Abstract
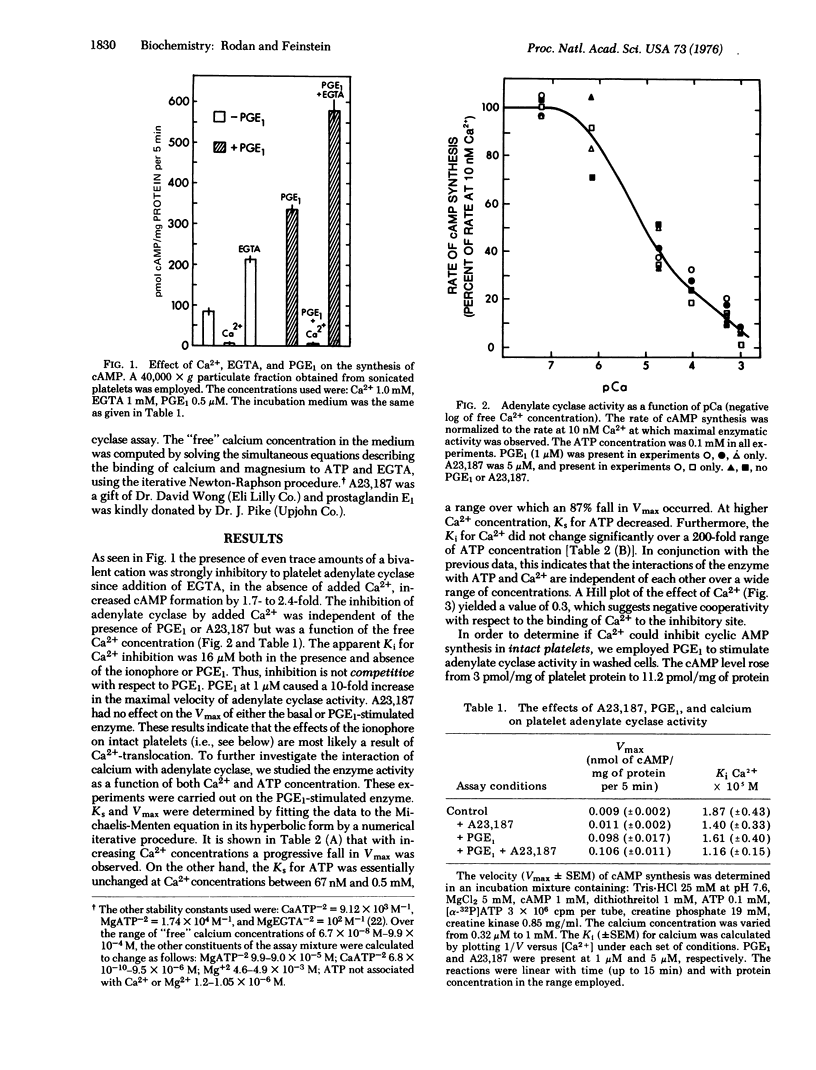
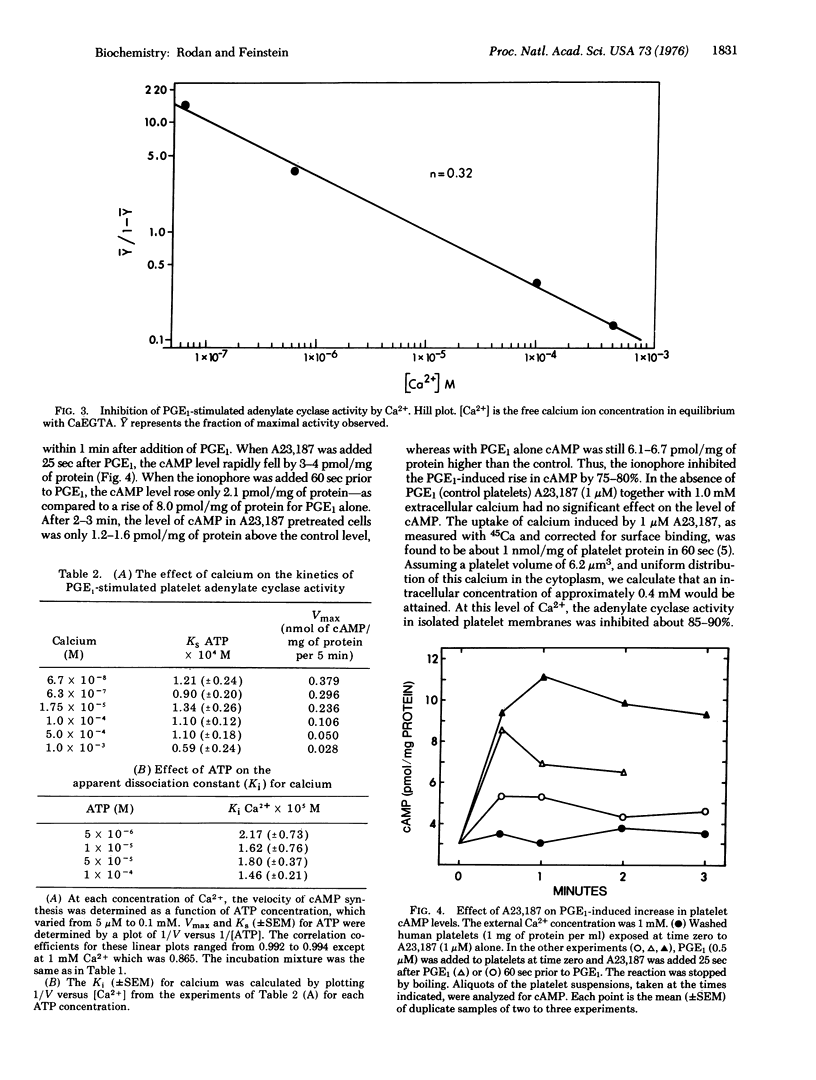
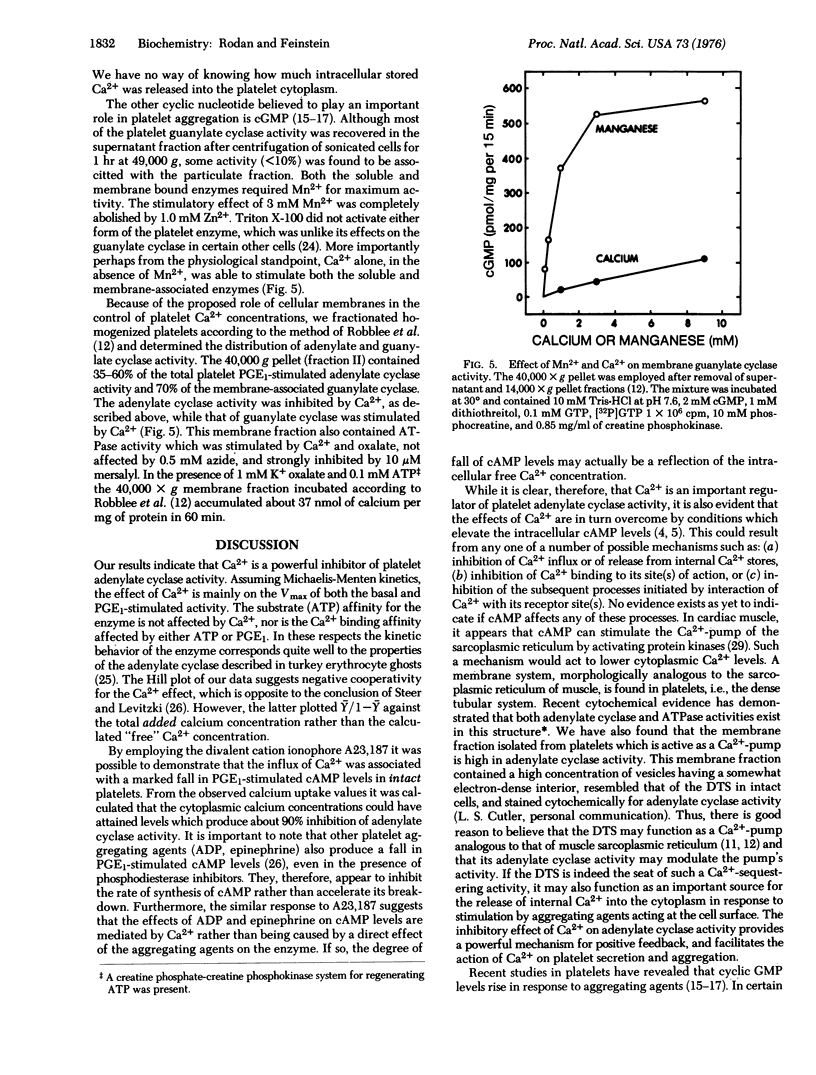
Ca2+ is a powerful inhibitor (Ki is congruent to 16 muM) of basal and prostaglandin E1 (PGE1)-stimulated adenylate cyclase [ATP pyrophosphate-lyase (cyclizing); EC 4.6.1.1] activity in membranes obtained from homogenized human platelets. Ca2+ (but not the ionophore A23,187) decreased V(max) of the reaction without an effect on the Ks for ATP. Neither ATP nor PGE1 affected Ki for Ca2+. In intact platelets A23,187 induced Ca2+ influx and markedly inhibited PGE1-stimulated rise in adenosine 3':5'-cyclic monophosphate (cAMP) levels. Guanylate cyclase [GTP pyrophosphate-lyase (cyclizing); EC 4.6.1.2] activity was mainly found in the soluble fraction (greater than 90%). Both soluble and membrane bound enzymes were stimulated by Mn2+ and Ca2+ and inhibited by Zn2+. Adenylate and guanylate cyclase activity were both present in a membrane fraction cyclase activity were both present in a membrane fraction which contained Ca2+ activated ATPase activity, and accumulated Ca2+ from the medium in the presence of ATP and oxalate. Other evidence indicates that these membranes originated in large part from the dense tubular system of the platelets. It is proposed that concurrent inhibition of adenylate cyclase and stimulation of guanylate cyclase facilitates the direct initiating effect of Ca2+ on platelet secretion and aggregation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs F. N., Fleishman M. Calcium binding by particle-free supernatants of homogenates of skeletal muscle. J Gen Physiol. 1965 Sep;49(1):131–149. [PMC free article] [PubMed] [Google Scholar]
- Detwiler T. C., Feinman R. D. Kinetics of the thrombin-induced release of adenosine triphosphate by platelets. Comparison with release of calcium. Biochemistry. 1973 Jun 19;12(13):2462–2468. doi: 10.1021/bi00737a015. [DOI] [PubMed] [Google Scholar]
- Feinstein M. B., Fraser C. Human platelet secretion and aggregation induced by calcium ionophores. Inhibition by PGE1 and dibutyryl cyclic AMP. J Gen Physiol. 1975 Nov;66(5):561–581. doi: 10.1085/jgp.66.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., White J. G., Rao G. H. Effects of the lonophore A23187 on the blood platelets II. Influence on ultrastructure. Am J Pathol. 1974 Nov;77(2):151–166. [PMC free article] [PubMed] [Google Scholar]
- Hardman J. G., Sutherland E. W. Guanyl cyclase, an enzyme catalyzing the formation of guanosine 3',5'-monophosphate from guanosine trihosphate. J Biol Chem. 1969 Dec 10;244(23):6363–6370. [PubMed] [Google Scholar]
- Haslam R. J. Interactions of the pharmacological receptors of blood platelets with adenylate cyclase. Ser Haematol. 1973;6(3):333–350. [PubMed] [Google Scholar]
- Haslam R. J., McClenaghan M. D. Effects of collagen and of aspirin on the concentration of guanosine 3':5'-cyclic monophosphate in human blood platelets: measurement by a prelabelling technique. Biochem J. 1974 Feb;138(2):317–320. doi: 10.1042/bj1380317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Murad F. Evidence for two different forms of guanylate cyclase in rat heart. J Biol Chem. 1974 Nov 10;249(21):6910–6916. [PubMed] [Google Scholar]
- MORSE E. E., JACKSON D. P., CONLEY C. L. ROLE OF PLATELET FIBRINOGEN IN THE REACTIONS OF PLATELETS TO THROMBIN. J Clin Invest. 1965 May;44:809–816. doi: 10.1172/JCI105193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massini P., Lüscher E. F. Some effects of ionophores for divalent cations on blood platelets. Comparison with the effects of thrombin. Biochim Biophys Acta. 1974 Nov 4;372(1):109–121. doi: 10.1016/0304-4165(74)90077-4. [DOI] [PubMed] [Google Scholar]
- Miller J. L., Katz A. J., Feinstein M. B. Plasmin inhibition of thrombin-induced platelet aggregation. Thromb Diath Haemorrh. 1975 Apr 30;33(2):286–309. [PubMed] [Google Scholar]
- Ogawa Y. The apparent binding constant of glycoletherdiaminetetraacetic acid for calcium at neutral pH. J Biochem. 1968 Aug;64(2):255–257. doi: 10.1093/oxfordjournals.jbchem.a128887. [DOI] [PubMed] [Google Scholar]
- Robblee L. S., Shepro D., Belamarich F. A. Calcium uptake and associated adenosine triphosphatase activity of isolated platelet membranes. J Gen Physiol. 1973 Apr;61(4):462–481. doi: 10.1085/jgp.61.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Ignarro L. J. Bioregulation of lysosomal enzyme secretion from human neutrophils: roles of guanosine 3':5'-monophosphate and calcium in stimulus-secretion coupling. Proc Natl Acad Sci U S A. 1975 Jan;72(1):108–112. doi: 10.1073/pnas.72.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statland B. E., Heagan B. M., White J. G. Uptake of calcium by platelet relaxing factor. Nature. 1969 Aug 2;223(5205):521–522. doi: 10.1038/223521a0. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Levitzki A. The control of adenylate cyclase by calcium in turkey erythrocyte ghosts. J Biol Chem. 1975 Mar 25;250(6):2080–2084. [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Repke D. I., Katz A. M. The stimulation of calcium transport in cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1974 Oct 10;249(19):6174–6180. [PubMed] [Google Scholar]
- Vigdahl R. L., Marquis N. R., Tavormina P. A. Platelet aggregation. II. Adenyl cyclase, prostaglandin E1, and calcium. Biochem Biophys Res Commun. 1969 Oct 22;37(3):409–415. doi: 10.1016/0006-291x(69)90930-9. [DOI] [PubMed] [Google Scholar]
- White A. A., Zenser T. V. Separation of cyclic 3',5'-nucleoside monophosphates from other nucleotides on aluminum oxide columns. Application to the assay of adenyl cyclase and guanyl cyclase. Anal Biochem. 1971 Jun;41(2):372–396. doi: 10.1016/0003-2697(71)90156-4. [DOI] [PubMed] [Google Scholar]
- White J. G. Interaction of membrane systems in blood platelets. Am J Pathol. 1972 Feb;66(2):295–312. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Rao G. H., Gerrard J. M. Effects of the lonophore A23187 on blood platelets I. Influence on aggregation and secretion. Am J Pathol. 1974 Nov;77(2):135–149. [PMC free article] [PubMed] [Google Scholar]



