Abstract
Human obesity-related diabetes and the accompanying metabolic disorders have been specifically linked to increased visceral adipose tissue mass. Understanding the differences in biology of the two human fat depots (visceral and subcutaneous) might hold the key to therapeutic strategies aimed at reducing obesity-induced insulin resistance and alleviating symptoms of the metabolic syndrome. Visfatin (pre-B-cell colony-enhancing factor, PBEF) is a novel adipokine that appears to be preferentially produced by visceral adipose tissue and has insulin-mimetic actions. Could this molecule hold the keytofuture treatments for type 1 and 2 diabetes? This article discusses the pros and cons of visfatin action and how it might affect future therapeutic strategies.
Introduction
Obesity, the excessive accumulation of fat, is a risk factor for the metabolic syndrome (i.e. diabetes, dyslipidaemia and cardiovascular complications). However, not every form of obesity poses a similar clinical threat. Strong epidemiological evidence indicates that the preferential accumulation of intra-abdominal (visceral) fat, surrounding the gastrointestinal organs, poses a greater cardiovascular risk than in other forms of obesity, in which fat is preferentially accumulated under the skin (subcutaneous) in the gluteal region [1]. Confronted with the fact that the distribution of body fat might be even more relevant than the total amount of stored fat, scientists have tried, with limited success, to identify the differences between these topographically distinct depots to understand what makes the intra-abdominal depot so deleterious (Table 1). In a recent issue of Science, Fukuhara et al. [2] identified visfatin as a new protein that is preferentially produced in the intra-abdominal adipose tissue of obese mice and humans, which, surprisingly, shares many of the anti-diabetic effects of insulin. This review discusses the pros and cons of visfatin action and how it might impact on future therapeutic strategies for diabetes.
Table 1. Known differences between visceral (VAT) and subcutaneous (SAT) adipose tissuea,b.
| VAT > SAT | VAT < SAT |
|---|---|
| PAI-1c | Leptind |
| Adiponectind | Adiponectind |
| IL-6c | PPARγd |
| TNFαc | RXR |
| Angiotensinogen | |
| HSD | |
| Visfatind | |
| Glut4 | Adipocyte size |
| Basal glucose uptake | Proximal insulin signalling |
| Insulin-stimulated glucose uptake | |
| Lipolytic response to catecholamines | Insulin-induced inhibition of lipolysis |
| Lipogenic response to TZDs |
Abbreviations: Glut, glucose transporter; HSD, hydroxysteroid dehydrogenase; PAI, plasminogen activator inhibitor; PPAR, peroxisome proliferator-activated receptor; RXR, retinoid X receptor; TZDs, thiazolidinediones.
Secreted adipokines that induce insulin resistance.
Secreted adipokines that enhance insulin sensitivity.
What is the molecular link between abdominal obesity and diabetes?
An intriguing unresolved scientific question is: how does the expansion of adipose tissue, which is typically observed in obesity, result in diabetes and increased cardiovascular risk? It has been suggested that the common feature linking these problems is insulin resistance (i.e. the inability of insulin to exert its metabolic effects). In fact, obese people, in particular those who have intra-abdominal obesity, develop marked insulin resistance. Two complementary theories have been proposed to explain why and how obesity induces insulin resistance. One theory proposes that when adipose tissue expands excessively, it reaches a threshold level at which its storage capacity becomes saturated. As a result, it becomes less able to amass more fat. Once this stage is reached, the excess fat is redirected towards other organs, such as the liver, pancreas or muscle. In these organs, lipid accumulation can be toxic and induce insulin resistance, a phenomenon known as lipotoxicity. The second hypothesis proposes that the excessive accumulation of fat in adipose tissue can change the repertoire of adipocyte-specific secreted molecules, also known as adipokines. Interestingly, some of these adipokines modulate insulin sensitivity not only in adipose tissue but also in other metabolically relevant organs, such as liver or muscle. This is the case for adiponectin, leptin, resistin, interleukin (IL)-6 and tumour necrosis factor (TNF)α. Therefore, the adipose tissue, in addition to being a specialized organ for fat storage, should be considered the largest endocrine gland in the body, capable of synthesizing and secreting hormones (adipokines) that can modulate insulin sensitivity locally and in other organs [3].
What is visfatin?
Visfatin is a protein that is preferentially produced in visceral adipose tissue and both its tissue expression and secreted plasma levels increase in parallel with obesity. Although visfatin is preferentially produced in visceral adipose tissue, it can be found in skeletal muscle, liver, bone marrow and lymphocytes, where it was initially identified as pre-B-cell colony-enhancing factor (PBEF). Interestingly, PBEF expression is regulated by cytokines that promote insulin resistance, such as lipopolysaccharide, IL-1β, TNFα and IL-6 [4,5]. Its levels are also increased in acute lung inflammation and sepsis, which is accompanied by an insulin-resistant state. The biological properties of visfatin (PEBF) are similar to the growth-factor-like properties of some of these cytokines (i.e. it is anti-apoptotic and promotes cell proliferation).
Could visfatin be the missing link between intra-abdominal obesity and diabetes?
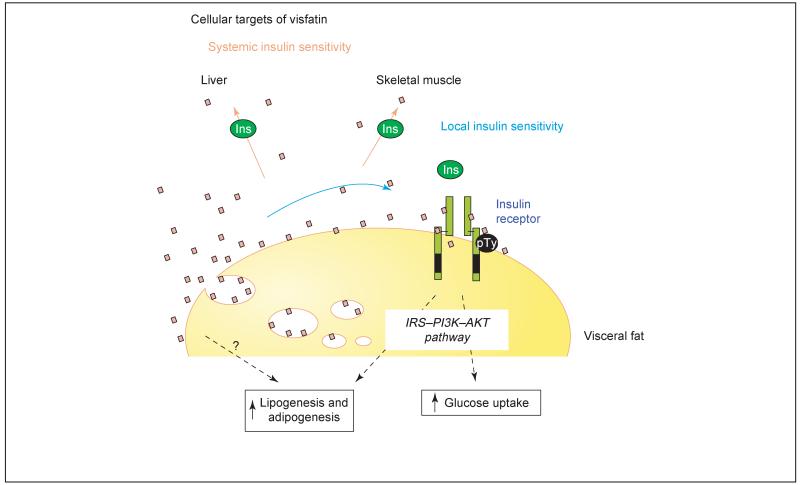
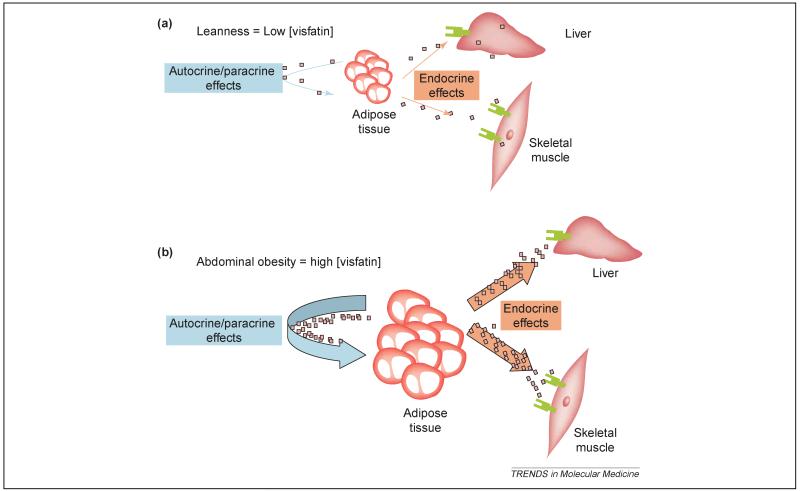
Several features about visfatin suggest that this molecule might be important for understanding the biological differences between intra-abdominal (visceral) and sub-cutaneous adipose tissue, and their contribution to the metabolic syndrome. First, visfatin is detected in the plasma and its concentration correlates with intra-abdominal fat mass but not with subcutaneous fat mass. Furthermore, the fact that visfatin increases in plasma following a high-fat diet suggests that it has an important role in diet- or obesity-induced insulin resistance. The early characterisation of visfatin suggested that it could be a secreted protein, in spite of not having the typical signal peptide that is common to other secreted proteins. Therefore, at first sight visfatin appears to be an excellent candidate for an adipokine that is preferentially secreted by the intra-abdominal adipose tissue and links the expansion of this adipose depot to insulin resistance. Although Fukuhara et al. [2] clearly suggested an endocrine role for visfatin, it cannot be excluded that visfatin might also have a paracrine effect on the visceral adipose tissue, facilitating the differentiation of the adipose tissue through its pro-adipogenic and lipogenic actions. In fact, the overexpression of visfatin in a preadipocyte cell line facilitates its differentiation to mature adipocytes and promotes the accumulation of fat through the activation of glucose transport and lipogenesis [2]. Precedence for paracrine action has previously also been proposed for PBEF action in foetal membranes [6]. A direct effect of visfatin on adipose tissue is further supported by its localization in the nucleus and cytoplasm, indicating a role in cell-cycle regulation [7]. Therefore, visfatin might have a double function: an autocrine/paracrine function on visceral adipose tissue, facilitating differentiation and fat deposition (Figure 1), and an endocrine role modulating insulin sensitivity in peripheral organs (Figure 2).
Figure 1.
Visfatin (PBEF) action. (a) In the lean state, visfatin production is low and its action on insulin sensitivity might be negligible. (b) Intra-abdominal obesity leads to increased visfatin production, which might simultaneously increase obesity and maintain insulin sensitivity in peripheral organs.
Figure 2.
Visceral fat and visfatin. Visceral fat is not only the site of visfatin production but is also a target organ for visfatin action. Here, visfatin might act on the insulin receptor to increase glucose uptake and lipogenic signals.
An unexpected twist: visfatin has insulin-mimetic properties
Further characterization of the metabolic properties of visfatin brought an unexpected new twist to the story. Contrary to the most intuitive hypothesis, visfatin treatment did not promote insulin resistance, but actually exhibited insulin-mimetic properties resulting in a glucose-lowering effect [2]. As does insulin, visfatin increases glucose transport and lipogenesis when administered to 3T3L1 preadipocytes and L6 myocytes and decreases glucose production by hepatocytes [2]. When delivered directly to diabetic mice, visfatin also improved insulin sensitivity in vivo, decreasing glucose and insulin levels [2]. The significance of endogenous visfatin controlling whole-body insulin sensitivity is reinforced by the phenotype of lean, heterozygous visfatin-knockout mice, which revealed mild but reproducible hyperglycaemia. Subsequent analysis of this insulin-mimetic effect revealed two surprising findings: that the effects of visfatin are mediated by the insulin receptor itself, with remarkably similar affinities to those of insulin but through a distinct binding site, and that this insulin-sensitizing effect of visfatin might be additive to the effect of insulin, suggesting that visfatin activates insulin-receptor-activated pathways through a novel mechanism.
What is the physiological relevance of visfatin for the metabolic syndrome?
The discovery of this curious new adipokine has great potential to significantly enhance our understanding of the metabolic syndrome. However, as with most new discoveries, these findings need to be reproduced. Indeed, there is already an indication that the correlation between visfatin gene expression and the metabolic syndrome might not be universal to all models [8]. Novel findings also raise several new questions that need to be addressed before their true significance can be appreciated. It will be necessary to determine the contribution of visfatin originating from visceral adipose tissue to the control of global insulin sensitivity. Although the affinity of visfatin for the insulin receptor appears to be similar to insulin, its concentration in plasma is much lower (3–10% of the insulin concentration) under physiological conditions. It is also not regulated by fasting and feeding. This raises doubts about the physiological importance of the systemic insulin-sensitizing effects of visfatin. However, the dramatic elevation of visfatin levels in the visceral adipose tissue of obese mice suggests that it has a significant role in the pathophysiology of obesity. Here, it remains to be established whether visfatin production is a compensatory response to tissue-specific insulin resistance or more simply a marker of tissue-specific inflammatory-cytokine action.
Nonetheless, the paracrine insulin-mimetic effects of visfatin in intra-abdominal adipose tissue might be more relevant, particularly if the concentration of visfatin in this tissue is higher. Therefore, it is likely that the autocrine/paracrine effects of visfatin, facilitating the expansion of the intra-abdominal fat depot, are more biologically relevant than its endocrine effects on improving global insulin sensitivity. Thus, one of the caveats about strategies directed to increase visfatin secretion from the adipose tissue to improve systemic insulin sensitivity is whether it might have the side effect of further expanding the visceral fat depot. Conversely, strategies directed to inhibit visfatin action might be relevant if its trophic effect on the visceral adipose tissue is confirmed. However, any strategies aiming to dramatically decrease visfatin levels should proceed with caution, because it is a putative housekeeping protein that is highly conserved in evolutionary terms and genetic ablation is lethal.
On the positive side, the identification of the insulin-mimetic properties of visfatin is extremely exciting. The excitement comes from the fact that these properties of visfatin are potentially additive to that of insulin and, as such, might be useful in synergistic strategies for treating insulin resistance. Alternatively, the insulin-mimetic properties of visfatin, together with its tonic plasma levels independent of feeding patterns, raise the compelling possibility of its potential therapeutic use in treating patients with type 1 diabetes.
Concluding remarks
Visfatin is a newly discovered adipokine that is produced by the intra-abdominal adipose tissue, which simultaneously facilitates adipogenesis and has insulin-mimetic properties. It is currently unclear what would be its physiological role or relevance in the context of the metabolic syndrome. Visfatin might be part of the mechanisms that facilitate the accumulation of fat in the intra-abdominal depot, a feedback mechanism preventing the deleterious effects of the expansion of the intra-abdominal depot in insulin sensitivity or simply an epiphenomenon that might be useful as a surrogate marker of increased visceral fat mass and cardiovascular risk. Its paradoxical effects facilitating fat accumulation and promoting insulin sensitivity suggest that visfatin is either good news for diabetes treatment or bad news for obesity treatment.
Acknowledgements
J. K. S. is a BBSRC David Phillips Research Fellow and A. V-P. is supported by The Wellcome Trust and the Medical Research Council.
References
- 1.Giorgino F, et al. Regional differences of insulin action in adipose tissue: insights from in vivo and in vitro studies. Acta Physiol. Scand. 2005;183:13–30. doi: 10.1111/j.1365-201X.2004.01385.x. [DOI] [PubMed] [Google Scholar]
- 2.Fukuhara A, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 3.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Ognjanovic S, et al. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J. Mol. Endocrinol. 2001;26:107–117. doi: 10.1677/jme.0.0260107. [DOI] [PubMed] [Google Scholar]
- 5.Kralisch S, et al. Interleukin-6 is a negative regulator of visfatin gene expression in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. doi: 10.1152/ajpendo.00090.2005. in press. [DOI] [PubMed] [Google Scholar]
- 6.Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am. J. Obstet. Gynecol. 2002;187:1051–1058. doi: 10.1067/mob.2002.126295. [DOI] [PubMed] [Google Scholar]
- 7.Kitani T, et al. Growth phase-dependent changes in the subcellular localization of pre-B-cell colony-enhancing factor. FEBS Lett. 2003;544:74–78. doi: 10.1016/s0014-5793(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 8.Kloting N, Kloting I. Visfatin: gene expression in isolated adipocytes and sequence analysis in obese WOKW rats compared with lean control rats. Biochem. Biophys. Res. Commun. 2005;332:1070–1072. doi: 10.1016/j.bbrc.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 9.Van Gaal LF, et al. Health risks of lipodystrophy and abdominal fat accumulation: therapeutic possibilities with leptin and human growth hormone. Growth Horm. IGF Res. 2003;13:S4–S9. doi: 10.1016/s1096-6374(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 10.Lihn AS, et al. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol. Cell. Endocrinol. 2004;219:9–15. doi: 10.1016/j.mce.2004.03.002. [DOI] [PubMed] [Google Scholar]