. We recorded clinical symptoms, mortality, food/water intake, body weight, and organ weights. For recovery testing, we observed five male and five female rats from the control and 2000 mg/kg dose groups for an additional 4 weeks after administration of the last dose of BHH10. This study was approved by the KFDA in compliance with the Testing Guidelines for the Safety Evaluation of Drugs (Notification No. A01-11009).
Experimental model of osteoporosis
Six-month-old female Sprague–Dawley specific pathogen-free rats were obtained from the Central Lab (Seoul, Korea) and housed in an approved facility under standard conditions (22°C with a 12-h light and 12-h dark cycle). During the experimental period, all rats were pair-fed and given food and water ad libitum. Body weight was recorded at regular intervals. All experiments were approved by the Animal Ethics Committee of Kyung Hee University and performed according to Guidelines for the Care and Use of Laboratory Animals (KHMC-IACUC-12-012). Acclimatized rats underwent either bilateral laparotomy (SHAM, n = 6) or bilateral ovariectomy (n = 34). After a 2-week recovery period, OVX rats were randomly divided into five groups: vehicle (OVX, n = 7); ALN (n = 6, 170 µg/kg); E2 (n = 6, 17 µg/kg); and BHH10 (250 mg/kg, n = 6 and 500 mg/kg, n = 7). According to the human-rat equivalent dose conversion principle (Reagan-Shaw et al., 2008), the experimental doses of BHH10, ALN, and E2 that were given to the rats were equivalent to the corresponding clinical prescribed daily doses for a human weighing 60 kg. The vehicle, BHH10, ALN, or E2 was administered orally via a custom-made stomach tube, starting 2 weeks after ovariectomy, and was continued for 12 weeks.
Measurement of BMD
The BMD of total femurs and femur necks was measured using dual-energy X-ray absorptiometry (General Electric, Madison, WI, USA), and assessed using the PIXImus2 software.
Microcomputed tomography analysis
The metaphyses of the proximal femur and femoral neck were analyzed using 3D microfocus computed tomography (micro-CT; Sky-Scan 1172TM; Skyscan, Kontich, Belgium) equipped with the appropriate software (CT-analyzer TM), and scanned at a resolution of 17 µm, with a tube voltage of 60 kV and a tube current of 167 μA. The bone region beginning 20 slices from the growth plate at the proximal end of the femur and up to 200 distal slices was selected as the volume of interest. 3D images were obtained for visualization and display. Microarchitectural parameters were automatically evaluated using the built-in micro-CT program with direct 3D morphometry. The parameters that were analyzed included bone volume fraction (BV/TV), connectivity density (Conn. D, 1/mm3), trabecular number (Tb. N, 1/mm), trabecular thickness (Tb. Th, mm), and trabecular separation (Tb. Sp, mm) on the trabecular bone of the femur.
Biomarkers of bone metabolism
Blood samples was collected in separator tubes and the serum analyzed for creatinine (CRE), blood urea nitrogen (BUN), total cholesterol (TC), triglycerides (TGs), and low-density lipoprotein (LDL) cholesterol by using an AU400 serum biochemistry analyzer (AU400; Olympus, Japan). We determined the levels of serum alkaline phosphatase (ALP), calcium (Ca), and inorganic phosphate (P) by using standard colorimetric methods using a Selectra 2 auto-analyzer (Vital Scientific, Netherlands) with commercial assay kits (Sigma Chemical, St. Louis, MO, USA). Serum osteocalcin (OCN) levels were determined using an enzyme-linked immunosorbent assay (ELISA) kit from Biomedical Technologies (MA, USA). Serum C-telopeptide type 1 collagen (CTX) levels were determined using a RatLaps ELISA kits (BioAssay System, CA, USA). Serum bone morphogenetic protein-2 (BMP-2) levels were determined using an ELISA kit from R&D System Inc. (MN, USA).
Statistical analysis
Data were expressed as the mean ± standard error of the mean (SEM) for each group. The statistical significance of differences between means was assessed using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test for multiple comparisons. Differences with p values <0.05 between groups were considered significant.
Results
The acute and chronic toxicity of BHH10
To evaluate the acute toxicity of a single oral dose of BHH10, we administered BHH10 at doses of 500, 1000, and 2000 mg/kg to male and female rats. These doses had no apparent effects on the mortality, clinical symptoms, body weight, or gross pathological findings in the male or female rats, which suggested that the lethal dose of BHH10 was higher than 2000 mg/kg in rats (data not shown). Furthermore, no mortality was observed in the rats receiving BHH10 at a daily dose of 500, 1000, or 2000 mg/kg for 13 weeks. During the 13 weeks of treatment and the four additional weeks of recovery, no marked abnormalities were found in clinical symptoms, food or water intake, biochemical tests of urine and blood samples, gross pathological examinations, or autopsy findings (data not shown).
The effect of BHH10 on body weight and uterine weight in OVX rats
All rats had similar initial mean body weights and increases in body weight after 14 weeks of surgery. Although the rats were pair-fed, the body weight of the rats in the OVX group was significantly higher than that in the sham group 3 weeks after the operation. Administration of BHH10 at 250 and 500 mg/kg markedly suppressed the ovariectomy-induced increase in body weight after 11 weeks. The final body weights of the rats receiving 250 and 500 mg/kg of BHH10 were significantly lower by 16.8 and 17.9%, respectively, than those in the OVX group (Fig. 2A). Results similar to those reported above were observed in the ALN and E2 groups. Compared with sham surgery, ovariectomy caused significant uterine tissue atrophy, which indicated success of the surgical procedure (Fig. 2B). The rats receiving BHH10 at 250 and 500 mg/kg had significantly higher uterine weights than those in the OVX group by 28.4 and 26.4%, respectively (Fig. 2B).
Figure 2.
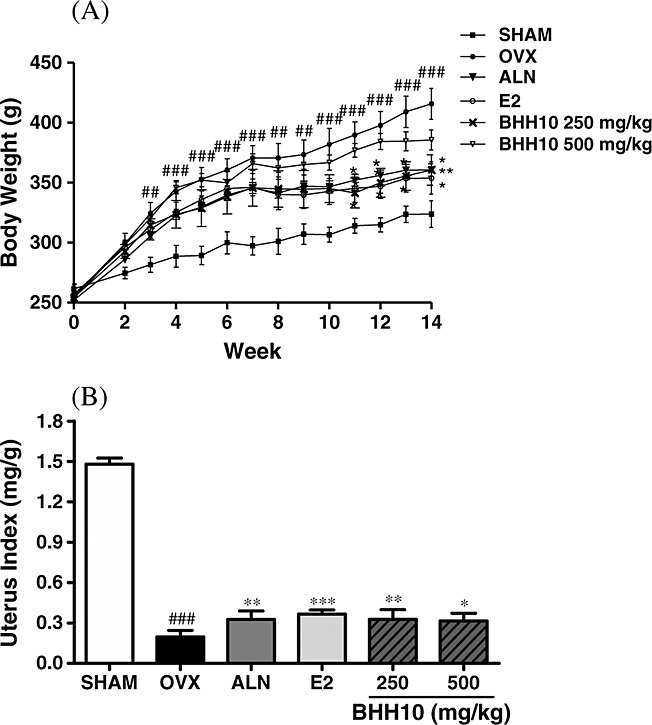
Effects of 12-week administration of BHH10 or 17β-estradiol (E2) on the body and uterine weights of ovariectomized rats. The six experimental groups are sham-operated (Sham), ovariectomized (OVX), and OVX rats treated with alendronate (ALN), 17β-estradiol (E2), or graded doses of BHH10 (250 and 500 mg/kg/day). Daily oral administration of BHH10 and E2 was initiated 2 weeks after ovariectomy and was continued for 12 weeks. (A) All rats were weighed once per week during the experimental period. (B) The uterine index was represented as the weight of the uterus divided by body weight. The data are expressed as the mean ± S.E.M. ##p < 0.01 and ###p < 0.001 compared with SHAM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with OVX.
The effect of BHH10 on BMD and bone morphometric analysis in OVX rats
We analyzed 3D micro-CT images of the entire femoral trabecular bone to determine BMD and specific differences in the microarchitecture of the trabecular bone (Fig. 3A and C). Compared with the OVX group, the group receiving BHH10 at 250 and 500 mg/kg showed a significant increase in the BMD of the entire femur by 18.4 and 13.2%; however, E2 increased the BMD of the entire femur only by 9.4%, which was not statistically significant (Fig. 3A). Compared with the OVX group, the group receiving BHH10 (250 and 500 mg/kg) showed a significant decrease in the whole femur bone volume over total volume (BV/TV; 45.0 and 28.1%, respectively), Conn. D (78.8 and 36.4%, respectively), Tb. N (25.6 and 18.5%, respectively), and Tb. Th (58.3 or 41.6%, respectively) and a marked decrease in Tb. Sp (24.2 and 21.2%, respectively) (Fig. 3A). Notably, treatment with 250 mg/kg BHH10 produced a much more marked response in BV/TV, Conn. D, Tb. N, and Tb. Th of the trabecular bone of the whole femur compared with that observed with ALN and E2 treatments (Fig. 3A). The effects of BHH10 on the BMD and microarchitecture of femoral neck were analyzed using micro-CT imaging (Fig. 3B and D). Compared with the OVX group, the group receiving BHH10 at a dose of 250 mg/kg showed a significant reversal of ovariectomy-induced effects on femoral neck BMD (24.5%), BV/TV (37.5%), Conn. D (26.1%), Tb. N (10.7%), Tb. Th (35.7%), and Tb. Sp (12.9%) (Fig. 3B). Treatment with BHH10 at a dose of 250 mg/kg had a significantly greater effect on BV/TV, Conn. D, and Tb. Th than that with ALN or E2 treatment (Fig. 3B).
Figure 3.
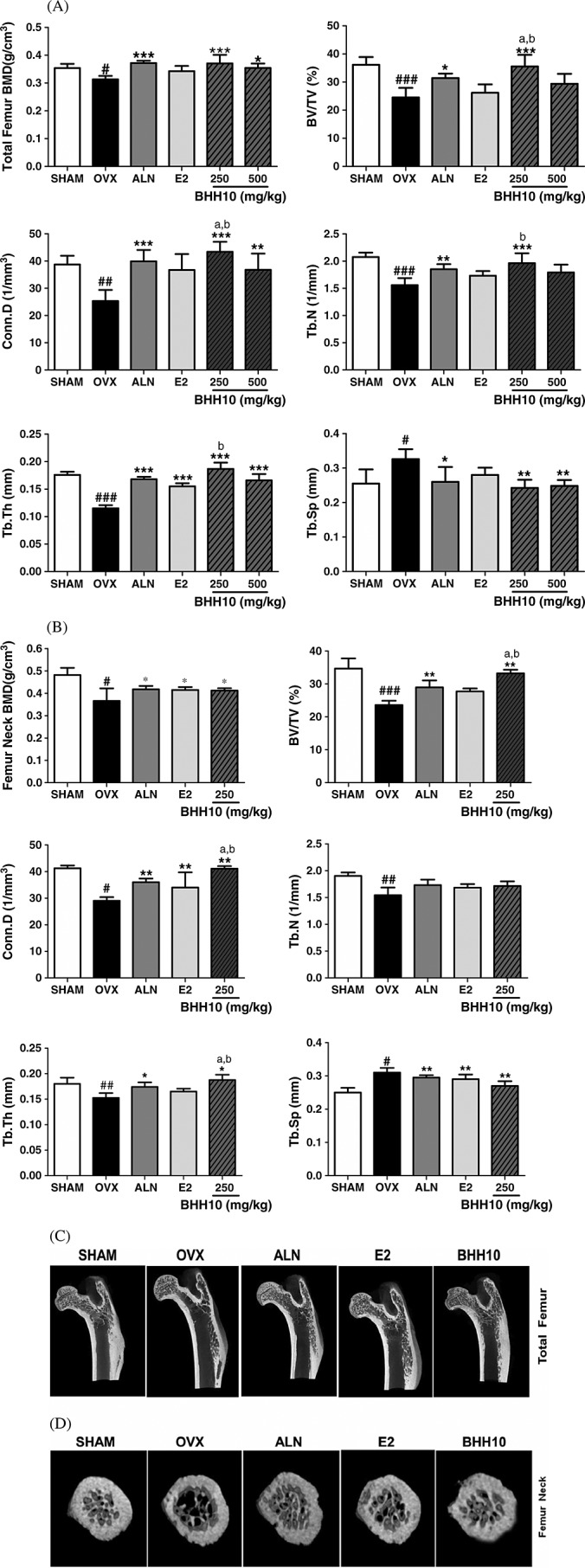
Effects of BHH10 on BMD and bone morphometric analysis of the whole femur (A) and femoral neck (B) in ovariectomized (OVX) rats. BHH10 altered the trabecular bone volume (BV/TV), connectivity density (Conn. D), trabecular number (Tb. N), trabecular thickness (Tb. Th), and trabecular separation (Tb. Sp) of the trabecular bone at the total femur (250, 500 mg/kg) and neck femur (250 mg/kg). 3D trabecular images of a vertical section of proximal femur (C) and femoral neck (D) in SHAM, OVX, alendronate (ALN), 17β-estradiol (E2), and 250 mg/kg BHH10 in rats. The trabecular bone loss was significantly higher in the OVX group than in the SHAM group. The trabecular bone volume was higher in the ALN, E2, and 250 mg/kg BHH10 groups than in the OVX group. The data are expressed as the mean ± S.E.M. #p < 0.05, ##p < 0.01, and ###p < 0.001 compared with SHAM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with OVX. ap < 0.01 compared with ALN. bp < 0.01 compared with E2.
The effect of BHH10 on serum biochemical parameters in OVX rats
The effects of BHH10 on serum biochemical parameters in OVX rats after 14 weeks are shown in Table 1. Administration of BHH10 (250 and 500 mg/kg) significantly prevented ovariectomy-induced changes in the serum levels of Ca (41.2 and 41.2%, respectively) and P (47.4 and 57.7%, respectively) and total cholesterol levels (20.3 and 20.0%, respectively). These levels of inhibition were similar to those obtained with either ALN or E2 treatment. Ovariectomy-induced increase in the serum LDL cholesterol levels in rats treated with 250 and 500 mg/kg of BHH10 was significantly reduced by 33.3 and 25.5%, respectively. However, neither ALN nor E2 treatment had a significant effect on the serum LDL cholesterol levels (5.7 and 13.6%, respectively; Table. 1).
Effects of 12-week administration of alendronate (ALN), 17β-estradiol (E2), and BHH10 on biochemical parameters in serum of ovariectomized (OVX) rats
| BHH10 (mg/kg) |
||||||
|---|---|---|---|---|---|---|
| SHAM | OVX | ALN | E2 | 250 | 500 | |
| (mg/dL) | 0.50 ± 0.02 | 0.60 ± 0.02 | 0.53 ± 0.07 | 0.59 ± 0.02 | 0.54 ± 0.02 | 0.56 ± 0.02 |
| BUN (mg/dL) | 17.92 ± 0.85 | 23.98 ± 1.04### | 23.42 ± 0.98 | 23.25 ± 0.60 | 22.40 ± 0.80 | 21.55 ± 0.76 |
| sCa (mg/dL) | 10.10 ± 0.14 | 11.22 ± 0.24### | 10.34 ± 0.22* | 10.40 ± 0.2* | 10.20 ± 0.11** | 10.20 ± 0.08*** |
| sP (mg/dL) | 6.52 ± 0.34 | 9.26 ± 0.45### | 6.80 ± 0.32* | 6.90 ± 0.43** | 6.28 ± 0.49** | 6.72 ± 0.32*** |
| TC (mg/dL) | 80.50 ± 5.60 | 149.0 ± 14.4### | 118.7 ± 0.40* | 114.3 ± 1.3* | 118.7 ± 4.28* | 119.2 ± 3.06* |
| LDL (mg/dL) | 7.67 ± 0.63 | 21.75 ± 0.50### | 20.50 ± 1.32 | 18.80 ± 1.32 | 14.50 ± 1.50** | 16.20 ± 0.49* |
The data are expressed as the mean ± standard error of mean (S.E.M).
p < 0.001 compared with SHAM.
p < 0.05,
p < 0.01, and
p < 0.001 compared with OVX,ap < 0.01 compared with ALN.bp < 0.01 compared with E2.
The effect of BHH10 on the levels of biomarkers of bone metabolism in OVX rats
Compared with the OVX group, the group receiving 250 and 500 mg/kg of BHH10 showed a significant decrease in the serum levels of ALP by 32.7 and 31.0%, OCN by 30.3 and 32.1%, CTX by 29.4 and 29.7%, and BMP-2 by 30.3 and 25.3%, respectively (Fig. 4A, B, C, and D). Treatment with ALN or E2 showed effects similar to those observed with BHH10 on the serum levels of ALP, OCN, and CTX (Fig. 4A, B, and C). However, the decrease in the serum BMP-2 levels was significantly greater with BHH10 treatment than with either ALN or E2 treatment (Fig. 4D).
Figure 4.
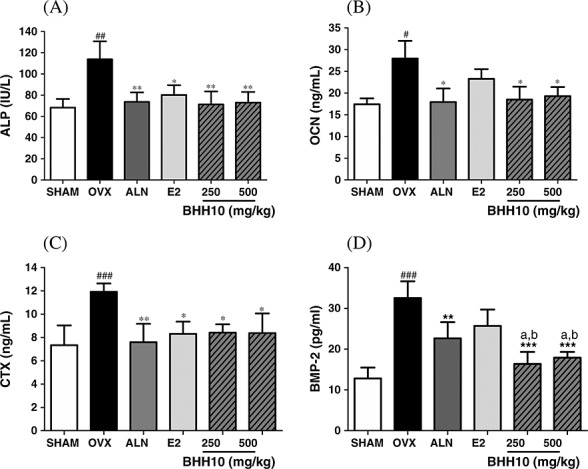
Effects of BHH10 on bone metabolic markers in the serum of ovariectomized (OVX) rats. The levels of serum alkaline phosphatase (ALP) (A), osteocalcin (OCN) (B), C-telopeptide type 1 collagen (CTX) (C), and bone morphogenetic protein-2 (BMP-2) (D) were determined using colorimetric methods or enzyme-linked immunosorbent assay in SHAM control rats, OVX rats, alendronate (ALN)-treated, 17β-estradiol (E2)-treated, or BHH10-treated rats. The data are expressed as the mean ± S.E.M. #p < 0.001 compared with SHAM. *p < 0.05 and ***p < 0.001 compared with OVX, ap < 0.01 compared with ALN. bp < 0.01 compared with ALN.
Discussion
In this study, we assessed whether BHH10 exerted protective effects against osteoporotic changes caused by the deficiency of ovarian hormone in rats. Additionally, we used ALN and E2 as positive controls to compare the bone metabolic response to BHH10 and established therapy. Our study showed that BHH10 is safe, has potent effects on bone metabolism, and decreases the deterioration in bone mass and microarchitecture in a postmenopausal model of osteoporosis. In OVX rats, BHH10 tended to have a greater effect on bone mass and metabolism than either ALN or E2.
The effects of ovariectomy in rats include a significant increase in body weight partially because of fat deposition. In addition, ovariectomy decreases the uterine mass because of a decline in the circulating estrogen levels (Nian et al., 2009). Our data showed that the characteristic increases in body weight and uterotrophic activity induced by ovariectomy were prevented by treatment with 250 or 500 mg/kg of BHH10 similar to that observed with ALN or E2. This uterotrophic effect of estrogen may be critically involved in gynecological tumor initiation and/or progression, because estrogen directly affects uterine parameters (Prelevic et al., 2005; Basha et al., 2013). The effect of BHH10 on uterine wet weight was lower than that of E2, which indicated a lower risk of hyperplasia than that associated with estrogen. These findings suggested that BHH10 treatment may not cause severe adverse risk associated with estrogen-like therapies, but further studies are required to confirm this finding.
Bone remodeling was evaluated on the basis of quantitative and histomorphometric evaluation of the femur and assessment of the mechanical properties of the entire femoral bone and femoral neck (Rachner et al., 2011). Altered bone turnover is associated with significant reductions in bone mass, predominantly at sites rich in trabecular bone, and with deterioration of the bone microarchitecture (Shen et al., 2013). BMD is an important determinant of bone strength and a primary indicator of bone biomechanical quality (Bouxsein, 2003). Our study showed that treatment with 250 or 500 mg/kg of BHH10 reversed ovariectomy-induced BMD loss at the proximal femur. Thus, BHH10 may contribute to an antiresorptive effect, similar to that of ALN, that affects the incorporation of bone matrix and inhibits osteoclasts, which leads to an increase in overall bone formation (Rodan and Reszka, 2002). These results suggested that BHH10 may have a protective effect on bone remodeling in postmenopausal women with osteoporosis. Similar trends in BMD were noted in the femoral neck, particularly in trabecular bone mass, where osteoporotic fractures may occur (Chen et al., 2013; Rachner et al., 2011). Our data showed that the femurs of BHH10-treated OVX rats may be more resistant to fractures and less susceptible to deformation than those of ALN-treated or E2-treated OVX rats. These findings indicate that BHH10 neutralized the progressive reduction in the bone mass of total femur and femoral neck.
In addition to BMD, preservation of metaphyseal microarchitecture contributes to the maintenance of bone structural integrity and reduces fracture risk (Ulrich et al., 1999). Micro-CT is a high-resolution digital imaging technique that provides detailed, quantitative, nondestructive analysis of 3D microscopic bone architecture (Chen et al., 2013). A recent micro-CT analysis showed that the normal trabecular bone structure was substantially impaired by ovariectomy (Qi et al., 2012). Measurements of trabecular bone microstructure, especially Conn. D, are indicators of bone strength, and increased Tb. Th represents an increase in the ratio of osteoblasts to osteoclasts (Ali et al., 2005). In our study, 250 mg/kg of BHH10 inhibited trabecular bone disruption, as evidenced by a significant increase in BV/TV, Conn. D, Tb. N, and Tb. Th. Compared with the OVX group, the group treated with BHH10 showed a decrease in Tb. Sp in the total femur diaphysis and femoral neck microarchitectural properties. These findings indicated that compared with ALN and E2, BHH10 treatment produced greater increases in bone volume, thickness, and connective density at the trabecular bone of total femur and femur neck, thereby attenuating the progression of osteoporosis.
The balance between bone formation and resorption is reflected by the relative levels of Ca and P (Civitelli and Ziambaras, 2011). BHH10 inhibited ovariectomy-induced increases in the serum Ca and P levels that are phenotypic markers of bone metabolism. These results suggested that BHH10 treatment maintained serum Ca and P homeostasis and contributed to alterations in BMD (Gaumet et al., 1997). Further, OVX rats displayed lower levels of circulating estrogens, which are associated with elevated TC and LDL cholesterol (Sato et al., 1998). Hypercholesterolemia associated with the pathogenesis of osteoporosis is closely linked to osteoclastogenesis and bone resorption (Tintut et al., 2004). Our results showed that compared with ovariectomy, treatment with BHH10 markedly decreased the levels of serum TC and serum LDL cholesterol. ALN or E2 administration did not significantly change the LDL cholesterol levels. These findings indicated that BHH10 was more efficacious than ALN and E2 in inhibiting bone resorption in osteoporosis.
The process of bone remodeling begins with the resorption of a volume of bone by osteoclasts followed by new bone formation by osteoblasts (Martin and Sims, 2005). Ovariectomy disturbs the bone remodeling process by markedly stimulating bone-resorbing osteoclasts, which results in a compensatory augmentation of bone-forming osteoblasts. This results in an overall increase in bone resorption on trabecular bone surface and subsequently in net bone loss, bone fragility, and osteoporotic fractures (Pacifici, 2007). ALP and OCN are osteoblast-related proteins that circulate during the bone-formation phase of the remodeling process and are sensitive markers of bone formation (De Leo et al., 2000). Conversely, CTX is the most prominent collagen-breakdown product generated by osteoclasts and is a marker of bone resorption (Khosla and Riggs, 2005). In addition, BMPs play a critical role in osteoblast differentiation and bone formation in the adult skeleton (Smith et al., 2014). In our study, the serum levels of ALP, OCN, BMP-2, and CTX significantly increased, which indicated an increased osteoblastic activity and enhanced osteoclastogenic activity in OVX rats compared with that in SHAM rats. Administration of BHH10 significantly decreased the serum levels of ALP, OCN, BMP-2, and CTX, which indicated better regulation of bone formation and bone resorption in OVX rats. In particular, BHH10 decreased the serum BMP-2 levels to a greater extent than ALN and E2 in OVX rats. These results suggested that BHH10 reversed the effects of ovariectomy on osteoblastic bone formation and bone loss without toxicity.
It has been postulated that the ability of BHH10, a modified prescription of Jasin-hwan, to both prevent bone loss and restore bone is because of the phytoestrogen content or a phenolic compound profile, which promotes osteogenesis and acts as an antioxidant and/or anti-inflammatory agent (Ma et al., 2013). A recent report from our laboratory showed that formononetin, a standard compound of BHH10, promotes fracture healing by stimulating osteogenesis and inhibits inflammatory cytokines in osteoblasts stimulated with IL-1β in a fracture model (Huh et al., 2011; Huh et al., 2009; Huh et al., 2010). Recent studies have shown that cinnamic acid and berberine, the major compounds of BHH10, promote osteoblast differentiation and inhibit bone resorption (Lee et al., 2008). However, further studies on the bioactive components of BHH10 are needed, and thus, it is premature to draw any specific conclusions.
In summary, our results showed that BHH10 has a remarkable osteoprotective effect against bone loss induced by estrogen deficiency in rats. This effect may result from either the enhancement of bone formation or the regulation of bone resorption. Therefore, we suggest that BHH10 could provide potential therapeutic benefits for osteoporosis through physiological bone remodeling and have a greater efficacy than ALN and E2.
Acknowledgments
This work was supported by a grant of the Oriental Medicine R&D Project, Ministry of Health & Welfare, Republic of Korea (HI11C2114).
Conflict of Interest
The authors have declared that there is no conflict of interest.
References
- Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146:1226–1235. doi: 10.1210/en.2004-0735. [DOI] [PubMed] [Google Scholar]
- Baron R, Hesse E. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab. 2012;97:311–325. doi: 10.1210/jc.2011-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha ME, Chang S, Burrows LJ, et al. Effect of estrogen on molecular and functional characteristics of the rodent vaginal muscularis. J Sex Med. 2013;10:1219–1230. doi: 10.1111/jsm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- Bouxsein ML. Mechanisms of osteoporosis therapy: a bone strength perspective. Clin Cornerstone Suppl. 2003;2:S13–21. doi: 10.1016/s1098-3597(03)90043-3. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhou X, Fujita H, Onozuka M, Kubo KY. Age-related changes in trabecular and cortical bone microstructure. Int J Endocrinol. 2013;2013:213234. doi: 10.1155/2013/213234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitelli R, Ziambaras K. Calcium and phosphate homeostasis: concerted interplay of new regulators. J Endocrinol Invest. 2011;34:3–7. [PubMed] [Google Scholar]
- De Leo V, Ditto A, la Marca A, Lanzetta D, Massafra C, Morgante G. Bone mineral density and biochemical markers of bone turnover in peri- and postmenopausal women. Calcif Tissue Int. 2000;66:263–267. doi: 10.1007/s002230010053. [DOI] [PubMed] [Google Scholar]
- Gal-Moscovici A, Sprague SM. Osteoporosis and chronic kidney disease. Semin Dial. 2007;20:423–430. doi: 10.1111/j.1525-139X.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- Gaumet N, Seibel MJ, Coxam V, Davicco MJ, Lebecque P, Barlet JP. Influence of ovariectomy and estradiol treatment on calcium homeostasis during aging in rats. Arch Physiol Biochem. 1997;105:435–444. doi: 10.1076/apab.105.5.435.3292. [DOI] [PubMed] [Google Scholar]
- Huh JE, Kwon NH, Baek YH, et al. Formononetin promotes early fracture healing through stimulating angiogenesis by up-regulating VEGFR-2/Flk-1 in a rat fracture model. Int Immunopharmacol. 2009;9:1357–1365. doi: 10.1016/j.intimp.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Huh JE, Nam DW, Baek YH, et al. Formononetin accelerates wound repair by the regulation of early growth response factor-1 transcription factor through the phosphorylation of the ERK and p38 MAPK pathways. Int Immunopharmacol. 2011;11:46–54. doi: 10.1016/j.intimp.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Huh JE, Seo DM, Baek YH, Choi DY, Park DS, Lee JD. Biphasic positive effect of formononetin on metabolic activity of human normal and osteoarthritic subchondral osteoblasts. Int Immunopharmacol. 2010;10:500–507. doi: 10.1016/j.intimp.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Imai K, Ohnishi I, Matsumoto T, Yamamoto S, Nakamura K. Assessment of vertebral fracture risk and therapeutic effects of alendronate in postmenopausal women using a quantitative computed tomography-based nonlinear finite element method. Osteoporos Int. 2009;20:801–810. doi: 10.1007/s00198-008-0750-8. [DOI] [PubMed] [Google Scholar]
- Kaczmarczyk-Sedlak I, Wojnar W, Zych M, Ozimina-Kaminska E, Taranowicz J, Siwek A. Effect of formononetin on mechanical properties and chemical composition of bones in rats with ovariectomy-induced osteoporosis. Evid Based Complement Alternat Med. 2013;2013:457052. doi: 10.1155/2013/457052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Riggs BL. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin North Am. 2005;34:1015–1030. doi: 10.1016/j.ecl.2005.07.009. , xi. [DOI] [PubMed] [Google Scholar]
- Lee HW, Suh JH, Kim HN, et al. Berberine promotes osteoblast differentiation by Runx2 activation with p38 MAPK. J Bone Miner Res. 2008;23:1227–1237. doi: 10.1359/jbmr.080325. [DOI] [PubMed] [Google Scholar]
- Lee KH, Choi EM. Stimulatory effects of extract prepared from the bark of Cinnamomum cassia blume on the function of osteoblastic MC3T3-E1 cells. Phytother Res. 2006;20:952–960. doi: 10.1002/ptr.1984. [DOI] [PubMed] [Google Scholar]
- Li LK, Kuang WJ, Huang YF, et al. Anti-tumor effects of Astragalus on hepatocellular carcinoma in vivo. Indian J Pharmacol. 2012;44:78–81. doi: 10.4103/0253-7613.91872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Ji W, Fu Q, Ma S. Formononetin inhibited the inflammation of LPS-induced acute lung injury in mice associated with induction of PPAR gamma expression. Inflammation. 2013;36:1560–1566. doi: 10.1007/s10753-013-9700-5. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11:76–81. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Maruotti N, Grano M, Colucci S, D'onofrio F, Cantatore FP. Osteoclastogenesis and arthritis. Clin Exp Med. 2011;11:137–145. doi: 10.1007/s10238-010-0117-2. [DOI] [PubMed] [Google Scholar]
- Nalbantsoy A, Nesil T, Yilmaz-Dilsiz O, Aksu G, Khan S, Bedir E. Evaluation of the immunomodulatory properties in mice and in vitro anti-inflammatory activity of cycloartane type saponins from Astragalus species. J Ethnopharmacol. 2012;139:574–581. doi: 10.1016/j.jep.2011.11.053. [DOI] [PubMed] [Google Scholar]
- Nian H, Ma MH, Nian SS, Xu LL. Antiosteoporotic activity of icariin in ovariectomized rats. Phytomedicine. 2009;16:320–326. doi: 10.1016/j.phymed.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- Pacifici R. T cells and post menopausal osteoporosis in murine models. Arthritis Res Ther. 2007;9:102. doi: 10.1186/ar2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp AW, Buffat H, Cavelti A, et al. Cortical bone loss at the tibia in postmenopausal women with osteoporosis is associated with incident non-vertebral fractures: Results of a randomized controlled ancillary study of HORIZON. Maturitas. 2014;77:287–293. doi: 10.1016/j.maturitas.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Prelevic GM, Kocjan T, Markou A. Hormone replacement therapy in postmenopausal women. Minerva Endocrinol. 2005;30:27–36. [PubMed] [Google Scholar]
- Qi W, Yan YB, Lei W, et al. Prevention of disuse osteoporosis in rats by Cordyceps sinensis extract. Osteoporos Int. 2012;23:2347–2357. doi: 10.1007/s00198-011-1842-4. [DOI] [PubMed] [Google Scholar]
- Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Rodan GA, Reszka AA. Bisphosphonate mechanism of action. Curr Mol Med. 2002;2:571–577. doi: 10.2174/1566524023362104. [DOI] [PubMed] [Google Scholar]
- Sato M, Turner CH, Wang T, Adrian MD, Rowley E, Bryant HU. LY353381.HCl: a novel raloxifene analog with improved SERM potency and efficacy in vivo. J Pharmacol Exp Ther. 1998;287:1–7. [PubMed] [Google Scholar]
- Shen CL, Chyu MC, Cao JJ, Yeh JK. Green tea polyphenols improve bone microarchitecture in high-fat-diet-induced obese female rats through suppressing bone formation and erosion. J Med Food. 2013;16:421–427. doi: 10.1089/jmf.2012.0199. [DOI] [PubMed] [Google Scholar]
- Shoback D. Update in osteoporosis and metabolic bone disorders. J Clin Endocrinol Metab. 2007;92:747–753. doi: 10.1210/jc.2007-0042. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Bu SY, Wang Y, et al. A comparative study of the bone metabolic response to dried plum supplementation and PTH treatment in adult, osteopenic ovariectomized rat. Bone. 2014;58:151–159. doi: 10.1016/j.bone.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Sung YY, Yoon T, Jang JY, Park SJ, Jeong GH, Kim HK. Inhibitory effects of Cinnamomum cassia extract on atopic dermatitis-like skin lesions induced by mite antigen in NC/Nga mice. J Ethnopharmacol. 2011;133:621–628. doi: 10.1016/j.jep.2010.10.043. [DOI] [PubMed] [Google Scholar]
- Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24:e6–10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Van Rietbergen B, Laib A, Ruegsegger P. The ability of three-dimensional structural indices to reflect mechanical aspects of trabecular bone. Bone. 1999;25:55–60. doi: 10.1016/s8756-3282(99)00098-8. [DOI] [PubMed] [Google Scholar]
- Zhang M, Long Y, Sun Y, et al. Evidence for the complementary and synergistic effects of the three-alkaloid combination regimen containing berberine, hypaconitine and skimmianine on the ulcerative colitis rats induced by trinitrobenzene-sulfonic acid. Eur J Pharmacol. 2011;651:187–196. doi: 10.1016/j.ejphar.2010.10.030. [DOI] [PubMed] [Google Scholar]


