Abstract
An inability of adipose tissue to expand consequent to exhausted capacity to recruit new adipocytes might underlie the association between obesity and insulin resistance. Adipocytes arise from mesenchymal precursors whose commitment and differentiation along the adipocytic lineage is tightly regulated. These regulatory factors mediate cross-talk between adipose cells, ensuring that adipocyte growth and differentiation are coupled to energy storage demands. The WNT family of autocrine and paracrine growth factors regulates adult tissue maintenance and remodelling and, consequently, is well suited to mediate adipose cell communication. Indeed, several recent reports, summarized in this review, implicate WNT signalling in regulating adipogenesis. Manipulating the WNT pathway to alter adipose cellular makeup, therefore, constitutes an attractive drug-development target to combat obesity-associated metabolic complications.
Introduction
Obesity is an energy balance disorder in which nutrient intake chronically exceeds energy expenditure, resulting in excessive white adipose tissue (WAT) accumulation. Aside from being a social stigma, obesity is frequently associated with insulin resistance, in turn linked to the development of type 2 diabetes, hypertension, hyperlipidaemia and atherosclerosis – the so-called metabolic syndrome (MS) [1]. We have proposed that rather than excessive WAT accumulation, an inability of subcutaneous WAT (see Glossary) to expand in the face of continuous positive energy balance might underlie the link between obesity and its metabolic complications [2,3] (Box 1).
Box 1. Impaired subcutaneous WAT expandability might cause obesity-associated insulin resistance.
This hypothesis is supported by several clinical and experimental observations. For instance, some morbidly obese (and up to 20% of obese) patients are unexpectedly metabolically healthy and, reciprocally, 13–18% of normal to slightly overweight individuals exhibit features of the MS [55,56]. The higher level of insulin sensitivity in so-called metabolically healthy obese subjects is associated with earlier onset obesity, which is characterized by an increased total adipocyte number [7] and lower levels of visceral fat. By contrast, so-called metabolically obese, normal-weight individuals often gain (modest) weight in adult life and tend to have centripetal fat distribution. Even more convincingly, patients (and mice) with lipodystrophy, a disease characterized by partial or complete absence of WAT, recapitulate several features of the MS [57]. Likewise, decreasing WAT mass with abdominal liposuction does not improve obesity-associated metabolic abnormalities contrasting the metabolic benefits of dietary weight loss [58]. However, administration of thiazolidinediones (TZDs), synthetic ligands for the master adipogenic transcription factor PPARG, to diabetic patients (and rodents) improves insulin sensitivity despite a paradoxical increase in adiposity [59]. Furthermore, subcutaneous fat transplantation ‘paradoxically’ improves glucose tolerance and insulin sensitivity in mice (albeit in association with reductions in body weight and total fat mass) [60]. Studies in leptin-deficient (ob/ob) mice also showed that uninhibited expansion of subcutaneous WAT by modest elevation of circulating adiponectin levels leads to complete normalization of the metabolic profile of these animals, despite morbid obesity [61]. The authors propose that by acting as a peripheral ‘starvation’ signal, adiponectin facilitates preferential storage of triglycerides in WAT with reduced lipid levels in liver and muscle conveying improved systemic insulin sensitivity. By contrast, although protected from obesity consequent to impaired adipogenesis, PPARG2-deficient ob/ob mice develop severe insulin resistance, dyslipidaemia and β-cell failure [62].
There is evidence that adipocytes have a central role in the pathogenesis of insulin resistance. First, they act as a ‘safe’ depot for free fatty acid (FFA) storage, being able to accumulate large amounts of lipid in a manner that is nontoxic to the cell or whole organism. When the capacity of WAT to store FFAs is exceeded, as occurs in some forms of obesity (when WAT expandability is exhausted) or in lipodystrophy (when WAT is partially or completely absent), extra-adipose lipid accumulation, lipotoxicity and insulin resistance ensues [4]. Second, adipocytes secrete multiple bioactive peptides termed ‘adipokines’ (e.g. leptin and adiponectin) that directly regulate whole-body insulin sensitivity [5]. Deregulated adipokine secretion from the expanded WAT of obese individuals contributes to the development of systemic insulin resistance and metabolic disease. Thus, understanding the molecular and cellular events regulating adipogenesis is crucial in designing rational therapies to prevent and treat the MS.
Adipocytes are present in distinct anatomical locations throughout the body, such as the subcutis, body cavity (in association with internal organs, intestinal mesentery and retroperitoneum) and bone marrow. Two main types of adipocytes exist: brown and white. White adipocytes primarily serve to store lipids, whereas brown adipocytes function predominantly to dissipate energy in the form of heat. Consequently, elucidating the molecular mechanisms controlling brown versus white adipocyte specificity holds enormous promise in treating obesity and the MS because these pathways could be pharmacologically exploited to induce a metabolic shift in the role of white adipocytes from lipid storage towards FFA oxidation. Even among white adipocytes, however, cells from different depots have different metabolic properties, which might account for their differential contribution to the pathogenesis of the MS. Visceral fat, for example, is more strongly linked to the development of insulin resistance than subcutaneous fat is [6]. Unravelling the molecular mechanisms underlying this heterogeneity offers additional avenues for the treatment of obesity-related metabolic complications.
WAT expansion during childhood obesity results from combined adipocyte hypertrophy and hyperplasia. By contrast, adults seem to have a fixed number of adipocytes, and changes in fat mass are primarily secondary to changes in fat-cell volume. Nonetheless, adult adipocytes exhibit a remarkably high and constant turnover. Furthermore, adipocyte number set during childhood might be a major determinant of adult WAT mass and expandability [7]. Adipocytes are thought to arise from multi-potent mesodermal stem cells residing in the adipose tissue stroma [8]. These mesenchymal stem cells (MSCs) become preadipocytes when they lose their ability to differentiate into other mesenchymal lineages and become ‘committed’ to the adipocytic lineage. This initial phase of adipocyte differentiation is known as determination and is poorly characterized.
The second phase of adipogenesis is terminal differentiation, whereby preadipocytes take on the characteristics of mature adipocytes, acquiring lipid droplets and the ability to respond to hormones such as insulin. Terminal differentiation consists of a cascade of transcriptional events [8]. The first wave involves the transient induction of CCAAT/enhancer-binding protein-β (CEBPB) and -δ (CEBPD), which, in turn, directly induce expression of CEBP-α (CEBPA) and peroxisome proliferator-activated receptor-γ (PPARG), the central transcriptional regulators of adipogenesis. CEBPA and PPARG subsequently feed back to induce their own expression, in addition to activating many downstream target genes whose expression defines the adipocyte. Although CEBPs and PPARG are integral to the transcriptional network controlling adipogenesis, other transcription factors also regulate this process [8,9].
WNT signalling: a network that might serve to tightly regulate adipose expansion to match energy storage demands
The adipogenic transcription factors outlined previously are positioned downstream of less-well-characterized signalling pathways relaying information about the suitability of intracellular and extracellular conditions for differentiation. Whereas some of these signals are hormonal in nature, local factors are also likely to be important. Such factors are necessary to mediate cross-talk between stromo-vascular cells and mature adipocytes to ensure that growth of existing adipocytes and differentiation of new adipocytes are tightly coupled to energy storage demands. Disruption of these local signalling networks could, in the context of positive energy balance, lead to compromised adipogenesis and WAT expandability and/or uncoupled hyperplastic and hypertrophic adipocyte responses resulting in metabolic dysfunction (Figure 1).
Figure 1.
Local factors regulate adipogenesis. (a) Under conditions of nutritional deprivation, anti-adipogenic signals emanating from both preadipocytes and mature adipocytes restrain preadipocyte differentiation. (b) In the face of continuous positive energy balance (overnutrition), hypertrophic adipocytes secrete factors that stimulate preadipocyte differentiation. These include both positive adipogenic factors and factors that block negative adipogenic signals. (c) Disruption of local signalling networks during chronic overnutrition leads to impaired adipogenesis and adipose expandability. This, in turn, results in adipocyte hypertrophy and dysfunction, and secondary adipose inflammation, in addition to ectopic lipid accumulation at extra-adipose sites (e.g. liver and muscle), which together, contribute to the pathogenesis of insulin resistance.
Wingless-type MMTV integration site family members (WNTs) are a family of secreted glycoproteins acting in autocrine and paracrine fashions to regulate adult tissue homeostasis and remodelling [10,11]. WNTs exert their effects by signalling through multiple so-called ‘canonical’ and ‘non-canonical’ pathways to control cell proliferation, survival, fate and behaviour. As such, the WNT signalling network seems well suited to mediate adipose cell cross-communication. Indeed, several reports have implicated WNT signalling in regulating MSC maintenance, proliferation, fate determination and preadipocyte differentiation. In the following sections, we provide a brief outline of the WNT pathways that are pertinent to adipocyte biology before subsequently addressing their relevance to adipogenesis.
Overview of WNT signalling
The canonical WNT signalling cascade converges on the transcriptional regulator β-catenin [10]. In the absence of WNTs, cytoplasmic β-catenin is recruited to a degradation complex nucleated by Axin and adenomatous polyposis coli (APC), which facilitates its sequential phosphorylation by casein kinase I and glycogen synthase kinase 3-β (GSK3β). This primes β-catenin for ubiquitination and proteasomal degradation. WNT binding to frizzled (FZD) receptors and low-density lipoprotein-receptor-related protein-5 or -6 (LRP5/6) co-receptors leads to inactivation of the degradation complex. This results in hypophosphorylation of β-catenin and its translocation in the nucleus where it binds to the lymphoid-enhancer-binding factor/T-cell-specific transcription factor (LEF/TCF) family of transcription factors to activate WNT target genes.
The signalling events of β-catenin-independent, noncanonical WNT signalling are poorly defined. In the WNT-cGMP/Ca2+ pathway, specific WNT and FZD homologues acting through heterotrimeric GTP-binding proteins trigger intracellular calcium release, which, in turn, activates the phosphatase calcineurin and the calcium-sensitive kinases calcium/calmodulin-dependent kinase II (CAMKII) and protein kinase C [12]. Recent data also indicate that in addition to FZD, the tyrosine kinases RYK receptor-like tyrosine kinase and Rar-related orphan receptor (ROR) are involved in WNT signal reception [13]. The downstream signalling pathways activated by such ligand–receptor interactions have yet to be delineated.
WNT signalling is modulated by several extracellular antagonists [14,15]. These include secreted frizzled-related proteins (sFRPs) and WNT inhibitory factor, which directly bind and sequester WNTs from their receptors. By contrast, dickkopf (Dkk) family members specifically inhibit canonical WNT signalling by binding as high-affinity antagonists to LRP co-receptors.
WNT/β-catenin signalling inhibits adipogenesis
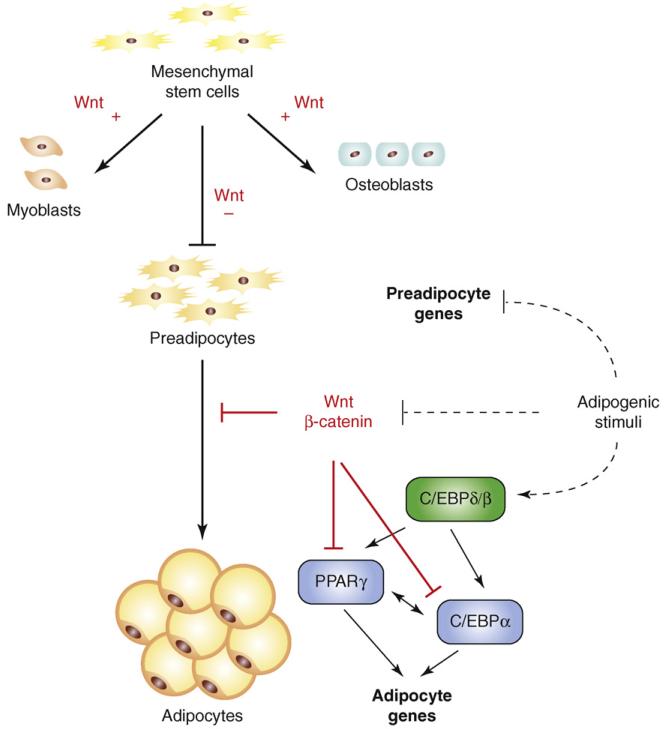
Studies from the MacDougald laboratory first implicated WNT signalling in the regulation of adipocyte differentiation. Specifically, canonical pathway activation in 3T3-L1 preadipocytes through overexpression of Wnt1 or a GSK3β phosphorylation-defective β-catenin mutant was shown to inhibit adipogenesis [16]. Likewise, treatment with pharmacological GSK3β inhibitors blocked adipocyte differentiation [17]. WNT signalling repressed adipogenesis by blocking induction of PPARG and CEBPA (Figure 2). Conversely, disruption of extracellular or intracellular WNT signalling with recombinant sFRP1 or sFRP2 or through constitutive expression of Axin or dominant-negative-transcription-factor-7-like 2 (dn TCF7L2 [formerly known as T-cell transcription factor 4, or TCF4]), respectively, led to spontaneous adipocyte differentiation[16,17]. These experiments established WNT signalling as a molecular switch that, when activated, represses adipogenesis.
Figure 2.
Canonical WNT signalling regulates mesenchymal stem cell fate. Activation of WNT/β-catenin signalling promotes differentiation of mesenchymal precursor cells into myocytes and osteocytes while suppressing commitment to the adipocytic lineage and terminal differentiation. WNT signalling restrains adipocyte differentiation by inhibiting the expression of PPARG and CEBPA, the central regulators of adipogenesis. These transcription factors are induced directly by CEBPB and CEBPD in response to adipogenic stimuli, which also serve to switch off the canonical WNT pathway. PPARG and CEBPA subsequently feed back to induce their own expression, in addition to activating many downstream target genes whose expression defines the adipocyte.
The finding that disruption of WNT/β-catenin signalling leads to spontaneous adipogenesis indicates that endogenous WNTs restrain preadipocyte differentiation. One likely candidate for such an anti-adipogenic WNT signal is Wnt10b. Wnt10b expression is highest in preadipocytes and declines rapidly after induction of differentiation[16,17]. Overexpression of Wnt10b in 3T3-L1 preadipocytes stabilizes β-catenin and blocks adipogenesis. More compellingly, Wnt10b anti-sera added to 3T3-L1 media promotes adipocyte differentiation [18]. In vivo, transgenic mice expressing Wnt10b under the control of the adipose-specific fatty-acid-binding protein-4 (FABP4) promoter (FABP4-Wnt10b) show a 50% decline in total body fat and resist high-fat-diet-induced WAT accumulation [19]. This adipose-specific expression of Wnt10b also protects against genetic obesity owing to leptin deficiency and ectopic agouti expression (Ay) [20]. Paradoxically, far from developing lipodystrophic (insulin-resistant) diabetes, FABP4-Wnt10b mice on either wild-type or Ay background are more glucose-tolerant and insulin-sensitive than control animals. This might be secondary to reduced adipose expression and circulating levels of resistin and/or decreased WAT inflammation [20]. Collectively, these studies show that Wnt10b constrains mouse WAT expansion by inhibiting preadipocyte differentiation (Figure 3). Wnt10b might also serve to modify the adipokine-secretion profile of WAT and/or have an immunomodulatory role in this tissue, thereby influencing systemic insulin sensitivity.
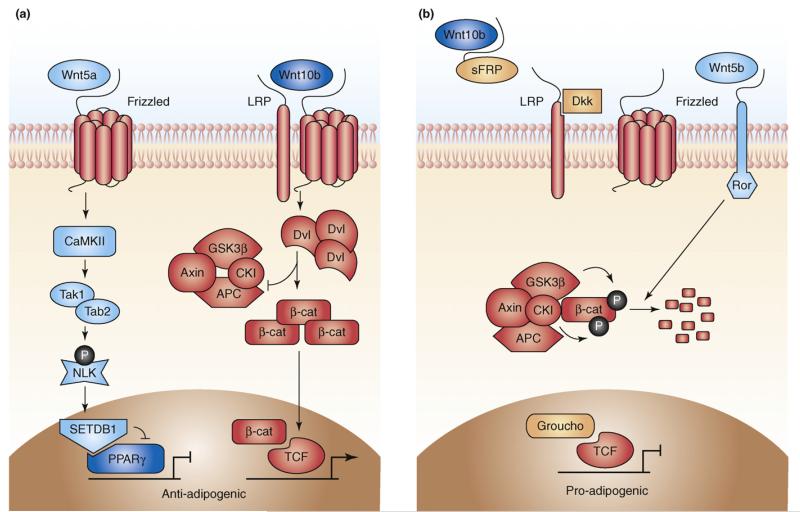
Figure 3.
Adipogenesis and WNT signalling. (a) In mesenchymal precursor cells, Wnt10b (and Wnt10a) binding to frizzled (FZD1) receptors and LRP5/6 co-receptors leads to dishevelled (DVL) phosphorylation and Axin degradation. This, in turn, results in hypophosphorylation of β-catenin, which accumulates in the cytoplasm and translocates to the nucleus, where it binds to TCF/LEF transcription factors to activate WNT target genes and inhibit adipogenesis by suppressing CEBPA and PPARG. Non-canonical WNT signalling, perhaps through Wnt5a acting via FZD2, might also inhibit adipocyte differentiation. Wnt5a stimulates a mitogen-activated protein kinase cascade mediated through CAMKII-TAK1-TAB2 (TAK1, TGFβ-activated protein kinase-1; TAB2, TAK1-binding protein-2) to activate Nemo-like kinase (NLK). NLK, in turn, phosphorylates a histone methyltransferase, SET domain bifurcated-1 (SETB1), leading to the assembly of a co-repressor complex, which inactivates ligand-bound PPARG through histone methylation. (b) Adipocyte differentiation is associated with secretion of sFRPs and Dkk1, which inhibit Wnt10b (and Wnt10a) signalling. sFRPs directly bind and sequester Wnt10b, whereas Dkk1 inhibits WNT signalling by binding as a high-affinity antagonist to LRP co-receptors. In the absence of WNT ligands, cytoplasmic β-catenin is recruited to a destruction complex that contains Axin and APC, which facilitates the GSK3β-dependent-phosphorylation and proteasomal degradation of β-catenin. In contrast to Wnt10b, Wnt5b promotes adipogenesis by inhibiting WNT/β-catenin signalling, perhaps by acting through ROR receptors.
WNT signalling regulates mesenchymal stem cell fate and susceptibility to obesity
FABP4-Wnt10b mice also express the Wnt10b transgene in bone marrow and, in addition to reduced adiposity, display an increase in bone mass [21]. In vitro, Wnt10b overexpression in ST2 bipotential mesenchymal cells and mouse embryonic fibroblasts (MEFs) blocks adipogenesis while stimulating osteoblastogenesis [21,22]. A role for WNT/β-catenin signalling in regulating MSC fate is also illustrated by genetic deletion of the WNT antagonist Sfrp1, which leads to a 20% reduction in body fat in aged male mice concomitant with increased bone mass [23]. By contrast, high-fat diet induces epigenetic activation of Sfrp5 expression in WAT that increases susceptibility to obesity (see subsequent paragraph) [24].
Canonical WNT signalling also controls the balance between myogenesis and adipogenesis. Myoblasts isolated from Wnt10b-null mice display increased adipogenic gene expression; furthermore, after muscle injury, high-fat-diet-fed Wnt10b-null mice accumulate excessive lipids in regenerating myofibres [25]. Similarly, conditional deletion of β-catenin in the developing mouse uterus mesenchyme results in progressive replacement of the myometrium by adipose tissue in adulthood [26]. Inhibition of WNT signalling also contributes to a switch from myogenesis to adipogenesis in the myocardium with pathological consequences [27] (Box 2). Collectively, the studies described previously provide compelling evidence that WNT/β-catenin signalling restrains mesenchymal precursors from differentiating into adipocytes while promoting osteoblastogenesis and myogenesis (Figure 2).
Box 2. WNT signalling regulation of myogenesis versus adipogenesis in the myocardium.
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an uncommon genetic disease, the pathologic hallmark of which is fibroadipocytic replacement of cardiac myocytes in conjunction with myocyte apoptosis. In a subset of families, ARVC is caused by mutations in desmosomal proteins including desmoplakin (DSP) and plakoglobin (PKGB). Of interest, siRNA-mediated suppression of Dsp expression in a mouse atrial myocyte cell line led to nuclear translocation of PKGB and inhibition of WNT/β-catenin signalling [27]. The ensuing phenotype was increased expression of adipogenic (and fibrogenic) genes, leading to lipid droplet accumulation in response to adipogenic media exposure. In vivo, cardiac-restricted heterozygous deletion of Dsp led to progressive fibroadipocytic replacement and increased apoptosis of cardiac myocytes in adulthood resulting in cardiac dysfunction and arrhythmias, recapitulating the phenotype of human ARVC. In accordance with the in vitro studies described previously, cardiomyocytes from DSP-deficient mice exhibited increased nuclear localization of PKGB along with enhanced expression of adipogenic and decreased levels of WNT target genes [27]. The investigators postulated that the cell source of excess adipocytes in the heart is likely to be composed of cardiac myoblasts or resident MSCs, which, in the absence of WNT signalling, could preferentially differentiate into adipocytes.
Epigenetic reprogramming of WNT signalling in WAT might also alter susceptibility to obesity by modulating adipogenesis. Genetically identical male B6 mice develop highly variable obesity after being fed a high-fat diet for four weeks with a fourfold range in WAT accumulation [24]. The adiposity phenotypes of these mice are evident from an early age and are not influenced by differences in litter size and wean weight. Furthermore, these differences are stable, persisting even after the obesogenic protocol is interrupted by caloric restriction, consistent with an epigenetic mechanism causing a permanent change in energy metabolism. Chronologically, differences in adiposity between high and low weight gainers precede those of feeding efficacy and food intake, indicating a causal link between primary alterations in WAT development and susceptibility to obesity. This was corroborated by gene-expression analyses showing differences in the expression of a large number of adipose genes between the two mouse groups. By contrast, hypothalamic gene expression was unchanged. In particular, highly upregulated Sfrp5 expression observed in multiple fat depots was postulated to be causally linked to WAT expansion. In support of this, differences in Sfrp5 gene expression were evident in epididymal fat biopsies in the pre-obese state and correlated with adiposity after high-fat diet. Furthermore, variation in epididymal fat Sfrp5 mRNA after four weeks of high-fat diet was associated with as much as 45% variance in adiposity [24]. In summary, sFRP5-mediated epigenetic silencing of WNT signalling in WAT leading to enhanced adipogenesis seems to increase susceptibility to diet-induced obesity in mice.
WNT signalling mediates anti-adipogenic actions of obesity-induced inflammatory cytokines
Obesity is associated with WAT macrophage infiltration, and increased local concentrations of interleukin 6 (IL6) and tumour necrosis factor-α (TNF) potentially contributing to the pathogenesis of insulin resistance by inhibiting adipogenesis [28,29]. Interestingly, the anti-adipogenic actions of IL6 and TNF seem to be mediated, at least partly, through WNT/β-catenin signalling [30,31]. Indeed, both knock-down of endogenous β-catenin and overexpression of dn Tcf7l2 reversed the anti-adipogenic effect of TNF, indicating that WNT signalling activation is a prerequisite for inhibiting adipogenesis by this cytokine [31]. Thus, cytokines might impair WAT expansion during obesity by hijacking the canonical WNT pathway, thereby exacerbating insulin resistance and metabolic deregulation.
Cross-regulation between PPARG and β-catenin signalling during adipogenesis
An emerging paradigm supported by work from several groups is the ability of PPARG and WNT/β-catenin signalling in WAT to cross-talk. Using a high-throughput assay to screen chemical libraries for pro-adipogenic compounds, Waki et al. [32] identified harmine as an adipocyte-specific inducer of Pparg expression. Administration of harmine to diabetic mice mimicked the effects of PPARG ligands on adipocyte gene expression and insulin sensitivity. The adipogenic activity of harmine results, at least partly, from its ability to block WNT/β-catenin signalling in adipocytes [32]. Work from the Farmer group [33,34] has also shown that a GSK3β-phosphorylation-defective β-catenin mutant inhibits expression of select adipogenic PPARG-target genes (Figure 2).
Reciprocally, PPARG activation has also been shown to suppress β-catenin protein levels in various cell types, including MEFs and preadipocytes [33-35]. PPARG-associated β-catenin degradation probably involves the proteasome system and requires GSK3β activity [33], although PPARG might also target β-catenin for proteasomal degradation through GSK3β-independent mechanisms [34]. PPARG interacts directly with the TCF/LEF-binding domain of β-catenin through a region within its ligand-binding domain [34]. This putative PPARG catenin-binding domain is involved in targeting β-catenin to the proteasome. PPARG might facilitate β-catenin degradation through direct association and/or indirectly through transcriptional regulation. In summary, PPARG and β-catenin signalling in adipose cells seems to be mutually antagonistic. Pharmacological inhibition of the WNT/β-catenin pathway in WAT, thus, might represent a complementary approach to PPARG ligands in the treatment of insulin resistance.
Nuclear WNT/β-catenin signalling and adipogenesis
The end result of canonical WNT signalling activation is nuclear translocation of β-catenin. In 3T3-L1 preadipocytes, nuclear β-catenin might act in concert with TCF7L2 to regulate WNT target-gene expression [16]. Interestingly, an alternatively spliced TCF7L2 isoform, Tcf7l2n, is also expressed in 3T3-L1 preadipocytes. TCF7L2N lacks the DNA-binding domain but retains the β-catenin-interacting domain. As predicted, TCF7L2N inhibited co-activation by β-catenin of a TCF/LEF-responsive promoter [36]. Remarkably, however, TCF7L2N potentiated co-activation by β-catenin of several non-TCF-responsive promoters. For example, β-catenin and TCF7L2N synergized with CEBPA to activate the leptin promoter. TCF7L2N also partially rescued the block in adipocyte differentiation caused by ectopic β-catenin [36]. Thus, by redirecting β-catenin from TCF/LEF to other transcription factors such as CEBPA, TCF7L2N might serve to suppress canonical WNT signalling during adipogenesis.
Other β-catenin-interacting proteins also contribute to the fine-tuning of nuclear WNT signalling during adipogenesis. For instance, Chibby (CBY), which inhibits WNT target-gene activation by competing with TCF/LEF for β-catenin binding [37], was shown recently to be required for adipocyte differentiation [38]. Cby is expressed in mouse WAT, and CBY protein levels increase during adipogenesis. Overexpression of Cby in 3T3-L1 cells led to inhibition of canonical WNT signalling and spontaneous adipogenesis. Reciprocally, RNAi-mediated depletion of Cby enhanced β-catenin transcriptional activity and potently suppressed adipocyte differentiation. Likewise, Cby-null MEFs displayed impaired adipogenic potential. Collectively, these experiments demonstrate that CBY promotes adipogenesis by repressing WNT/β-catenin-dependent transcription. Furthermore, these studies indicate that during adipocyte differentiation, multiple nuclear interacting partners sequester β-catenin from TCF/LEF, while redirecting it to other transcription factors.
Non-canonical WNT signalling and adipogenesis
In addition to signalling through β-catenin, WNT family members can also activate non-canonical pathways. Genetic evidence indicates that non-canonical signalling through Wnt5a can antagonize the canonical pathway[39,40] (Figure 3a). Wnt5a might act via the ROR2 receptor [41]. Accordingly, adenoviral overexpression of the related Wnt5b impaired β-catenin nuclear translocation and enhanced 3T3-L1 cell differentiation [42,43]. In contrast to Wnt10b, Wnt5b expression transiently increases during adipogenesis (Figure 3b).
The studies described previously indicate that non-canonical WNT signalling promotes adipogenesis by antagonising the WNT/β-catenin pathway. However, a report demonstrating that Wnt5a-mediated non-canonical signalling inhibits adipogenesis in ST2 cells by attenuating the transcriptional activity of PPARG has provided additional complexity [44] (Figure 3a). Likewise, the MacDougald laboratory showed that non-canonical signalling inhibits adipogenesis [45]. Fzd1 and Fzd2 are highly expressed in preadipocytes and associated with canonical and β-catenin-independent WNT signalling, respectively. This study used constitutively active chimeras of Xenopus Wnt8 and mouse FZD1 or FZD2 to investigate the effects of these receptors on mesenchymal cell fate. Of note, both chimeras partially blocked adipogenesis by preventing Pparg and Cebpa expression. Interestingly, the anti-adipogenic action of Wnt-FZD2 was partially rescued by calcineurin inhibitors. It is likely, therefore, that distinct non-canonical pathways exert opposing effects on adipogenesis.
WNT signalling and brown adipogenesis
Activation of WNT/β-catenin signalling through Wnt10b overexpression also inhibits brown adipogenesis [46]. WNT signalling blocks adipogenesis through repression of CEBPA and PPARG, and blocks the thermogenic programme through suppression of PPARG co-activator 1-α (PGC1A). Wnt10a and/or Wnt10b are likely to be endogenous inhibitory WNT(s) because both are expressed in brown adipose tissue (BAT) and decline during brown adipogenesis. Furthermore, in vivo expression of Wnt10b from the FABP4 promoter blocks BAT development [19].
In differentiated brown adipocytes, WNT signalling activation suppresses uncoupling protein 1 (UCP1) expression through PGC1A repression without influencing common adipocyte markers. Similarly, in vivo expression of Wnt10b from the UCP1 promoter results in BAT with histological and molecular characteristics of WAT, indicating that WNT signalling converts brown adipocytes into white. This is also supported by the reciprocal expression of Wnt10b with Ucp1 and Pgc1a in BAT from cold-challenged and ob/ob mice. The latter have BAT with characteristics of WAT and increased Wnt10b expression [46].
Work from the Kahn laboratory also indicates a role for Wnt10a in regulating brown adipogenesis [47,48]. Specifically, levels of Wnt10a expression in Irs1–4 knockout (KO) brown preadipocytes correlated inversely with their ability to differentiate. Expression of Wnt10a in Irs1 KO preadipocytes was blocked by ectopic Irs1 and partially reduced by ectopic Irs4, which correlated with restoration of differentiation [48]. Finally, Wnt10a gene expression progressively increased in BAT of Irs1 KO and Irs1/3 double-KO mice, which exhibit increasing defects in the development of adipose tissues [47]. Taken together, these data indicate that canonical WNT signalling through Wnt10b and/or Wnt10a blocks differentiation of brown preadipocytes, while stimulating conversion of mature brown adipocytes into white.
WNT signalling and human adipogenesis
The studies outlined previously demonstrate the involvement of WNT pathways in the regulation of murine adipocyte differentiation in vitro and in vivo. More recently, we and others have begun addressing the relevance of WNT signalling in human adipogenesis. Dkk1 specifically inhibits canonical WNT signalling by binding as a high-affinity antagonist to LRP5/6 co-receptors. Expression of Dkk1 gene and protein is transiently induced during differentiation of human preadipocytes, and constitutive expression of DKK1 in 3T3-L1 cells blocks WNT/β-catenin signalling and promotes adipogenesis [49]. Furthermore, in vitro studies showed that activating LRP5 mutations (owing to reduced affinity to and inhibition by Dkk1 [50]) inhibit adipogenic differentiation of human MSCs, whereas inactivating LRP5 mutations exert the opposite effect [51]. Collectively, these experiments indicate that Dkk1 promotes human adipogenesis by antagonising LRP5/6 signalling (Figure 3b).
sFRP1 has also been implicated in the regulation of human adipogenesis. Graves’ thyroid ophthalmopathy (GO) results partly from intra-orbital WAT expansion. Using gene expression profiling, Kumar et al. [52] identified SFRP1 as one of the most highly upregulated transcripts in orbital WAT from patients with severe GO. In vitro recombinant sFRP1 enhanced differentiation of GO orbital preadipocytes, indicating a pathogenetic role in GO.
WNT signalling, with other developmental pathways, has also been implicated in the regulation of body fat distribution in humans (and mice) [53]. Finally our group has sought to determine whether WNTs contribute to human obesity by screening two independent populations of obese subjects for mutations in the WNT10B gene [54]. One proband with early-onset obesity was found to be heterozygous for a loss-of-function WNT10B mutation, which was not found in 600 control alleles. Although pedigree analysis did not provide definitive proof of a causal link of this variant with obesity, all relatives of the proband who carried this allele were either overweight or obese.
Concluding remarks
Abundant evidence, mostly derived from mouse studies, links WNT signalling to the regulation of adipogenesis (Figure 3). It must be mentioned that data derived from in vitro work utilizing overexpression experiments should be interpreted with caution. However, there is now compelling evidence that the canonical WNT pathway regulates MSC fate determination in vivo in humans and mice. Additionally, WNT signalling might have a role in the regulation of body fat distribution and, to a degree, susceptibility to obesity. Modulation of this pathway, therefore, might provide novel therapies for obesity and, by enhancing WAT functionality, the MS. Given the increasing prevalence and enormous worldwide impact on health and health care resources of disorders of adipose mass, further insights into the mechanisms by which WNTs regulate adipogenesis and MSC fate determination, specifically in humans, are a priority.
Acknowledgements
This research is funded by the Medical Research Council (MRC), Biotechnology and Biological Science Research Council (BBSRC), Diabetes UK and the European Union Sixth Framework Programme on Hepatic and Adipose tissue functions in the Metabolic Syndrome (EU-FP6 HEPADIP) (A.V.-P. and J.K.S.). Personal support is also acknowledged as follows: MRC Clinical Research Training Fellowship (C.C.), ALFEDIAM (Association de Langue Française pour l’Etude du Diabète et des Maladies Métaboliques) and Marie Curie Intra-European Fellowship (EIF) Postdoctoral fellowship (C.L.), MRC Career Establishment Award (A.V.-P.) and BBSRC David Phillips.
Glossary
- Agouti
peptide, the expression of which is normally restricted to hair follicles, where it regulates coat colour by antagonising melanocortin-1-receptor signalling. Ectopic agouti expression consequent to genomic rearrangement leads to autosomal dominant severe obesity in humans and mice as a result of hypothalamic melanocortin-4-receptor antagonism, which potently stimulates appetite and reduces energy expenditure
- FFA oxidation
the oxidative degradation of fatty acids in mitochondria to produce energy in the form of ATP
- Harmine
naturally occurring β-carboline alkaloid found in numerous plants, common plant-derived foods and human tissues
- Positive energy balance
state in which calorie consumption exceeds energy expenditure
- Stromo-vascular cells
the non-adipocyte fraction of WAT, consisting of a heterogeneous cell population that includes MSCs, preadipocytes and vascular and immune cells
- Subcutaneous WAT
adipose tissue found in the subcutaneous layers between the muscle and dermis
- Thermogenic programme
the production of heat energy in brown adipose tissue facilitated by UCP-1, which enables energy in mitochondria to be dissipated as heat without generating ATP
- Visceral fat
WAT associated with the internal organs, intestinal mesentery and retroperitoneum within the body cavity
- WAT expandability
the capacity of WAT to expand its size and, consequently, lipid storage capacity through adipocyte hypertrophy and hyperplasia in response to chronic overnutrition
References
- 1.Kahn BB, Flier JS. Obesity and insulin resistance. J. Clin. Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J. Lipid Res. 2007;48:1253–1262. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray SL, Vidal-Puig AJ. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr. Rev. 2007;65:S7–S12. doi: 10.1111/j.1753-4887.2007.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 4.Savage DB, et al. Mechanisms of insulin resistance in humans and possible links with inflammation. Hypertension. 2005;45:828–833. doi: 10.1161/01.HYP.0000163475.04421.e4. [DOI] [PubMed] [Google Scholar]
- 5.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montague CT, O’Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–888. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- 7.Spalding KL, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 8.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 9.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 11.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 12.Semenov MV, et al. SnapShot: noncanonical Wnt signaling pathways. Cell. 2007;131:1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25:7461–7468. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- 14.Jones SE, Jomary C. Secreted frizzled-related proteins: searching for relationships and patterns. Bioessays. 2002;24:811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- 15.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 16.Ross SE, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 17.Bennett CN, et al. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan V, et al. Regulation of bone mass by Wnt signaling. J. Clin. Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longo KA, et al. Wnt10b inhibits development of white and brown adipose tissues. J. Biol. Chem. 2004;279:35503–35509. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- 20.Wright WS, et al. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes. 2007;56:295–303. doi: 10.2337/db06-1339. [DOI] [PubMed] [Google Scholar]
- 21.Bennett CN, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang S, et al. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein α and peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 23.Bodine PV, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol. Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 24.Koza RA, et al. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006;2:e81. doi: 10.1371/journal.pgen.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vertino AM, et al. Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Mol. Biol. Cell. 2005;16:2039–2048. doi: 10.1091/mbc.E04-08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arango NA, et al. Conditional deletion of β-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev. Biol. 2005;288:276–283. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Gras E, et al. Suppression of canonical Wnt/β-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J. Clin. Invest. 2006;116:2012–2021. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagathu C, et al. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem. Biophys. Res. Commun. 2003;311:372–379. doi: 10.1016/j.bbrc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Cawthorn WP, Sethi JK. TNF-α and adipocyte biology. FEBS Lett. 2008;582:117–131. doi: 10.1016/j.febslet.2007.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gustafson B, Smith U. Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. J. Biol. Chem. 2006;281:9507–9516. doi: 10.1074/jbc.M512077200. [DOI] [PubMed] [Google Scholar]
- 31.Cawthorn WP, et al. Tumour necrosis factor-α inhibits adipogenesis via a β-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ. 2007;14:1361–1373. doi: 10.1038/sj.cdd.4402127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waki H, et al. The small molecule harmine is an antidiabetic cell-type-specific regulator of PPARγ expression. Cell Metab. 2007;5:357–370. doi: 10.1016/j.cmet.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Farmer SR. Regulating the balance between peroxisome proliferator-activated receptor γ and β-catenin signaling during adipogenesis. A glycogen synthase kinase 3β phosphorylation-defective mutant of β-catenin inhibits expression of a subset of adipogenic genes. J. Biol. Chem. 2004;279:45020–45027. doi: 10.1074/jbc.M407050200. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, et al. Functional interaction between peroxisome proliferator-activated receptor γ and β-catenin. Mol. Cell. Biol. 2006;26:5827–5837. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moldes M, et al. Peroxisome-proliferator-activated receptor γ suppresses Wnt/β-catenin signalling during adipogenesis. Biochem. J. 2003;376:607–613. doi: 10.1042/BJ20030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennell JA, et al. T-cell factor 4N (TCF-4N), a novel isoform of mouse TCF-4, synergizes with β-catenin to coactivate C/EBPα and steroidogenic factor 1 transcription factors. Mol. Cell. Biol. 2003;23:5366–5375. doi: 10.1128/MCB.23.15.5366-5375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takemaru K, et al. Chibby, a nuclear β-catenin-associated antagonist of the Wnt/Wingless pathway. Nature. 2003;422:905–909. doi: 10.1038/nature01570. [DOI] [PubMed] [Google Scholar]
- 38.Li FQ, et al. Chibby promotes adipocyte differentiation through inhibition of β-catenin signaling. Mol. Cell. Biol. 2007;27:4347–4354. doi: 10.1128/MCB.01640-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topol L, et al. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J. Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westfall TA, et al. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/β-catenin activity. J. Cell Biol. 2003;162:889–898. doi: 10.1083/jcb.200303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits β-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanazawa A, et al. Association of the gene encoding wingless-type mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. Am. J. Hum. Genet. 2004;75:832–843. doi: 10.1086/425340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanazawa A, et al. Wnt5b partially inhibits canonical Wnt/β-catenin signaling pathway and promotes adipogenesis in 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2005;330:505–510. doi: 10.1016/j.bbrc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Takada I, et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-γ transactivation. Nat. Cell Biol. 2007;9:1273–1285. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- 45.Kennell JA, MacDougald OA. Wnt signaling inhibits adipogenesis through β-catenin-dependent and -independent mechanisms. J. Biol. Chem. 2005;280:24004–24010. doi: 10.1074/jbc.M501080200. [DOI] [PubMed] [Google Scholar]
- 46.Kang S, et al. Effects of Wnt signaling on brown adipocyte differentiation and metabolism mediated by PGC-1α. Mol. Cell. Biol. 2005;25:1272–1282. doi: 10.1128/MCB.25.4.1272-1282.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tseng YH, et al. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nat. Cell Biol. 2005;7:601–611. doi: 10.1038/ncb1259. [DOI] [PubMed] [Google Scholar]
- 48.Tseng YH, et al. Differential roles of insulin receptor substrates in brown adipocyte differentiation. Mol. Cell. Biol. 2004;24:1918–1929. doi: 10.1128/MCB.24.5.1918-1929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christodoulides C, et al. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J. Cell Sci. 2006;119:2613–2620. doi: 10.1242/jcs.02975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ai M, et al. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol. Cell. Biol. 2005;25:4946–4955. doi: 10.1128/MCB.25.12.4946-4955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiu W, et al. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J. Bone Miner. Res. 2007;22:1720–1731. doi: 10.1359/jbmr.070721. [DOI] [PubMed] [Google Scholar]
- 52.Kumar S, et al. Gene expression profiling of orbital adipose tissue from patients with Graves’ ophthalmopathy: a potential role for secreted frizzled-related protein-1 in orbital adipogenesis. J. Clin. Endocrinol. Metab. 2005;90:4730–4735. doi: 10.1210/jc.2004-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gesta S, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Christodoulides C, et al. WNT10B mutations in human obesity. Diabetologia. 2006;49:678–684. doi: 10.1007/s00125-006-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karelis AD, et al. Metabolic and body composition factors in subgroups of obesity: what do we know? J. Clin. Endocrinol. Metab. 2004;89:2569–2575. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 56.Ruderman N, et al. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 57.Agarwal AK, Garg A. Genetic basis of lipodystrophies and management of metabolic complications. Annu. Rev. Med. 2006;57:297–311. doi: 10.1146/annurev.med.57.022605.114424. [DOI] [PubMed] [Google Scholar]
- 58.Klein S, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N. Engl. J. Med. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 59.Yki-Jarvinen H. Thiazolidinediones. N. Engl. J. Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 60.Tran TT, et al. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim JY, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Medina-Gomez G, et al. PPAR γ 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3:e64. doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]