Summary
Secretion of Wnts by adipose cells has an important role in the control of murine adipogenesis. We present the first evidence that a Wnt antagonist, Dickkopf 1 (Dkk1), is secreted by human preadipocytes and promotes adipogenesis. DKK1 mRNA increases six hours after onset of human adipogenesis and this is followed by an increase in Dkk1 protein. With further differentiation, the mRNA and protein levels progressively decline such that they are undetectable in mature adipocytes. The transient induction in DKK1 correlates with downregulation of cytoplasmic and nuclear β-catenin levels, this being a surrogate marker of canonical Wnt signalling, and Wnt/β-catenin transcriptional activity. In addition, constitutive expression of Dkk1 in 3T3-L1 preadipocytes promotes their differentiation, further supporting the functional significance of increased Dkk1 levels during human adipogenesis. Concomitant downregulation of the Dkk1 receptors LRP5 and LRP6 is likely to potentiate the ability of Dkk1 to inhibit Wnt signalling and promote differentiation. Notably, Dkk1 is not expressed in primary murine preadipocytes or cell lines. The involvement of Dkk1 in human but not murine adipogenesis indicates that inter-species differences exist in the molecular control of this process. Given the public health importance of disorders of adipose mass, further knowledge of the pathways involved specifically in human adipocyte differentiation might ultimately be of clinical relevance.
Keywords: Adipocyte, Adipogenesis, Wnt, Dickkopf 1, LRP5, Human
Introduction
Induction of preadipocyte differentiation in vivo is influenced by a balance of positive and negative factors (MacDougald and Mandrup, 2002). Whereas part of the signal is endocrine in nature, local signals originating from both preadipocytes and mature adipocytes are also important. Understanding the signals that regulate the balance between growth of existing and differentiation of new adipocytes might provide novel strategies to prevent obesity and related metabolic complications.
Wnts are a family of lipid-modified secreted glycoproteins comprising 19 members in humans (Miller, 2002; Willert et al., 2003). They act in autocrine and paracrine fashions to direct pattern specification during embryonic development and adult tissue remodelling (Logan and Nusse, 2004; Taipale and Beachy, 2001; Wodarz and Nusse, 1998). Wnts exert their effects by signalling through multiple pathways to regulate cell differentiation, cell growth and apoptosis. In the canonical pathway, binding of Wnts to Frizzled receptors and low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6) co-receptors inhibits the activity of glycogen synthase kinase-3β (GSK-3β) (Hagen et al., 2004; Hagen and Vidal-Puig, 2002). Inactivation of GSK-3β leads to hypophosphorylation of β-catenin, which accumulates in the cytoplasm and translocates to the nucleus, where it binds to the lymphoid enhancer-binding factor/T-cell-specific transcription factor (LEF/TCF) family of transcription factors to activate Wnt target genes.
Wnt signalling can be modulated by extracellular antagonists (Jones and Jomary, 2002; Kawano and Kypta, 2003). Secreted Frizzled-related proteins (SFRPs), Wnt inhibitory factor (WIF) and Cerberus directly bind and sequester Wnt proteins from their receptors. By contrast, Dickkopf (Dkk) proteins, such as Dkk1, inhibit Wnt signalling by binding as high-affinity antagonists to LRP5/6 co-receptors, thus preventing Wnt-induced Frizzled-LRP5/6 complex formation (Bafico et al., 2001; Mao et al., 2001; Semenov et al., 2001). In addition, Dkk1 also interacts with another receptor class, Kremen1 or 2 (Krm1/2) to inhibit LRP6 synergistically (Davidson et al., 2002; Mao et al., 2002). The Dkk family has at least four members – Dkk1, Dkk2, Dkk3 and Dkk4. However, not all Dkks inhibit Wnt signalling. Like Dkk1, Dkk4 can cooperate with Krm2 to inhibit Wnt/β-catenin signalling through binding and sequestering LRP6. Dkk2 can either stimulate or inhibit Wnt signalling in a context-dependent manner. By contrast, the function of Dkk3 is less clear; there is no evidence that it can bind either of these receptors or modulate Wnt/β-catenin signalling (Kawano and Kypta, 2003).
Recently, evidence has emerged for the involvement of Wnts in murine adipogenesis (Bennett et al., 2002; Ross et al., 2000). It has been shown that Wnt signalling, probably mediated by WNT10B, prevents differentiation of the 3T3-L1 murine preadipocyte cell line by inhibiting expression of the master adipogenic transcription factors CCAAT/enhancer binding protein-α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ). Reciprocally, disruption of extracellular or intracellular Wnt signalling results in spontaneous adipogenesis. Furthermore, transgenic mice expressing Wnt10b under the control of the fat-specific Fabp4 promoter have reduced body fat content without lipodystrophic diabetes (Longo et al., 2004). To date, no study has directly addressed the relevance of Wnt signalling in human adipogenesis. In the present study, we have identified DKK1 as a novel gene that is significantly upregulated during early human adipogenesis. In addition, we present evidence suggesting that coordinate regulation of Dkk1 and its receptors might facilitate human adipocyte differentiation by inhibiting Wnt signalling.
Results
DKK1 mRNA and protein is transiently upregulated in early human adipogenesis
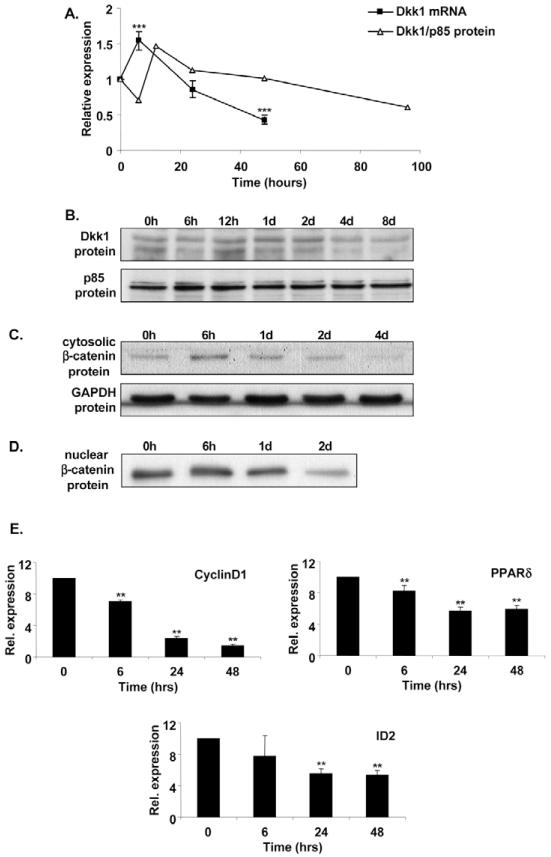
While interrogating microarray expression profiles of early human adipogenesis, we identified Dkk1, a secreted Wnt antagonist, as one of the most upregulated transcripts six hours following onset of differentiation. Given that Wnt signalling has been shown to inhibit murine adipocyte differentiation, we reasoned that Dkk1 might be an important endogenous inducer of human adipogenesis. To verify the microarray expression data, we analysed DKK1 gene expression during differentiation of subcutaneous preadipocytes from eight additional, unrelated subjects using quantitative real-time reverse transcription (RT)-PCR. As illustrated in Fig. 1A, we confirmed that DKK1 mRNA is present in confluent preadipocytes and its expression increases six hours following induction of differentiation. Thereafter, DKK1 mRNA levels progressively decline.
Fig. 1.
DKK1 mRNA and Dkk1 protein expression is transiently upregulated during human adipogenesis and correlates with inhibition of canonical Wnt signalling. (A) DKK1 mRNA and protein expression during human adipogenesis. Subcutaneous preadipocytes from eight unrelated subjects were differentiated in vitro and total RNA extracted at the time points indicated. DKK1 mRNA levels were determined by real-time RT-PCR. A plot of the ratio of Dkk1 to p85 protein obtained by densitometry (see Fig. 1B) is also shown alongside the RNA data for comparison. Results are expressed as fold difference relative to baseline (time 0). (B-D) Dkk1 and β-catenin protein expression during human adipogenesis. Subcutaneous preadipocytes were differentiated in vitro and whole-cell lysates (B), cytosolic (C), or nuclear extracts (D) were obtained at the time points indicated and subjected to SDS-PAGE and western blot analysis. p85 PI 3-kinase and GAPDH were used as loading controls. Results are representative of at least two independent experiments. 0h indicates onset of differentiation (3 days post-confluence); 6h, 12h, 1d, 2d, 4d, 8d respectively indicate 6 hours, 12 hours, 1 day, 2 days, 4 days and 8 days post-induction of differentiation. (E) Expression of Wnt target genes during human adipogenesis. CyclinD1, PPARδ and ID2 mRNA levels were determined by real-time RT PCR using RNA from six of the subjects used in A. Rel., relative. **P<0.01, ***P<0.001.
To confirm that the changes in DKK1 mRNA levels were translated to changes in Dkk1 protein, we performed western blot analysis on identically treated samples (Fig. 1B). As with the expression of DKK1 mRNA, Dkk1 protein was also found to be upregulated transiently, peaking 12 hours following onset of differentiation (Fig. 1A,B). Furthermore, the induction of Dkk1 protein correlated with downregulation of both cytosolic and nuclear β-catenin, which is a downstream effector of canonical Wnt signalling (Fig. 1C,D). The induction of Dkk1 protein also correlated with reduced expression of cyclinD1, PPARδ and ID2, known transcription targets of Wnt/β-catenin (Fig. 1E). These results suggest that increased expression of Dkk1 during human adipogenesis is associated with inhibition of canonical Wnt signalling.
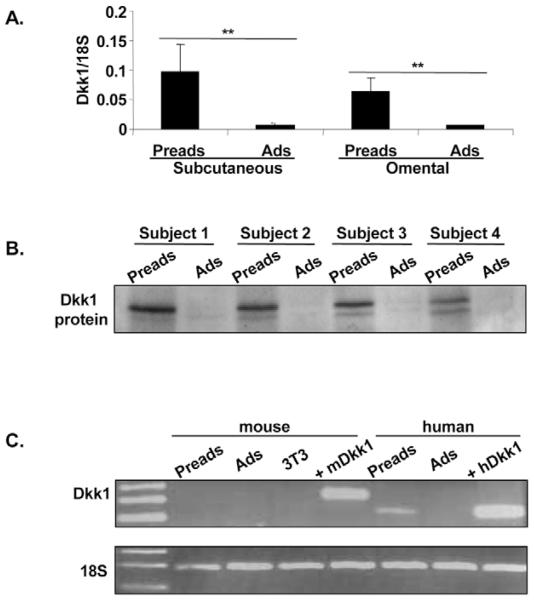
DKK1 gene and protein expression is restricted to the stromal-vascular fraction of human adipose tissue
We next examined the distribution of DKK1 mRNA in human adipose tissue. As shown in Fig. 2A, DKK1 mRNA was expressed in stromal-vascular cells and was essentially absent in mature adipocytes in both subcutaneous and omental adipose depots. Similarly, Dkk1 protein was present in stromal-vascular cells (Fig. 2B). Parallel experiments were also conducted on murine samples. However, in this species, we could not detect Dkk1 gene expression in stromal-vascular cells and mature adipocytes (Fig. 2C) or during differentiation of murine primary cultures (our unpublished data). Dkk1 transcript was also undetectable in 3T3-L1 preadipocytes (Fig. 2C), although the same primer pair gave a positive signal when whole mouse embryo cDNA was used as PCR template. Similarly, using a mouse-specific antibody, we could not detect Dkk1 protein in murine stromal-vascular cells or 3T3-L1 preadipocytes. Nevertheless, the same antibody, which crossreacts with human protein, readily detected recombinant human (r)Dkk1 protein and human (h)Dkk1 protein ectopically expressed in 3T3-L1 cells (see below). Hence, adipose expression of Dkk1 appears to be species specific.
Fig. 2.
DKK1 mRNA and protein expression is restricted to the stromal-vascular fraction of human adipose tissue. (A) DKK1 mRNA levels were measured using real-time RT PCR in subcutaneous and omental stromal-vascular cells and mature adipocytes from eight unrelated subjects. (B) Dkk1 protein expression in vivo. Western blot analysis of whole-cell lysates from subcutaneous stromal-vascular cells and mature adipocytes from four unrelated subjects. (C) DKK1 mRNA expression in human and mouse adipose tissues. Total RNA was extracted from stromal-vascular cells and mature adipocytes from four mice (pooled samples), confluent 3T3-L1 cells and one human subject, and RT-PCR for mouse and human DKK1 was performed. RNA isolated from whole mouse embryo and 3T3-L1 cells stably overexpressing human DKK1 were used as positive controls for mouse and human PCRs, respectively. Data were normalised using 18S control. Preads, preadipocytes; Ads, adipocytes; 3T3, 3T3-L1 cells; m, mouse; h, human; +, positive control; **P<0.01.
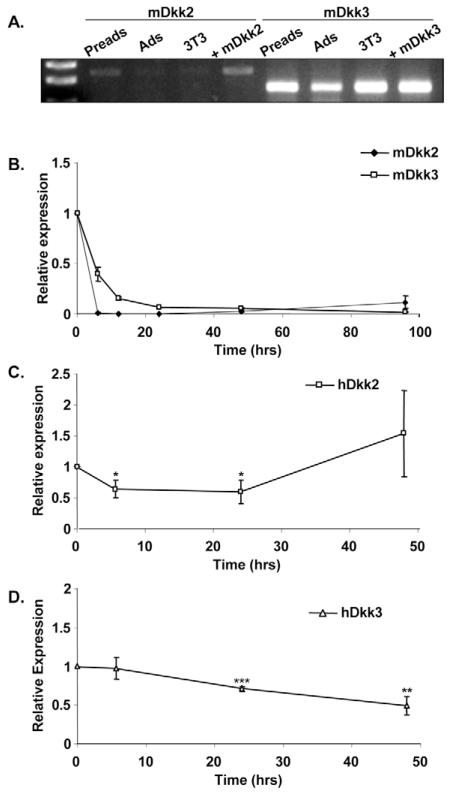
Other Dkk family members are downregulated during adipogenesis
Given the lack of Dkk1 expression in murine adipose cells, we examined whether other members of the Dkk family are present in mouse adipose tissue and regulated during adipogenesis. Fig. 3A shows that both Dkk2 and Dkk3 are expressed in primary preadipocytes and 3T3-L1 cells, and to a lesser extent in mature adipocytes. Dkk4 was not detected in 3T3-L1 or primary preadipocytes (our unpublished data). The expression of Dkk2 and Dkk3 during differentiation of 3T3-L1 cells was also analysed using quantitative real-time RT-PCR. As shown in Fig. 3B, both Dkk2 and Dkk3 mRNA are rapidly downregulated within six hours of induction of differentiation and thus do not show the same profile as DKK1 during human adipogenesis. This suggests that Dkk2 and Dkk3 are not substitutes for Dkk1 in murine adipogenesis. DKK2 and DKK3 mRNAs are also downregulated during human adipogenesis, albeit with different kinetics (Fig. 3C,D).
Fig. 3.
Expression of DKK2 and DKK3 during adipogenesis. (A) Expression of Dkk2 and Dkk3 mRNA in mouse adipose tissue was determined using RNA obtained in Fig. 2C. (B) 3T3-L1 preadipocytes were induced to differentiate and total RNA extracted at the time points indicated. Dkk2 and Dkk3 mRNA levels were measured using real-time RT-PCR. Results are expressed as fold difference relative to the baseline (time 0). All results the mean ± s.e.m. of four independent experiments. (C) Human subcutaneous preadipocytes from six unrelated subjects were differentiated in vitro and total RNA extracted at the time points indicated. mRNA levels of DKK2 and DKK3 were determined by real-time RT-PCR. Results are expressed as fold difference relative to baseline (time 0). Preads, preadipocytes; Ads, adipocytes; 3T3, 3T3-L1 cells; m, mouse; h, human; +, positive control. *P<0.05, **P<0.01, ***P<0.001.
Ectopic expression of hDkk1 inhibits Wnt signalling in 3T3-L1 preadipocytes
To assess the functional consequences of Dkk1 upregulation, we generated 3T3-L1 cells constitutively expressing hDkk1. After confirming hDkk1 protein expression in our Dkk1 cell line (Fig. 4A), we determined whether hDkk1 could inhibit Wnt signalling in these cells. Both control (empty vector) and hDkk1-expressing preadipocytes were induced to differentiate, and cytosolic protein extracts collected at the times indicated (Fig. 4B). Ectopic expression of hDkk1 led to constitutive inhibition of Wnt signalling, as demonstrated by decreased levels of cytosolic β-catenin throughout differentiation in these cells. This was further confirmed with promoter assays using the luciferase reporter construct TOPflash (Fig. 4C). This reporter contains multiple TCF-binding sites and is a read-out for Wnt/β-catenin transcriptional activity. Control and hDkk1-expressing cells were transfected with TOPflash 8 hours after induction of differentiation and luciferase activity was determined at 48 hours. Overexpression of hDkk1 led to a significant reduction in promoter activity, confirming that hDkk1 inhibits canonical Wnt signalling in differentiating 3T3-L1 preadipocytes.
Fig. 4.
hDkk1 inhibits Wnt signalling and promotes differentiation of 3T3-L1 preadipocytes. (A) Western blot analysis of whole-cell lysates from 3T3-L1 preadipocytes expressing empty vector (EV) or human Dkk1 (hDkk1) and pooled stromal-vascular cells from four mice (mPreads). Human recombinant Dkk1 protein (rDkk1) was used as positive control, and loading efficiency was assessed using p85 PI 3-kinase. (B) Western blot analysis of cytosolic β-catenin levels during differentiation of control and hDkk1-expressing 3T3-L1 preadipocytes. (C) Effect of hDkk1 on TOPflash reporter activity in 3T3-L1 cells expressing empty vector (EV) or hDkk1. Results are expressed as fold difference relative to EV control. All results are the mean ± s.e.m. of three independent experiments. (D) Oil-Red O staining of EV and hDkk1-expressing 3T3-L1 adipocytes. Cells were induced to differentiate for 8 days using either differentiation medium lacking IBMX (sub-differentiation) or the full differentiation cocktail. (E) Effect of hDkk1 on adipogenic gene expression. Control and hDkk1-expressing 3T3-L1 cells were differentiated sub-maximally using DI and total RNA extracted at the time points indicated. PPARγ1, PPARγ2 and aP2 mRNA levels were measured using real-time PCR. Results are expressed as fold difference relative to the basal (time 0) value for control. All results are the mean ± s.e.m. of three independent experiments. All comparisons were made against control using Student’s t test. PC, pre-confluent; C, confluent; 0, onset of differentiation (2 days post-confluence); 2, 4, 8 indicate 2, 4 and 8 days post-induction of differentiation respectively; RU, relative units; 3T3, 3T3-L1 cells; hDkk1, human Dkk1. *P<0.05, ***P<0.001.
Ectopic expression of hDkk1 in 3T3-L1 preadipocytes promotes adipogenesis
We next determined whether inhibition of Wnt signalling by constitutive expression of hDkk1 affected adipogenesis. Canonical Wnt signalling is rapidly suppressed upon induction of differentiation of 3T3-L1 cells in response to 1-methyl-3-isobutylxanthine (IBMX) (Bennett et al., 2002; Moldes et al., 2003). As shown in Fig. 4D,E, when cells were induced to differentiate sub-maximally by omitting IBMX from the differentiation cocktail, ectopic expression of hDkk1 led to increased lipid accumulation and expression of the adipogenic markers PPARγ1, PPARγ2 and aP2, compared with control. With maximal stimulation using 1-methyl-3-isobutylxanthine, dexamethasone and insulin (MDI), both cell lines differentiated to the same extent (Fig. 4D). Our results demonstrate that Dkk1 can facilitate differentiation of 3T3-L1 cells through inhibition of canonical Wnt signalling.
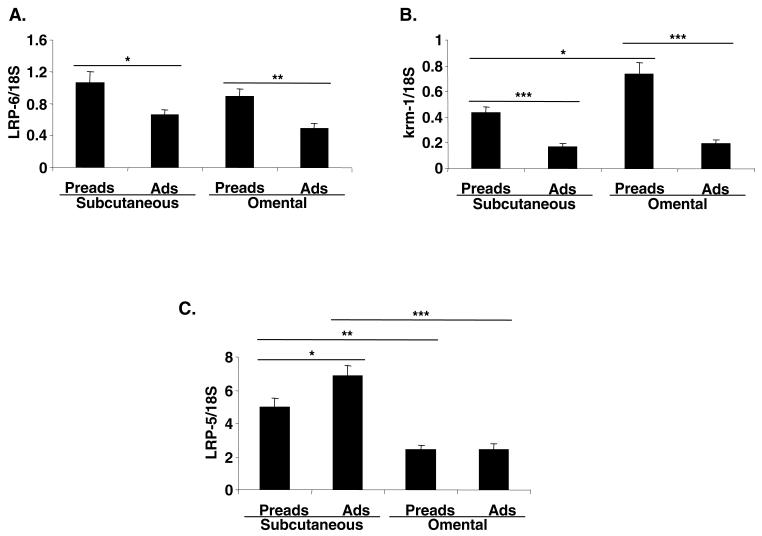
Dkk receptors are present in human adipose tissue and are transiently downregulated during human adipogenesis
Dkk1 uses a network of receptors to inhibit Wnt signalling. Hence, we investigated the presence of these receptors in human adipose tissue. Like DKK1, LRP6 and Krm1 are predominantly expressed in stromal-vascular cells from both subcutaneous and omental adipose depots (Fig. 5A,B). By contrast, LRP5 is present at similar levels in mature adipocytes and stromal-vascular cells (Fig. 5C).
Fig. 5.
Expression of Dkk1 receptors in human adipose tissue. (A) LRP6, (B) KRM1 and (C) LRP5 mRNA levels were measured using real-time RT PCR in subcutaneous and omental, stromal-vascular cells and mature adipocytes from 7-8 unrelated subjects. Preads, preadipocytes; Ads, adipocytes. *P<0.05, **P<0.01, ***P<0.001.
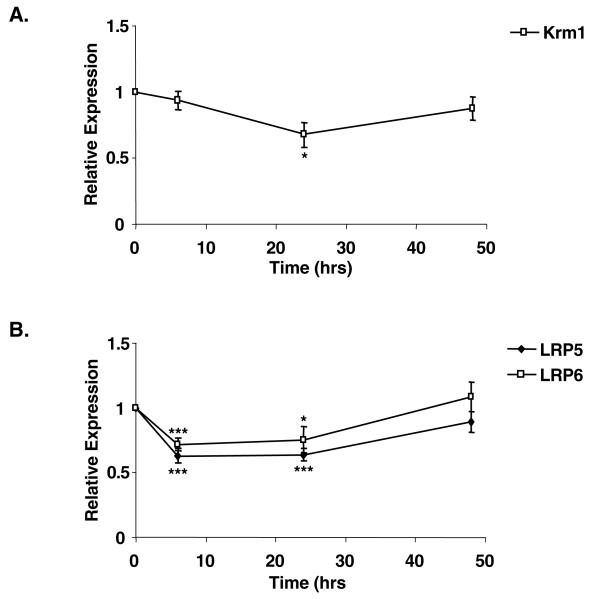
Given that human preadipocytes express the necessary repertoire of receptors required to respond to Dkk1, we assessed whether KRM1, LRP5 and LRP6 gene expression was also regulated during the early stages of human preadipocyte differentiation. Fig. 6A and Table 1 show that there was little change in expression of KRM1 mRNA throughout the 48-hour time course. However, the levels of LRP5 mRNA were significantly and transiently repressed six hours following treatment of human preadipocytes with adipogenic medium (Fig. 6B). A similar trend was observed for LRP6 (Fig. 6B and Table 1). Our results demonstrate that Dkk receptors are expressed in human adipose tissue. Furthermore, DKK1 and LPR5/6 are reciprocally regulated during early human adipogenesis.
Fig. 6.
Expression of Dkk1 receptors is regulated during early human adipogenesis. Subcutaneous preadipocytes from eight unrelated subjects were differentiated in vitro and total RNA extracted at the time points indicated (see Table 1). mRNA levels of (A) KRM1 and (B) LRP5 and LRP6 were determined by real-time RT PCR. Results are expressed as fold difference relative to baseline (time 0). *P<0.05, ***P<0.001.
Table 1. Expression of Dkkl receptors is regulated during early human adipogenesis.
| Gene (Rel. expr.) | 0 hours | 6 hours | 24 hours | 48 hours |
|---|---|---|---|---|
| KRM1 | 1 | 0.94±0.07 | 0.68±0.09* | 0.87±0.09 |
| LRP5 | 1 | 0.63±0.06*** | 0.64±0.05*** | 0.89±0.08 |
| LRP6 | 1 | 0.72±0.05*** | 0.75±0.1* | 1.09±0.11 |
Subcutaneous preadipocytes from eight unrelated subjects were differentiated in vitro and total RNA extracted at the time points indicated (see Fig. 6). mRNA levels of the genes KRM1, LRP5 and LRP6 were determined by real-time PCR. Results are expressed as fold difference relative to baseline (time=0). Rel. expr., relative expression.
P<0.05
P<0.001.
Discussion
We interrogated a microarray database for genes upregulated during the early stages of human adipogenesis and identified DKK1 as a transcript that was significantly induced within six hours of differentiation. Given the evidence from studies on murine preadipocytes suggesting that Wnt signalling functions as an early adipogenic switch (Bennett et al., 2002; Ross et al., 2000), we investigated the role of Dkk1, a secreted Wnt antagonist, in human adipogenesis. Here, we demonstrate that Dkks and their receptors are expressed in human preadipocytes and stromal-vascular cells, indicating that they might modulate human adipogenesis in vitro and in vivo. Our in vitro studies also show that Dkk1 expression is transiently upregulated in human preadipocytes following adipogenic stimulation and this correlates with inhibition of canonical Wnt signalling. In addition, we observe coordinate downregulation of Dkk receptors LRP5 and LRP6 that is likely to potentiate the ability of Dkk1 to inhibit Wnt signalling.
We also show that constitutive expression of Dkk1 can modulate the adipogenic program as demonstrated by its ability to promote differentiation of 3T3-L1 preadipocytes. This is likely to occur through inhibition of canonical Wnt signalling as it is accompanied by a reduction in cytosolic β-catenin levels, leading to reduced TCF promoter activity. Furthermore, the pro-adipogenic effect of Dkk1 is most pronounced when IBMX, an agent that suppresses Wnt signalling within hours of onset of differentiation of 3T3-L1 cells (Bennett et al., 2002), is omitted from the differentiation cocktail. Given that Dkk1 is necessary for re-entry of mesenchymal stem cells into the cell cycle (Gregory et al., 2003) and considering that Wnt signalling was shown to block differentiation of 3T3-L1 cells in part through inhibiting mitotic clonal expansion (Ross et al., 2002), it is possible that Dkk1 promotes adipogenesis by driving this process.
We considered adopting a loss-of-function approach to substantiate our findings. However, the surprising lack of Dkk1 expression in murine models made this approach unfeasible. The absence of Dkk1 from murine adipose tissue indicates that its proadipogenic role might be species specific. We considered the possibility that other members of the Dkk family could be involved in the regulation of murine adipogenesis. In this respect, both mouse stromal-vascular cells and 3T3-L1 preadipocytes express Dkk2 and Dkk3 but not Dkk4. However, both Dkk2 and Dkk3 are downregulated following differentiation of murine as well as human preadipocytes. Thus, it seems unlikely that these Dkk family members are direct substitutes for Dkk1 in murine adipogenesis.
LRP5, LRP6 and KRM1, the receptors of Dkk1, are highly expressed in stromal-vascular cells. Both, LRP5 and LRP6 are downregulated upon induction of differentiation. LRP6 has been shown to ‘titrate’ the ability of Dkk1 to inhibit Wnt signalling in vitro in HEK 293 T cells (Bafico et al., 2001; Mao et al., 2001) and in vivo (MacDonald et al., 2004). Hence, it is likely that downregulation of LRP5/6 at a time when Dkk1 expression is induced would enhance the ability of Dkk1 to inhibit Wnt signalling (Bafico et al., 2001; Mao et al., 2001). Furthermore, as the changes in DKK1, LRP5 and LRP6 levels occur simultaneously, it is likely that these events are coordinated to decrease Wnt signalling in human preadipocytes undergoing differentiation.
Adipocytes are derived from mesenchymal stem cells. These precursors can also differentiate into a variety of other cell types including osteoblasts (Nuttall and Gimble, 2004; Prockop et al., 2003). A key signal thought to regulate the balance between these lineages is canonical Wnt signalling. In addition to inhibiting adipogenesis, recent studies have demonstrated that Wnt/β-catenin signalling is required for osteoblast lineage differentiation. Specifically, it has been shown that loss-of-function mutations in LRP5 cause the autosomal recessive osteoporosis-pseudoglioma syndrome (OPPG) (Gong et al., 2001). Conversely, patients expressing gain-of-function mutations in LRP5 as a result of impaired affinity and antagonism by Dkk1 exhibit high bone density (Ai et al., 2005; Boyden et al., 2002; Little et al., 2002; Van Wesenbeeck et al., 2003; Zhang et al., 2004). Whether such patients or indeed mice engineered to carry mutations in LRP5/6 exhibit adipose phenotypes is as yet unreported. Furthermore, increased production of Dkk1 by myeloma cells inhibits the differentiation of osteoblast precursor cells in vitro and is associated with the presence of lytic bone lesions in patients with multiple myeloma (Tian et al., 2003). As ours is the first report to suggest that a secreted Wnt antagonist, Dkk1, might promote human adipocyte differentiation, we hypothesise that, through inhibition of Wnt signalling, Dkk1 might direct human mesenchymal stem cell fate towards adipogenesis while preventing commitment to the osteoblast lineage.
In summary, we have shown that Dkk1, a Wnt antagonist, is secreted by human preadipocytes and promotes adipogenesis in vitro by inhibiting Wnt signalling. Coordinated downregulation of Dkk1 receptors LPR5 and LRP6 might potentiate the pro-adipogenic action of Dkk1. In vivo, Dkk1 is appropriately expressed in the stromal-vascular fraction of human adipose tissue and might function as an endogenous trigger for recruiting new preadipocytes into the program of adipocyte differentiation. Our results also show that, although Wnt inhibition is a mechanism promoting adipocyte differentiation in humans and mice, there are inter-species differences in the molecular control of adipogenesis. Given the public health importance of human obesity, specific knowledge of the molecular pathways unique to human adipogenesis should aid better understanding of disease pathogenesis and guide the development of effective therapies.
Materials and Methods
Human adipocyte and preadipocyte isolation
Adipose tissue samples were obtained from subjects undergoing elective open-abdominal or thigh surgery. All subjects were fasted for 6 hours prior to the operation and all underwent general anaesthesia. Cambridge Research Ethics Committee approval was obtained and all patients gave their informed consent. Adipose tissue biopsies were placed in PBS (Sigma-Aldrich) and processed within 30 minutes. Samples were finely diced and digested in collagenase solution [Hank’s balanced salt solution containing 3 mg/ml type II collagenase (Sigma-Aldrich) and 1.5% bovine serum albumin] at 37°C for 1 hour. Subsequently, the digest was filtered through a stainless steel mesh and centrifuged at 400 g for 5 minutes to separate mature adipocytes from the stromal-vascular cells.
Human preadipocyte culture
The cell pellet containing the stromal-vascular fraction was treated with red cell lysis buffer (154 mmol/l NH4Cl, 10 mmol/l KHCO3, 0.1 mmol/l EDTA) for 5 minutes at room temperature and centrifuged (5 minutes, 400 g). The pellet was then re-suspended and cultured in DMEM:Hams F12 (1:1) medium supplemented with 10% foetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin at 37°C in a humidified incubator at 5% CO2. Cells were passaged 2-3 times before being grown to confluence. Differentiation was induced in 3-day post-confluent monolayers, by adding serum-free differentiation medium [DMEM:Hams F12 (1:1), 2 mM L-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 33 μM Biotin, 17 μM panthothenic acid, 10 μg/ml human apotransferrin, 0.2 nM tri-iodothyronine, 100 nM cortisol, 500 nM insulin] supplemented with 10−7 M BRL 49653 and 10−7 M LG 100268. For the first 3 days of culture, 0.25 mM 1-methyl-3-isobutylxanthine (IBMX) was also added to the medium. Differentiation medium was replaced after 3 days on the first occasion and thereafter every 2 days.
Culture, differentiation and infection of 3T3-L1 preadipocytes
3T3-L1 cells were cultured and differentiated into adipocytes as described previously (Nugent et al., 2001). Human DKK1 cDNA was a kind gift from S. E. Millar (University of Pennsylvania, Philadelphia, USA). Human Dkk1-overexpressing and control 3T3-L1 cell lines were generated using the pBabe-Puro retroviral vector system as described previously (Xu et al., 1999). Cells were kept in puromycin-containing medium except during differentiation experiments.
RT-PCR
Total RNA was prepared using the RNeasy Mini Kit (Qiagen) and 500 ng RNA was reverse transcribed using Moloney murine leukaemia virus reverse transcriptase and random hexamer primers (Promega). The cDNAs provided templates for human and mouse DKK PCRs using Taq DNA polymerase (Invitrogen) and the following primers: human DKK1 (forward 5′-CGGGAATTACTGCAAAAATGGA-3′, reverse 5′-GCACAGTCTGATGACCGGAGA-3′); mouse Dkk1 (forward 5′-GTACTGCTCCAGCCCCAGC-3′, reverse 5′-GAGGCAGACGGAGCCTTCTT-3′); mouse Dkk2 (forward 5′-CAGTCACTGAGAGCATCCTCA-3′, reverse 5′-CCTGATGGAGCACTGGTTTGC-3′); mouse Dkk3 (forward 5′-CGAGAGGTGGAGGAGCTGATG-3′, reverse 5′-GTCTCCGTGCTGGTCTCATTG-3′). The cycle conditions were as follows: denaturation, 30 seconds at 95°C; annealing, 30 seconds at 62°C (human and mouse Dkk1) or 50°C (mouse Dkk2 and Dkk3); elongation, 30 seconds at 72°C. PCR synthesis was quantified at 40 cycles. Data were subsequently normalised using 18S control. For 18S PCRs, cDNA samples were diluted 1:10 and amplified using a two-step PCR as follows: denaturation, 15 seconds at 95°C; annealing and elongation, 1 minute at 60°C. PCR synthesis was quantified at 18, 20, 22, 24, 26 and 28 cycles in a test reaction to ensure that the quantitative PCR amplification was in the linear range.
TaqMan® quantitative real-time reverse transcription PCR
RNA preparation, reverse transcription and conditions for TaqMan real-time reverse transcription (RT)-PCR were performed as described previously (Sewter et al., 2002). Primers and probes were designed using Primer Express software (Applied Biosystems) and sequences from the GenBank database, and are available upon request. In the case of LRP5 and LRP6, TaqMan reagents were specifically designed to amplify transcripts coding for full-length receptors only. Primers and probes for 18S and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) internal controls were purchased from Perkin Elmer.
Western blot analysis
Cells were washed with cold PBS and scraped into either lysis buffer or hypotonic lysis buffer to obtain cytosolic protein extracts for immunoblots of β-catenin as described (Culbert et al., 2001; Laudes et al., 2004). For immunoblots of Dkk1 in stromal-vascular cells and mature adipocytes, adipose tissue samples were processed as outlined above and the cell pellet containing the stromal-vascular fraction or the floating mature adipocytes were solubilised in lysis buffer. After centrifugation at 4°C at 10,000 g for 10 minutes, equal amounts of protein were dissolved in Laemmli buffer, heated to 95°C and separated by SDS-PAGE. Proteins were transferred to polyvinylidene fluoride membranes (Millipore) and immunoblotted with the following antibodies according to the manufacturer’s instructions: anti-human Dkk1 (AF 1096; R&D Systems), anti-mouse Dkk1 (AF 1765; R&D Systems), anti-β-catenin (Transduction Laboratories), anti-p85 phosphoinositide 3-kinase (PI 3-kinase; kindly provided by K. Siddle, University of Cambridge, Cambridge, UK) and anti-GAPDH (Abcam). All secondary antibodies were purchased from DAKO (Denmark) and used as 1:5000 dilutions in PBS + 1% milk or BSA.
Nuclear extracts
Nuclear extracts were prepared using the NucBuster™ protein extraction kit (MERCK Biosciences) as recommended by the manufacturer. Briefly, cells were washed with, and subsequently scraped into, 200 μl of cold PBS. After centrifugation at 4°C at 500 g for 5 minutes, cell pellets were resuspended in 150 μl of NucBuster extraction reagent 1 for 5 minutes on ice to release nuclei. The nuclei were harvested by centrifugation (16,000 g for 5 minutes at 4°C) and resuspended in 75 μl of NucBuster extraction reagent 2 for 5 minutes on ice. Nuclear extracts were separated from cell debris by centrifugation (16,000 g for 5 minutes at 4°C).
Gene promoter reporter assay
Human Dkk1-overexpressing and control 3T3-L1 cells were seeded in 24-well plates (5×104 cells per well) and grown to confluence. At 2 days post-confluence, cells were induced to differentiate, and 8 hours later transfected with 1 μg/well of the TOPflash promoter-reporter gene construct using FuGENE® (Roche Applied Science). To correct for transfection efficiency, 10 ng/well pRL-CMV (Promega) was co-transfected. 48 hours after onset of differentiation, cells were lysed in passive lysis buffer (Promega) and luciferase activity was determined by luminometry (EG Berthold) using the dual luciferase reporter assay (Promega).
Statistical analysis
All results are presented as mean ± s.e.m. Unless otherwise stated, statistical significance was assessed using the Mann-Whitney test (*P<0.05, **P<0.01, ***P<0.001).
Acknowledgments
We thank the Addenbrooke’s hospital surgeons for generously providing adipose tissue biopsies and T. Hagen and M. López for useful advice and discussions. C. Christodoulides and W. Cawthorn are sponsored by the Medical Research Council. M. Laudes and S. Schinner are sponsored by Deutsche Forschungsgemeinschaft. This research is funded by the Wellcome Trust Integrative Physiology Program (A.V.P., S.O’R.), MRC Career Establishment Award (A.V.-P.), and BBSRC David Phillips Fellowship (J.K.S.).
References
- Ai M, Holmen SL, Van Hul W, Williams BO, Warman ML. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol. Cell. Biol. 2005;25:4946–4955. doi: 10.1128/MCB.25.12.4946-4955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Culbert AA, Brown MJ, Frame S, Hagen T, Cross DA, Bax B, Reith AD. GSK-3 inhibition by adenoviral FRAT1 overexpression is neuroprotective and induces Tau dephosphorylation and beta-catenin stabilisation without elevation of glycogen synthase activity. FEBS Lett. 2001;507:288–294. doi: 10.1016/s0014-5793(01)02990-8. [DOI] [PubMed] [Google Scholar]
- Davidson G, Mao B, del Barco Barrantes I, Niehrs C. Kremen proteins interact with Dickkopf1 to regulate anteroposterior CNS patterning. Development. 2002;129:5587–5596. doi: 10.1242/dev.00154. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Singh H, Perry AS, Prockop DJ. The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J. Biol. Chem. 2003;278:28067–28078. doi: 10.1074/jbc.M300373200. [DOI] [PubMed] [Google Scholar]
- Hagen T, Vidal-Puig A. Characterisation of the phosphorylation of beta-catenin at the GSK-3 priming site Ser45. Biochem. Biophys. Res. Commun. 2002;294:324–328. doi: 10.1016/S0006-291X(02)00485-0. [DOI] [PubMed] [Google Scholar]
- Hagen T, Sethi JK, Foxwell N, Vidal-Puig A. Signalling activity of beta-catenin targeted to different subcellular compartments. Biochem. J. 2004;379:471–477. doi: 10.1042/BJ20031749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Jomary C. Secreted Frizzled-related proteins: searching for relationships and patterns. BioEssays. 2002;24:811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Laudes M, Christodoulides C, Sewter C, Rochford JJ, Considine RV, Sethi JK, Vidal-Puig A, O’Rahilly S. Role of the POZ zinc finger transcription factor FBI-1 in human and murine adipogenesis. J. Biol. Chem. 2004;279:11711–11718. doi: 10.1074/jbc.M310240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Longo KA, Wright WS, Kang S, Gerin I, Chiang SH, Lucas PC, Opp MR, MacDougald OA. Wnt10b inhibits development of white and brown adipose tissues. J. Biol. Chem. 2004;279:35503–35509. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Adamska M, Meisler MH. Hypomorphic expression of Dkk1 in the doubleridge mouse: dose dependence and compensatory interactions with Lrp6. Development. 2004;131:2543–2552. doi: 10.1242/dev.01126. [DOI] [PubMed] [Google Scholar]
- MacDougald OA, Mandrup S. Adipogenesis: forces that tip the scales. Trends Endocrinol. Metab. 2002;13:5–11. doi: 10.1016/s1043-2760(01)00517-3. [DOI] [PubMed] [Google Scholar]
- Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302:179–183. doi: 10.1016/s0378-1119(02)01106-x. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Miller JR. The Wnts. Genome Biol. 2002;3 doi: 10.1186/gb-2001-3-1-reviews3001. REVIEWS3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem. J. 2003;376:607–613. doi: 10.1042/BJ20030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent C, Prins JB, Whitehead JP, Savage D, Wentworth JM, Chatterjee VK, O’Rahilly S. Potentiation of glucose uptake in 3T3-L1 adipocytes by PPAR gamma agonists is maintained in cells expressing a PPAR gamma dominant-negative mutant: evidence for selectivity in the downstream responses to PPAR gamma activation. Mol. Endocrinol. 2001;15:1729–1738. doi: 10.1210/mend.15.10.0715. [DOI] [PubMed] [Google Scholar]
- Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr. Opin. Pharmacol. 2004;4:290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc. Natl. Acad. Sci. USA. 2003;100(Suppl. 1):11917–11923. doi: 10.1073/pnas.1834138100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Ross SE, Erickson RL, Gerin I, DeRose PM, Bajnok L, Longo KA, Misek DE, Kuick R, Hanash SM, Atkins KB, et al. Microarray analyses during adipogenesis: understanding the effects of Wnt signaling on adipogenesis and the roles of liver X receptor alpha in adipocyte metabolism. Mol. Cell. Biol. 2002;22:5989–5999. doi: 10.1128/MCB.22.16.5989-5999.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Sewter CP, Blows F, Vidal-Puig A, O’Rahilly S. Regional differences in the response of human pre-adipocytes to PPARgamma and RXRalpha agonists. Diabetes. 2002;51:718–723. doi: 10.2337/diabetes.51.3.718. [DOI] [PubMed] [Google Scholar]
- Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Benichou O, Scopelliti D, Key L, Renton T, Bartels C, Gong Y, et al. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am. J. Hum. Genet. 2003;72:763–771. doi: 10.1086/368277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Xu H, Sethi JK, Hotamisligil GS. Transmembrane tumor necrosis factor (TNF)-alpha inhibits adipocyte differentiation by selectively activating TNF receptor 1. J. Biol. Chem. 1999;274:26287–26295. doi: 10.1074/jbc.274.37.26287. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Li X, Zhang J, Mao J, Li Z, Zheng J, Li L, Harris S, Wu D. The LRP5 high-bone-mass G171V mutation disrupts LRP5 interaction with Mesd. Mol. Cell. Biol. 2004;24:4677–4684. doi: 10.1128/MCB.24.11.4677-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]