Abstract
Objective
To determine long-term safety and efficacy of adjunctive clobazam for patients with Lennox-Gastaut syndrome (LGS).
Methods
Eligible patients from two randomized controlled trials (Phase II OV-1002 and Phase III OV-1012) were able to enroll in open-label extension (OLE) study OV-1004 beginning in December 2005 and received clobazam until they discontinued (mandatory at 2 years for patients outside the United States) or until study completion in March 2012. Patients in the United States could have received clobazam for 6 years before it became commercially available. Efficacy assessments included changes in rates of drop seizures and total seizures, responder rates (≥50%, ≥75%, or 100% decreases in seizure frequency vs. baseline), sustained efficacy over time, concomitant antiepileptic drug (AED) use, and global evaluations. Safety assessments included exposure to clobazam, laboratory assessments, physical and neurologic examinations, vital sign monitoring, electrocardiography monitoring, and adverse event reporting.
Results
Of 267 patients who enrolled in the OLE, 188 (70%) completed the trial. Two hundred seven patients were from the United States, which was the only country in which patients could be treated with clobazam for >2 years. Forty-four patients were treated with clobazam for 5 years, and 11 for 6 years. Because of the low number of Year 6 patients, this group is not reported separately. Improvements in baseline seizure rates were very stable over the course of the study, with a median 85% decrease in drop seizures at Year 1, 87% at Year 2, 92% at Year 3, 97% at Year 4, and a 91% decrease for patients who had reached Year 5. Similar results were observed for total seizures (79% decrease at both Years 1 and 2, 82% decrease at Year 3, 75% decrease at Year 4, and 85% decrease at Year 5). Responder rates were also stable for the duration of the trial. Of patients who had achieved a ≥50% decrease in median drop-seizure frequency from baseline to Month 3, 86% still had that degree of drop-seizure reduction at Year 3 (and 14% lost their initial responses), and 47% were drop-seizure–free. Most patients who had achieved drop-seizure freedom in the original controlled trials remained drop-seizure–free in the OLE. Based on parents' and physicians' ratings of global evaluations, 80% of patients were “very much improved” or “much improved” after 3 years. Of the 43 patients with concomitant AED data who were treated for 5 years, 30% increased, 19% decreased, and 51% had no change in numbers of AEDs versus their Week 4 regimens. The mean modal clobazam dosage was 0.90 mg/kg/day at Year 1 and 0.97 mg/kg/day at Year 5, suggesting that study patients did not need significant increases in dosage over time. The safety profile was what would be expected for clobazam for LGS patients over a 5-year span, and no new safety concerns developed over time.
Significance
In this largest and longest-running trial in LGS, adjunctive clobazam sustained seizure freedom and substantial seizure improvements at stable dosages through 3 years of therapy in this difficult- to-treat patient population.
Keywords: Drop seizures, Antiepileptic drug, Benzodiazepines, Clinical trials, Epilepsy
Lennox-Gastaut syndrome (LGS) is a complex, chronic epileptic encephalopathy that often requires decades of polytherapy.1,2 The most common seizure types associated with LGS are tonic and atonic seizures (also referred to as “drop attacks” because they may result in falls), occurring in at least 50% of LGS patients. Drop attacks are often the first seizure-associated manifestation in LGS, and have the greatest potential for bodily harm.3,4 The substantial prevalence of drop seizures may also contribute to the substantial morbidity and injury in LGS patients versus other epilepsy syndromes.4–6 Several other seizure types are also experienced by patients with LGS (e.g., clonic, atypical absence, myoclonic, partial [focal], and generalized tonic–clonic seizures), and episodes of nonconvulsive status epilepticus are also common among patients with LGS. These multiple seizure types are one of the key reasons that patients with LGS often require increasing antiepileptic drug (AED) dosages or polytherapeutic regimens.1,7,8 Peak age at onset of LGS is between 3 and 5 years, and treatment-related adverse events, which can be common with polytherapy, are of particular concern in this young population.1 Because seizure freedom is rarely achieved in patients with LGS, the goal of treatment for LGS patients is to maximize quality of life through the combination of minimizing both seizure frequency and treatment-related adverse events.7,8 Optimizing treatment is inherently a challenge for clinicians who are treating patients with LGS, with the lack of quality long-term trial data adding to the challenge.1,7,8
Clobazam is a novel 1,5-benzodiazepine approved by the U.S. Food and Drug Administration (FDA) in October 2011 for the adjunctive treatment of seizures associated with LGS for patients 2 years and older. Clobazam, first approved in Australia in 1970 and in France in 1974,9,10 is also approved outside the United States for the treatment of anxiety and other forms of epilepsy. FDA approval of clobazam for LGS was based on two randomized controlled trials: OV-100211 (NCT00162981) and OV-101212 (also known as the CONTAIN trial, NCT00518713).
After participation in studies OV-1002 or OV-1012, patients were eligible to enroll in a long-term, open-label extension (OLE) trial, OV-1004 (NCT01160770). An interim data analysis of OV-1004 (the period from December 28, 2005, through July 1, 2010) was published,13 and these data were used in the FDA's review of clobazam for approval. The OV-1004 trial continued until March 2012, and the final analyses of these data, with some patients receiving clobazam for 6 years, are presented here.
Methods
Study design
Qualifying patients 2–60 years of age from two randomized controlled studies (Phase II OV-1002 trial,11 and Phase III OV-1012 trial12) were given the option of enrolling in OV-1004, a multicenter, OLE study of clobazam as adjunctive therapy in patients with LGS. Detailed methodology has been published in an interim analysis of this OLE trial.13
Qualifying patients from the two randomized controlled trials returned to their investigational sites at Week 1 (OV-1012 patients only) and Months 1, 2, 3, 6, 9, and 12, and then every 6 months thereafter. For patients outside the United States (India, Europe, and Australia), the treatment period was limited to 2 years. Patients in the United States were allowed to remain in the trial until clobazam was commercially available, which was a maximum of 6 years. During the week preceding or following each study visit, the parent/caregiver, with the assistance of the patient, if able, maintained a seizure diary in which daily seizure counts (including drop seizures) were recorded. In the OV-1012 trial, a drop seizure was defined as any drop attack or spell involving the entire body, trunk, or head that led to a fall, injury, slumping in a chair, or the patient's head hitting a surface, or that could have led to a fall or injury, depending on the patient's position at the time of the attack or spell.12
During the Day 1 visit (first visit in the OV-1004 OLE), the patient or the patient's legally authorized representative signed and dated the institutional review board (IRB)/independent ethics committee (IEC)–approved informed consent form/Health Insurance Portability and Accountability Act (HIPAA) authorization form and assent, if appropriate. Demographic data for all patients were re-collected upon entry into OV-1004.
Additional methodology details are provided in Appendix 2.
Statistical analyses
The Efficacy Analysis Set consists of all patients who received ≥1 dose of clobazam and had ≥1 efficacy measurement during the study. The Safety Analysis Set consists of all patients who received ≥1 dose of clobazam in OV-1004 unless otherwise indicated.
The subsets of patients who received clobazam for a minimum of 1, 2, 3, 4, or 5 years were used for various efficacy and safety measures as indicated. Clobazam exposure during the randomized trials was included when determining these subsets. All analyses were calculated for the Year 3 patient subset. Selected analyses, determined on a post hoc basis, were calculated for the Year 4 and Year 5 patient subsets.
Efficacy analyses
All data are observed values and were summarized by study visit with descriptive statistics.
Reduction in drop seizures was calculated as the weekly number of drop seizures during baseline minus the weekly number of drop seizures at each study visit. The weekly number of drop seizures could have been the 7 days prior to the study visit or 7 days immediately following the study visit, whichever was applicable, as the assessment period changed by protocol amendment during the course of the study.
Safety analyses
For each patient, the most common (modal) and maximum dosages of clobazam were calculated. Descriptive summaries of the mean modal and mean maximum dosages were provided over 5 years for the 265 patients who received clobazam for >22 days (i.e., postdosage titration). In addition, the numbers and percentages of patients were cross-tabulated by modal dosage (dose given on most treatment days) and years of clobazam exposure.
Results
Patient disposition
The first patient enrolled in this open-label study in December 2005, and the last patient completed in March 2012. Of the 267 patients enrolled in OV-1004, 207 were from the United States and had the opportunity to continue beyond 2 years of treatment (Table1). Time to discontinuation for U.S. patients is presented in Figure1. The longest any patient was treated was 6 years, although this group includes only 11 patients and therefore is not presented separately. The most common reason for discontinuation (12%) was patient, caregiver, or parent request (Table1).
Table 1.
Patient disposition, n (%)
| Enrolled | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|---|
| USA | 207 | 181 (87) | 171 (83) | 121 (59) | 54 (26) | 44 (21) |
| Outside USA | 60 | 48 (80) | 39 (65) | 0 (0) | 0 (0) | 0 (0) |
| Treated: 267 (100) | ||||||
| Completed: 188 (70) | ||||||
| Discontinued: 79 (30) | ||||||
| Patient, parent, or caregiver request: 33 (12) | ||||||
| Lack of efficacy: 15 (6) | ||||||
| Adverse event: 10 (4) | ||||||
| Death: 9 (3) | ||||||
| Other reasons: 12 (4) | ||||||
Figure 1.
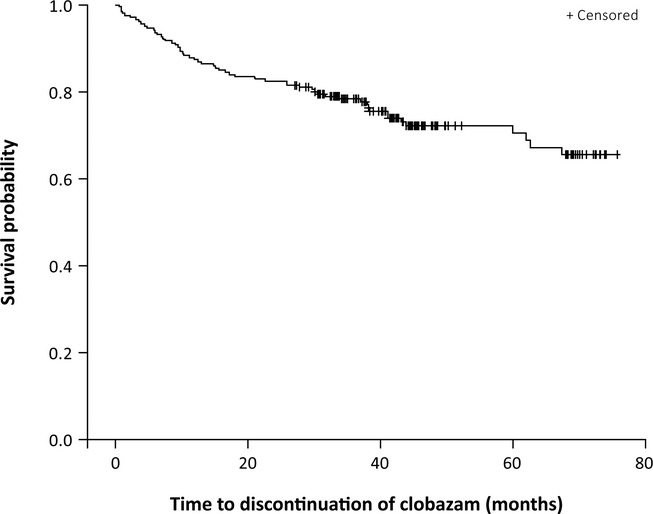
Time to study discontinuation for U.S. patients (N = 207). +: Patients who completed the study are censored at their dates of completion, since they did not have the event of discontinuation.
Patient demographics
Demographics and baseline clinical characteristics from the first days patients received clobazam (either in preceding randomized controlled trial or the OLE) are presented in Table2. The changing patient population over time as non-U.S. patients completed Year 2 had an impact on race, but not on other baseline data. The median patient age at study start was 9 years, with a range from 2 to 54 years throughout the span of the study.
Table 2.
Demographics and baseline clinical characteristicsa
| Total | |
|---|---|
| Patients, N | 267 |
| Age, mean (SD), years | 11.3 (7.8) |
| Male (%) | 61 |
| Region (%) | |
| United States | 78 |
| India | 17 |
| Rest of world | 5 |
| Race (%) | |
| White | 66 |
| Asian | 20 |
| Black | 12 |
| Other | 2 |
| Ethnicity, non-Hispanic/Latino (%) | 88 |
| Time since LGS diagnosis, mean (SD), years | 6.4 (7.7) |
| Most common concomitant AEDs (%)b | |
| Valproic acid | 52 |
| Levetiracetam | 38 |
| Lamotrigine | 37 |
| Topiramate | 33 |
| Diazepam | 30 |
| Rufinamide | 22 |
| Felbamate | 18 |
| Zonisamide | 18 |
| Lorazepam | 17 |
| Phenobarbital | 12 |
| Phenytoin | 9 |
| Lacosamide | 8 |
AEDs, antiepileptic drugs; OLE, open-label extension; LGS, Lennox-Gastaut syndrome; SD, standard deviation.
Demographics and baseline clinical characteristics were determined by the first day on study drug (either in a preceding randomized controlled trial or the OLE).
Received at baseline by ≥10% of the patients remaining in the trial at Year 3.
Efficacy results
Primary efficacy outcome
The high median percentage decrease from baseline in average weekly rate of drop seizures (85–91%) was maintained through Year 5 (Fig.2). The median percentage decrease in total seizures was also maintained, with an 85% reduction from baseline in those patients who had reached Year 5 (Fig.2).
Figure 2.
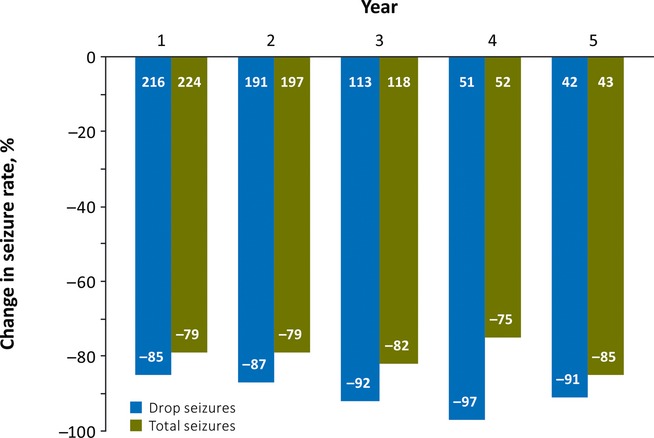
Median percentage reduction from baseline in average weekly seizure rate (Efficacy Analysis Subsets). Number of patients is indicated at the base of each column.
Treatment responders
The percentages of patients with decreases of ≥50%, ≥75%, or 100% in their average weekly seizure rates from the previous blinded study baseline were consistent over the 5-year trial span for both drop and total seizures (Fig.3A,B, respectively). Over 5 years, 62–69% achieved at least a 75% reduction in drop seizures, and 50–65% attained a 75% or more reduction in total seizures while treated with clobazam. Drop-seizure freedom was achieved by ≥32% of patients over the course of the study, and complete seizure freedom was achieved by ≥18% of patients in the yearly subsets.
Figure 3.
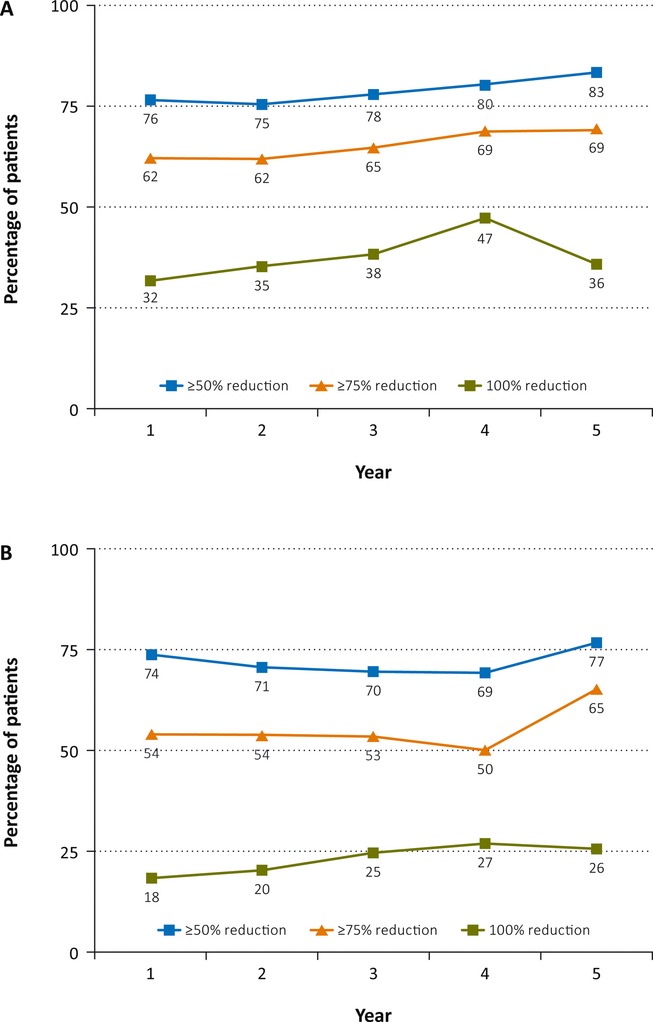
Responder rates for (A) drop and (B) total seizures (Efficacy Analysis Subsets). Responder categories are not mutually exclusive.
Sustainment of response
As a proxy assessment of tolerance to clobazam, patients who had at least a 50% decrease in drop seizures from baseline at Month 3 (n = 156) were assessed for continued response over the duration of the OLE trial. Of the 74 initial ≥50% treatment responders who had remained in the study at Year 3, 64 (86%) maintained this degree of response, and 10 (14%) lost their initial responses (Table3). In addition, at Years 1 and 3, respectively, 56 (40%) of 139 and 35 (47%) of 74 patients who had a ≥50% decrease in seizures at 3 months and had remained in the study were now seizure-free (100% responders), indicating a clinically relevant sustained or increased response. Of the 74 patients who achieved 100% seizure reduction at Month 3, 23 (64%) of 36 who had remained in the study at Year 3 maintained this degree of response, and 13 (36%) of 23 no longer had 100% responses (Table3).
Table 3.
Drop-seizure responder rates in subset of patients with initial drop-seizure responsea and seizure freedomb,c
| Duration of exposure, n (%) |
||||
|---|---|---|---|---|
| Month 3 | Year 1 | Year 2 | Year 3 | |
| Responders remaininga | 156 | 139/156 (89) | 124/156 (79) | 74/156 (47) |
| With ≥50% reduction | 156/156 (100) | 120/139 (86) | 105/124 (85) | 64/74 (86) |
| With <50% reduction | 19/139 (14) | 19/124 (15) | 10/74 (14) | |
| With 100% reduction | 74/156 (47) | 56/139 (40) | 57/124 (46) | 35/74 (47) |
| With <100% reduction | 83/139 (60) | 67/124 (54) | 39/74 (53) | |
| Of 100% responders remainingb | ||||
| With 100% reduction | 74/74 (100) | 40/65 (62) | 39/57 (68) | 23/36 (64) |
| With <100% reduction | 25/65 (38) | 18/57 (32) | 13/36 (36) | |
| End of phase III study | ||||
| Seizure-free patients remainingc | 18 | 16/18 (89) | 16/18 (89) | 7/18 (39) |
| With ≥50% reduction | 18/18 (100) | 14/16 (88) | 14/16 (88) | 7/7 (100) |
| With 100% reduction | 18/18 (100) | 11/16 (69) | 12/16 (75) | 6/7 (86) |
Response, ≥50% seizure reduction at Month 3 of OV-1004 (n = 156).
Response, 100% seizure reduction at Month 3 of OV-1004 (n = 74).
100% decrease in drop seizures (seizure freedom) at end of 15-week maintenance phase of Phase III OV-1012 (CONTAIN) trial (n = 18).
In the Phase III OV-1012 trial, there were 18 patients with complete freedom from drop seizures (100% decrease vs. baseline drop-seizure rate). Of these 18 patients, 11 (61%) remained seizure-free until their last measurements in OV-1004. All seven of the patients who had achieved complete freedom from drop seizures during the OV-1004 OLE who were still enrolled at Year 3 had at least a 50% reduction in their seizure rates compared with baseline, and six (86%) of seven were still seizure-free (Table3).
The Year 3 analysis in Table3 is complicated by the 39 patients outside the United States who were not permitted to continue beyond Year 2 by study design. Therefore, the decrease in Year 3 results includes the 39 who left the study, by study design, rather than because of loss of response.
Physician and patient caregiver global evaluations
The majority of patients were assessed by both their physicians and caregivers as “very much improved” or “much improved” after 1, 2, and 3 years of treatment (Table4). The ratings improved slightly over time, and after 3 years of treatment, both physicians and caregivers rated 80% of patients as “very much” or “much improved.”
Table 4.
Physician and parent global evaluations
| Physician |
Parent |
|||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 1 | Year 2 | Year 3 | |
| N | 227 | 204 | 137 | 229 | 206 | 137 |
| Very much/much improved (%) | 76 | 74 | 80 | 76 | 72 | 80 |
| Minimal improvement/worsening or no change (%) | 23 | 25 | 18 | 22 | 27 | 17 |
| Very much/much worse (%) | 1 | 1 | 2 | 2 | 1 | 3 |
Concomitant antiepilepsy treatments
Few patients were on a ketogenic diet (3.4%), and 25.8% of patients used vagus nerve stimulation during clobazam treatment in the OLE. Modification of AED therapy (addition or subtraction of therapies, and/or adjustment of dosage) was unrestricted throughout the study. The percentages of patients receiving concomitant AEDs indicated for the treatment of seizures associated with LGS during clobazam therapy included lamotrigine (36.7%), topiramate (33.0%), rufinamide (22.5%), felbamate (17.6%), and clonazepam (7.9%). The percentages of patients receiving other common concomitant antiepileptic therapies during clobazam therapy included valproic acid/valproate semisodium (79.7%), levetiracetam (38.2%), and diazepam (30.3%).
At Week 4, patients receiving 1, 2, 3, or >3 concomitant AEDs were grouped, and their changes in AED intake were calculated at the end of each year of the trial. In Years 1, 2, and 3, more patients decreased than increased their number of concomitant AEDs versus their Week 4 regimen (Table5). By Year 4, slightly more patients increased than decreased their numbers of concomitant AEDs. At Year 5, 7 (16%) of 43 patients were on clobazam monotherapy.
Table 5.
Patients with a net increase, decrease, or no change in numbers of concomitant AEDs from week 4
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| n | 228 | 207 | 109 | 46 | 43 |
| Increase, n (%) | 21 (9) | 34 (16) | 24 (22) | 13 (28) | 13 (30) |
| Same, n (%) | 161a (71) | 119a (57) | 51a (47) | 24 (52) | 22 (51) |
| Decrease, n (%) | 37 (16) | 46 (22) | 31 (28) | 9 (20) | 8 (19) |
AED, antiepileptic drug.
Because of the grouping of all patients with >3 AEDs in these analyses, it cannot be determined if patients who began and ended in the >3 AEDs group remained on the same number of AEDs, so they have been excluded from this calculation; n = 9 at Year 1, n = 8 at Year 2, n = 3 at Year 3.
Exposure over time
At Year 5, the mean modal and mean maximum dosages of clobazam in patients who received clobazam for >22 days (N = 265) were 0.97 and 1.19 mg/kg/day, respectively, and there was no substantive change over time (Table6). The modal clobazam dosage by duration of exposure was stable over time (Table7). Very few patients (<8%) received a modal dosage below 0.375 mg/kg/day, and the majority of patients received 0.375–1.25 mg/kg/day of clobazam through Year 5.
Table 6.
Modal and maximum daily dosages of clobazam for patients exposed up to 5 yearsa
| Week 4 BL | Up to Year 1 | Up to Year 2 | Up to Year 3 | Up to Year 4 | Up to Year 5 | |
|---|---|---|---|---|---|---|
| Modal | ||||||
| Mean (SD), mg/kg | 0.66 (0.32) | 0.90 (0.50) | 0.95 (0.52) | 0.96 (0.53) | 0.96 (0.53) | 0.97 (0.53) |
| Median, mg/kg | 0.61 | 0.79 | 0.85 | 0.87 | 0.87 | 0.87 |
| Q1, Q3 | 0.44, 0.87 | 0.50, 1.18 | 0.51, 1.30 | 0.52, 1.33 | 0.54, 1.36 | 0.54, 1.36 |
| Maximum | ||||||
| Mean (SD), mg/kg | 0.67 (0.32) | 1.06 (0.57) | 1.14 (0.61) | 1.17 (0.61) | 1.19 (0.62) | 1.19 (0.62) |
| Median, mg/kg | 0.61 | 0.96 | 1.00 | 1.06 | 1.07 | 1.07 |
| Q1, Q3 | 0.44, 0.88 | 0.62, 1.40 | 0.65, 1.49 | 0.69, 1.58 | 0.69, 1.60 | 0.69, 1.65 |
BL, baseline; Q1, 25th percentile; Q3, 75th percentile; SD, standard deviation.
Two patients received clobazam for <22 days and were excluded from this analysis.
(N = 265)
Table 7.
Modal clobazam dosagea by duration of clobazam exposure
| Dosage, mg/kg/day | Duration of clobazam exposure, % of patients |
||||
|---|---|---|---|---|---|
| 1 year (n = 229) | 2 years (n = 210) | 3 years (n = 121) | 4 years (n = 54) | 5 years (n = 44) | |
| ≤0.375 | 17 (7) | 15 (7) | 9 (7) | 4 (7) | 3 (7) |
| >0.375–≤0.75 | 73 (32) | 66 (31) | 35 (29) | 14 (26) | 12 (27) |
| >0.75–≤1.25 | 66 (29) | 61 (29) | 39 (32) | 20 (37) | 19 (43) |
| >1.25 | 73 (32) | 68 (32) | 38 (31) | 16 (30) | 10 (23) |
Dosage received on the greatest number of days.
Safety results
Incidence of adverse events
During the open-label study, 60% of patients experienced ≥1 treatment-related adverse event. The most common adverse events, in order of descending overall incidence, are provided in Table S1. The most common adverse events during the OLE were upper respiratory tract infection (28%) and pyrexia (19%). The upper respiratory tract infection and pneumonia events occurred predominantly in pediatric patients.
Severity of adverse events
The majority of treatment-emergent adverse events were mild or moderate. Severe adverse events were reported for 85 patients (32%) during the OLE. Severe treatment-emergent adverse events reported for ≥2% of patients were pneumonia (6%), status epilepticus and convulsion (3% each), and dehydration and pneumonia aspiration (2% each).
Serious adverse events
Serious adverse events were reported by 115 patients (43%) during exposure to clobazam in the OLE study. Serious adverse events that occurred in ≥2% of patients were convulsion (11%), pneumonia (10%), pneumonia aspiration (6%), status epilepticus (4%), urinary tract infection (3%), and dehydration (2%). Fourteen patients (5%) reported a treatment-related serious adverse event during the OLE, of which pneumonia (three patients), convulsion (three patients), and status epilepticus (two patients) were the only serious severe adverse events reported by >1 patient.
Discontinuations due to adverse events
Eighteen patients (7%) reported adverse events that led to premature discontinuation of clobazam during the OLE. Adverse events that led to premature discontinuation in >1 patient were death, pneumonia, convulsion, and acute respiratory distress syndrome (ARDS), each reported for two patients.
Deaths
Ten patients (4%) died during this OLE study, including the two patients described earlier. One patient prematurely discontinued study drug because of patient/parent/caregiver request and died following discontinuation. Fatal adverse events were pneumonia (three patients); death of unknown origin or etiology (two patients); epilepsy (one patient); the combination of pneumonia, ARDS, and sepsis (one patient); pneumonia and cardiopulmonary arrest (one patient); the combination of preexisting seizure increase, atelectasis, and hypoxic respiratory failure (one patient); and ARDS and right leg hematoma (one patient). Nine of the 10 deaths were deemed “unlikely related” or “not related” to clobazam by investigators. One patient experienced a convulsion with an outcome of death that was considered “possibly related” to clobazam by the investigator.
Discussion
The clobazam OV-1004 trial is, by far, the largest and longest-running trial in patients with LGS published to date. As of May 2013, only three other long-term drug extension trials in LGS patients had been published: a 6-month extension of a topiramate trial with 97 patients,14 a 1-year extension of a felbamate trial with 73 patients,15 and a 3-year extension of a rufinamide trial with 124 patients.16 No other adjunctive therapy for LGS has demonstrated this degree of sustained seizure freedom and substantial seizure improvements for this length of time. Moreover, the stable dosages observed over time indicate a lack of tolerance that has not been observed in this difficult-to-treat patient population, and make these findings unprecedented in the epilepsy field and important to those treating this chronic illness.
The stability of clobazam dosing over time was carefully monitored in this open-label extension trial. Dosages were generally stable and remained <2 mg/kg maximum for most patients at the interim cutoff,13 and these final results. From Year 1 through Year 5, mean modal clobazam dosages increased by a mere 8%. In addition, at Years 3, 4, and 5, approximately 75% of patients who had reached those time points were receiving either the same or a fewer number of concomitant AEDs (with approximately 25% of patients having increased their numbers of concomitant AEDs).
Development of tolerance has been known to occur with prolonged 1,4-benzodiazepine use.17 Although tolerance was not specifically evaluated in this study, these final data support the interim analyses findings of limited development of tolerance, as suggested by the following: (1) Response rates did not diminish; (2) mean modal clobazam dosages did not increase with time; (3) 70% of patients remained in treatment long term, with only 6% discontinuing because of lack of efficacy; and (4) some patients were able to decrease their numbers of concomitant AEDs. For a few patients (approximately 15%), seizure control was possible with clobazam monotherapy.
At Years 1 and 3, 14% of patients who were initial responders (≥50% seizure reduction from baseline to Month 3) had lost their responses (potentially indicating tolerance). This percentage is notably lower than that of an independent, retrospective chart review of 46 LGS patients at the pediatric neurology department of Asan Medical Center in Seoul, Korea (2000–2009), which reported tolerance for 12 (48%) of 25 patients.18 Our summary outcome of sustained therapeutic responses, as measured by numbers and percentages of patients sustaining their initial responses at Years 1, 2, and 3, as well as our analyses of mean modal dosages over time, does not exclude the possibility of the development of tolerance. Some patients may improve later and compensate or even overcompensate for the loss of initial efficacy in other patients. It is important to note that patients outside the United States were limited to 2 years of therapy. Therefore, 39 patients outside the United States who had completed 2 years in the study were not permitted to continue in Year 3, and this accounts for the notable decrease in patient numbers from Year 2 to Year 3.
In phase II and phase III randomized controlled trials, clobazam was found to be well-tolerated and efficacious for treatment of seizures associated with LGS.11,12 Decreases in average weekly drop attacks and total seizures observed in these trials that led into this long-term, open-label extension were sustained through 2 years (as reported in an interim analysis13), and have continued to improve or remain steady throughout the entire ≥5 years. Responder rates, defined as at least 50%, 75%, or 100% decreases in drop-seizure and total-seizure frequencies with clobazam treatment, were substantially greater than previously published long-term LGS trials, with at least 4 years' longer clobazam treatment data.19
The type and incidence of adverse events reported during this trial were similar to those reported during the double-blind studies. The elevated incidences of upper respiratory tract infections and pyrexia were expected given the duration of the trial and a predominantly pediatric patient population. Nine of the 10 deaths were deemed “unlikely related” or “not related” to clobazam by investigators. One death was considered only “possibly related” to clobazam. Given that patients with LGS often require chronic treatment with one or more AEDs, the safety data observed during this study suggest that clobazam has a favorable long-term safety profile. Because LGS is a chronic syndrome that requires a lifetime of treatment, the lack of change in clobazam's safety profile through 6 years is reassuring for the long-term management of LGS.
Although vulnerable to the limitations of all OLE trials, the final OV-1004 data support the interim findings13 of high patient retention rate, stable dosages, and continued substantial seizure improvements in LGS patients treated with clobazam. Data from long-term, open-label extension trials are useful, but they should be interpreted with caution. Patients who discontinue because of a lack of efficacy may enrich the pool of patients for whom the drug is efficacious. However, at the data cutoff date of the interim analysis (July 1, 2010), <5% of patients had discontinued the trial for lack of efficacy,12 and only 6% of patients at this final analysis had done so. In addition, the most common reason for discontinuation (12%) in this final analysis of the study was patient, caregiver, or parent request. We had no additional information about these patients, and this limited our analyses. Study investigators could optimize treatment regimens for patients by adding, removing, or adjusting dosages of concomitant AEDs and by employing other treatment options, such as vagus nerve stimulation and the ketogenic diet. Finally, the natural course of LGS varies widely and may be less severe for older patients. Concomitant therapies; responder bias; the indeterminate patient, caregiver, or parent request reason for discontinuation; and the variable natural course of the disease are potential confounders of the results presented here.
The data from the 6-year, open-label OV-1004 trial in LGS patients indicate that clobazam is efficacious over the long term and can be used safely to treat this chronic disorder. Although results from studies of different adjunctive AEDs for LGS cannot be compared directly, clobazam's efficacy rates were greater and were sustained for longer periods than for other adjunctive LGS AEDs studied. The substantial and sustained efficacy observed in this extension, the stable dosages required to do so, and the stable and predictable safety profile provide evidence that clobazam is a valuable long-term treatment option for LGS.
Acknowledgments
Manuscript preparation, including editing and formatting the manuscript, incorporating author comments, preparing tables and figures, and coordinating submission requirements, was provided by Neva West, PhD, of Prescott Medical Communications Group (Chicago, IL), and Michael A. Nissen, ELS, of Lundbeck LLC (Deerfield, IL). This editorial support was funded by Lundbeck LLC.
Biography
Dr. Joan Conry was the coprimary lead investigator for the clobazam open-label extension study.

Appendix I
OV-1004 Study Investigators (by Country)
Australia: Terence O'Brien, MD (Royal Melbourne Hospital, Parkville, Victoria), Ingrid Scheffer, MD (The Austin and Repatriation Hospital, West Heidelberg, Victoria). Belarus: Halina Navumava, MD (Vitebsk Regional Diagnostic Center, Vitebsk). India: Arijit Chattopadhyay, MD (Apollo Gleneagles Hospitals Limited, Kolkata), Anand Prakash Dubey, MD (Maulana Azad Medical College and Associated Lok Nayak Hospital, New Delhi), Anaita Hegde, MD (Jaslok Hospital and Research Centre, Mumbai, Maharashtra), Lekha Pandit, MD (Justice K.S. Hegde Charitable Hospital, Mangalore, Karnataka), Surekha Rajadhyaksha, MD (Deenanath Mangeshkar Hospital and Research Centre, Pune, Maharashtra), Gosala R. Sarma, MD (St. Johns Medical College Hospital, Bangalore), Shankar Nellikunja, MD (Mallikatta Neuro Center, Mangalore, Karnataka), Maneesh Kumar Singh, MD (Chhatrapati Shah Ji Maharaj Medical University, Uttar Pradesh), Vrajesh Udani, MD (P.D. Hinduja Hospital and Medical Research Center, Veer Savarkar Marg, Mahim, Mumbai, Maharashtra), Devananthan Vasudevan, MD (Dr. Kamakshi Memorial Hospital, Pallikaranai, Chennai), Nandan Yardi, MD (KEM Hospital, Pune, Mahara). Lithuania: Nerija Vaiciene, MD (Kaunas University of Medicine Hospital, Kaunas). U.S.A.: Susan T. Arnold, MD (University of Texas SW Medical Center, Dallas, TX), Blaise Bourgeois (Harvard Medical School Children's Hospital, Boston, MA), Martina Bebin, MD (University of Alabama at Birmingham, Birmingham, AL), Meriem Bensalem-Owen, MD (University of Kentucky Medical Center, Lexington, KY), Jeffrey Buchhalter, MD, PhD (Phoenix Children's Hospital, Phoenix, AZ), Tsao Chang-Yong, MD (Nationwide Children's Hospital, Columbus, OH), Steve S. Chung, MD (St. Joseph's Hospital and Medical Center, Phoenix, AZ), Joan A. Conry, MD (Children's National Medical Center, Washington, DC), Roy Elterman, MD (Dallas Pediatric Neurology Associates, Dallas, TX), Jose Ferreira, MD (Pediatric Epilepsy & Neurology Specialists, Tampa, FL), Matthew Frank, MD (Children's Hospital of the King's Daughter, Norfolk, VA), Angel Hernandez, MD (Cook Children's Health Care System, Fort Worth, TX), Gregory L. Holmes, MD (Dartmouth-Hitchcock Medical Center, Lebanon, NH), Andres M. Kanner, MD (Rush University Medical Center, Chicago, IL), Lydia Kernitsky, MD (Virginia Commonwealth University, Richmond, VA), Pavel Klein, MD (Mid-Atlantic Epilepsy and Sleep Center, Bethesda, MD), Paul M. Levisohn, MD (The Children's Hospital, Aurora, CO), Edwin Liu, MD (Pediatric Neurology and Epilepsy Center, Loxahatchee, FL), Eric Marsh, MD, PhD (Children's Hospital of Philadelphia, Philadelphia, PA), Mark Mintz, MD (Clinical Research Center of New Jersey, Voorhees, NJ), Wendy Mitchell, MD (Children's Hospital Los Angeles, Los Angeles, CA), Yu-tze Ng, MD (former study investigator, St. Joseph's Hospital and Medical Center, Phoenix, AZ), Dipakumar Pandya, MD (St. Joseph's Regional Medical Center, Patterson, NJ), Juliann Paolicchi, MD (former investigator, Nationwide Children's Hospital, Columbus, OH), Yong D. Park, MD (Medical College of Georgia Neurology, Augusta, GA), J. Ben Renfroe, MD (Children's Neurology Center of NW FL, Gulf Breeze, FL), Anthony R. Riela, MD (Texas Child Neurology, LLP, Plano, TX), Rosario Maria Riel-Romero, MD (Louisiana State University, Shreveport, LA), Frank J. Ritter, MD (Minnesota Epilepsy Group, PA, St. Paul, MN), William Rosenfeld, MD (The Comprehensive Epilepsy Care Center for Children and Adults, Chesterfield, MO), Michael Schwabe, MD (Children's Hospital of Wisconsin, Milwaukee, WI), Michael R. Sperling, MD (Thomas Jefferson University, Philadelphia, PA), Elizabeth A. Thiele, MD, PhD (Massachusetts General Hospital, Boston, MA), David Wang, MD (University of Rochester Medical Center, Rochester, NY), Robert Wechsler, MD, PhD (Consultants in Epilepsy & Neurology, Boise, ID), James W. Wheless, MD (University of Tennessee Medical Group, Memphis, TN), Angus Wilfong, MD (Texas Children's Hospital, Houston, TX), Brenda Y. Wu, MD (University of Medicine and Dentistry of NJ, Robert Wood Johnson Medical School, New Brunswick, NJ).
Appendix 2
Methods
Clobazam and concomitant AED dosing
Clobazam was given twice daily, in the morning and at bedtime. The maximum allowed daily dosage for all patients in OV-1004 was 2.0 mg/kg/day (maximum 80 mg/day). To optimize patient care, investigators were able to start, adjust, and discontinue other AEDs, and initiate other forms of treatment, including the ketogenic diet and vagus nerve stimulator placement. Concomitant medications were classified by the World Health Organization Drug Dictionary, Version June 1, 2009. Concomitant medications were those received during the clobazam dosing period. The summary for concomitant medications was separated for AEDs and non-AEDs. Week 4 AED regimens (based on Week 4 of the OLE) were used as the baseline comparators. At the initiation of the OLE, all patients were started at a dosage of 0.5 mg/kg/day. During the first 4 weeks of the OLE, dosages were titrated to efficacy. Therefore, Week 4 was chosen, since it represented a beginning point of stable, maintenance therapy. Rescue medications (i.e., medications with preferred terms of “clonazepam,” “diazepam,” “lorazepam,” “midazolam,” “midazolam hydrochloride,” “phenytoin,” “phenytoin sodium,” “phenobarbital,” and “phenobarbital sodium”) received for only 1 day or one dose were not considered concomitant therapies in determining the total number of AEDs a patient was receiving. Rescue medications were permitted no more than once per month.
Efficacy assessments
The primary efficacy outcome was the percentage decrease in the average weekly rate of drop seizures at yearly time intervals compared with baseline values. The percentage decrease in the average weekly rate of total seizures was also measured.
Baseline values for seizure analyses were defined as follows: (1) For patients who received placebo in OV-1012, baseline corresponded to the last week of recorded seizures prior to receiving their first doses of clobazam; or (2) For patients who received clobazam in OV-1002 or OV-1012, baseline corresponded to the last week of recorded seizures from the baseline period of the blinded study. For all other parameters (e.g., exposure), baseline was defined as the last value before the first dose of clobazam, whether in the previous blinded study or OV-1004.
Additional efficacy outcomes included percentage of treatment responders, percentage of patients maintaining initial response (indicating lack of development of tolerance), global evaluations of patients' overall changes in symptoms, and changes in concomitant AED use. Treatment responders were defined as patients with ≥50%, ≥75%, and 100% decreases in drop seizures or total seizures from baseline. As a proxy measure of the development of tolerance to clobazam, patients who had achieved a ≥50% decrease in drop seizures from baseline at Month 3 were assessed for a decrease in response rate. Investigator and parent/caregiver global evaluations of patients' overall changes in symptoms assessments were based on a 7-point scale from 1 (very much improved) to 7 (very much worse). At Week 4, patients receiving 1, 2, 3, or >3 concomitant AEDs were grouped, and changes in their AED intakes were calculated at each year (360 days) of the trial. Ketogenic diet, vagus nerve stimulation, and rescue medications (previously defined in Methods and used for 1 day or single dose) were not considered concomitant AEDs for this calculation.
Safety assessments
The primary overall objective of the OV-1004 open-label trial was collection of safety information. Measures included clobazam exposure, laboratory assessments (e.g., hematology tests, blood chemistry evaluations, and urinalyses), physical and neurologic examinations, vital sign monitoring, electrocardiography monitoring, and adverse event reporting.
Treatment-emergent adverse events were summarized over time by relationship to treatment (not related, possible, or probable), severity (mild, moderate, or severe), and seriousness. A serious adverse event was defined as any adverse event that met one of five predefined criteria previously described.13
Conflict of Interest
This study was funded by Lundbeck LLC (Deerfield, IL). Dr. Conry has served as a consultant, study investigator, and scientific advisory board member for Lundbeck and has received travel funding and speaker honoraria. Dr. Ng has served as a consultant, study investigator, and scientific advisory board member for Lundbeck and has received consulting fees, travel funding, and speaker honoraria. Drs. Paolicchi, Kernitsky, and Mitchell have all served as study investigators for Lundbeck. In addition, Dr. Mitchell has received travel funding from Lundbeck. Drs. Veidemanis, Drummond, Isojarvi, and Lee are Lundbeck employees. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Most frequently reported adverse events (≥10% of patients) during any exposure to clobazam (Safety Analysis Set).
References
- 1.Arzimanoglou A, French J, Blume WT, et al. Lennox-Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. Lancet Neurol. 2009;8:82–93. doi: 10.1016/S1474-4422(08)70292-8. [DOI] [PubMed] [Google Scholar]
- 2.Camfield PR. Definition and natural history of Lennox-Gastaut syndrome. Epilepsia. 2011;52(Suppl. 5):3–9. doi: 10.1111/j.1528-1167.2011.03177.x. [DOI] [PubMed] [Google Scholar]
- 3.Chevrie JJ, Aicardi J. Childhood epileptic encephalopathy with slow spike-wave. A statistical study of 80 cases. Epilepsia. 1972;13:259–271. doi: 10.1111/j.1528-1157.1972.tb05260.x. [DOI] [PubMed] [Google Scholar]
- 4.Berg AT, Shinnar S, Testa FM, et al. Mortality in childhood-onset epilepsy. Arch Pediatr Adolesc Med. 2004;158:1147–1152. doi: 10.1001/archpedi.158.12.1147. [DOI] [PubMed] [Google Scholar]
- 5.Crumrine PK. Lennox-Gastaut syndrome. J Child Neurol. 2002;17(Suppl. 1):S70–S75. doi: 10.1177/08830738020170011001. [DOI] [PubMed] [Google Scholar]
- 6.Trevathan E. Infantile spasms and Lennox-Gastaut syndrome. J Child Neurol. 2002;17(Suppl. 2):2S9–2S22. doi: 10.1177/08830738020170021201. [DOI] [PubMed] [Google Scholar]
- 7.Arzimanoglou A, Resnick T. All children who experience epileptic falls do not necessarily have Lennox-Gastaut syndrome … but many do. Epileptic Disord. 2011;13(Suppl. 1):S3–S13. doi: 10.1684/epd.2011.0422. [DOI] [PubMed] [Google Scholar]
- 8.Montouris GD. Rational approach to treatment options for Lennox-Gastaut syndrome. Epilepsia. 2011;52(Suppl. 5):10–20. doi: 10.1111/j.1528-1167.2011.03178.x. [DOI] [PubMed] [Google Scholar]
- 9.Ng YT, Collins SD. Clobazam. Neurotherapeutics. 2007;4:138–144. doi: 10.1016/j.nurt.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sankar R. GABAA Receptor physiology and its relationship to the mechanism of action of the 1,5-benzodiazepine clobazam. CNS Drugs. 2012;26:229–244. doi: 10.2165/11599020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Conry JA, Ng YT, Paolicchi JM, et al. Clobazam in the treatment of Lennox-Gastaut syndrome. Epilepsia. 2009;50:1158–1166. doi: 10.1111/j.1528-1167.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- 12.Ng YT, Conry JA, Drummond R, et al. Randomized, phase III study results of clobazam in Lennox-Gastaut syndrome. Neurology. 2011;77:1473–1481. doi: 10.1212/WNL.0b013e318232de76. [DOI] [PubMed] [Google Scholar]
- 13.Ng YT, Conry J, Paolicchi J, et al. Long-term safety and efficacy of clobazam for Lennox-Gastaut syndrome: interim results of an open-label extension study. Epilepsy Behav. 2012;25:687–694. doi: 10.1016/j.yebeh.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 14.Glauser TA, Levisohn PM, Ritter F, et al. Topiramate in Lennox-Gastaut syndrome: open-label treatment of patients completing a randomized controlled trial. Topiramate YL Study Group. Epilepsia. 2000;41(Suppl. 1):S86–S90. doi: 10.1111/j.1528-1157.2000.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 15.Dodson WE. Felbamate in the treatment of Lennox-Gastaut syndrome: results of a 12-month open-label study following a randomized clinical trial. Epilepsia. 1993;34(Suppl. 7):S18–S24. doi: 10.1111/j.1528-1157.1993.tb04590.x. [DOI] [PubMed] [Google Scholar]
- 16.Kluger G, Glauser T, Krauss G, et al. Adjunctive rufinamide in Lennox-Gastaut syndrome: a long-term, open-label extension study. Acta Neurol Scand. 2010;122:202–208. doi: 10.1111/j.1600-0404.2010.01334.x. [DOI] [PubMed] [Google Scholar]
- 17.Riss J, Cloyd J, Gates J, et al. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008;118:69–86. doi: 10.1111/j.1600-0404.2008.01004.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee EH, Yum MS, Choi HW, et al. Long-term use of clobazam in Lennox-Gastaut syndrome: experience in a single tertiary epilepsy center. Clin Neuropharmacol. 2013;36:4–7. doi: 10.1097/WNF.0b013e3182770730. [DOI] [PubMed] [Google Scholar]
- 19.Wheless JW, Vazquez B. Rufinamide: a novel broad-spectrum antiepileptic drug. Epilepsy Curr. 2010;10:1–6. doi: 10.1111/j.1535-7511.2009.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Most frequently reported adverse events (≥10% of patients) during any exposure to clobazam (Safety Analysis Set).


