Abstract
Background: Nonalcoholic fatty liver disease is positively associated with obesity and cardiovascular disease risk. Apo-10′-lycopenoic acid (APO10LA), a potential oxidation product of apo-10′-lycopenal that is generated endogenously by β-carotene-9′,10′-oxygenase (BCO2) cleavage of lycopene, inhibited hepatic steatosis in BCO2-expressing mice.
Objective: The present study evaluated lycopene and APO10LA effects on hepatic steatosis in mice without BCO2 expression.
Methods: Male and female BCO2-knockout (BCO2-KO) mice were fed a high saturated fat diet (HSFD) with or without APO10LA (10 mg/kg diet) or lycopene (100 mg/kg diet) for 12 wk.
Results: Lycopene or APO10LA supplementation reduced hepatic steatosis incidence (78% and 72%, respectively) and severity in BCO2-KO male mice. Female mice did not develop steatosis, had greater hepatic total cholesterol (3.06 vs. 2.31 mg/g tissue) and cholesteryl ester (1.58 vs. 0.86 mg/g tissue), but had lower plasma triglyceride (TG) (229 vs. 282 mg/dL) and cholesterol (97.1 vs. 119 mg/dL) than male mice. APO10LA-mitigated steatosis in males was associated with reduced hepatic total cholesterol (18%) and activated sirtuin 1 signaling, which resulted in reduced fatty acids (FAs) and TG synthesis markers [stearoyl-coenzyme A (CoA) desaturase protein, 71%; acetyl-CoA carboxylase phosphorylation, 79%; AMP-activated protein kinase phosphorylation, 67%], and elevated cholesterol efflux genes (cytochrome P450 family 7A1, 65%; ATP-binding cassette transporter G5/8, 11%). These APO10LA-mediated effects were not mimicked by lycopene supplementation. Intriguingly, steatosis inhibition by lycopene induced peroxisome proliferator–activated receptor (PPAR)α- and PPARγ-related genes in mesenteric adipose tissue (MAT) that increases mitochondrial uncoupling [cell death–inducing DNA fragmentation factor, α subunit-like effector a, 55%; PR domain-containing 16, 47%; uncoupling protein 3 (Ucp3), 55%], FA β-oxidation (PPARα, 53%; very long chain acyl-CoA dehydrogenase, 38%), and uptake (FA transport protein 4, 29%; lipoprotein lipase 43%). Expressions of 10 MAT PPAR-related genes were inversely correlated with steatosis score, suggesting that lycopene reduced steatosis by increasing MAT FA utilization.
Conclusions: Our data suggest that lycopene and APO10LA inhibit HSFD-induced steatosis in BCO2-KO male mice through differential mechanisms. Sex disparity of BCO2-KO mice was observed in the outcomes of HSFD-induced liver steatosis and plasma lipids.
Keywords: nonalcoholic fatty liver disease, hepatic cholesterol, lipid metabolism, sirtuin 1, obesity, plasma lipids, mitochondrial uncoupling, PPAR, carotenoid metabolism, mesenteric adipose tissue
Introduction
Nonalcoholic fatty liver disease (NAFLD)8 is a chronic liver disease that is observed in 75–100% of overweight and obese adults and children (1). NAFLD describes a series of related disorders that can progress in stages from simple steatosis to fibrosis and cirrhosis (2). Patients with NAFLD are often associated with metabolic syndrome including insulin resistance and hypertriglyceridemia, as well as with increased cardiovascular disease (CVD) and related risk factors (3), which include an atherogenic lipid profile (high in serum TGs and LDL cholesterol; low in HDL cholesterol) and systemic inflammation (4, 5). Liver has the capacity to synthesize lipids via de novo lipogenesis and secrete lipoprotein particles. Thus, liver dysfunction can modify risk factors of CVD (3, 4). In light of the current obesity epidemic and increasing prevalence of NAFLD, the prevention of NAFLD-associated metabolic disorders through dietary means represents an important strategy.
Observational data indicates that the intake of lycopene-rich foods is inversely associated with CVD risk (6, 7) and with CVD risk indicators such as dyslipidemia and systemic inflammation (6, 7). In vivo and in vitro studies have suggested that lycopene may use multifaceted mechanisms to reduce CVD and NAFLD risks (8–10), mechanisms that include modulating cholesterol metabolism, serving as an antioxidant, and inhibiting inflammation (8–10). Of note, NAFLD patients have substantially reduced plasma lycopene concentration (11), suggesting potential interactions between low lycopene status and CVD risk.
We and others have demonstrated that the enzyme β-carotene-9′,10′-oxygenase (BCO2) plays a critical role in the endogenous metabolism of nonprovitamin A carotenoids including lycopene (12, 13). BCO2 metabolizes lycopene through asymmetric cleavage at the 9′,10′ double bond and generates metabolites, which include apo-10′-lycopenal, apo-10′-lycopenol, and apo-10′-lycopenoic acid (APO10LA; chemical structure shown in Supplemental Figure 1) (12, 13). Evidence suggests that these metabolites may exhibit more important biological roles than lycopene itself (9, 14, 15), and BCO2 ablation in mice altered lycopene metabolism (16, 17). In particular, APO10LA supplementation was effective in inhibiting hepatic steatosis in genetically induced obese (ob/ob) mice (15) and attenuating high saturated fat diet (HSFD)-induced liver inflammation as well as tumor number and volume in C57Bl/6J mice (18). An important question remains as to whether the biological activity of lycopene could be different from its metabolite APO10LA in the absence of BCO2 expression. This information is critically needed because 19 single nucleotide polymorphisms of BCO2 have been found in humans (19), and BCO2 gene variants can account for the differential expression and activity of the BCO2 enzyme among individuals. BCO2 single nucleotide polymorphisms have been associated with increased circulatory proinflammatory IL-18 expression (19) and with decreased HDL cholesterol concentrations (19), suggesting a gene-diet interaction between the BCO2 enzyme and dietary lycopene on human health outcomes. We hypothesize that the lycopene biological effects would be different from APO10LA in mice when BCO2 enzyme expression is absent.
Using a murine model that lacks BCO2 protein expression, we investigated the separate effects of lycopene or APO10LA supplementation on HSFD-induced hepatic steatosis and determined the underlying mechanisms thereof.
Methods
Animal experimental design.
The animal experimental protocol was approved by the Institutional Animal Care and Use Committee at the Jean Mayer-USDA Human Nutrition Research Center on Aging at Tufts University. BCO2-knockout (BCO2-KO) mice with BCO2 protein ablation were generated as previously described (20). The schematic for the animal experimental design is shown in Supplemental Figure 2. Mice were fed the standard laboratory chow until the start of experiments (7012 Teklad LM-485; Harlan Laboratories), maintained on a 12-h light/dark cycle in a controlled temperature and humidity room, and consumed water ad libitum. Two separate experiments were conducted concurrently to evaluate APO10LA and lycopene effects. Six-wk-old male and female BCO2-KO mice were randomly assigned to 1 of the 4 diets: 1) an obesogenic HSFD [Bio-Serv; information cited in Ip et al. (21)], in which 60% of energy is lard derived; 2) an HSFD supplemented with APO10LA (HSFD+APO10LA; 10 mg/kg diet); 3) an HSFD supplemented with lycopene placebo beadlet (HSFD+LyP; 1000 mg beadlet/kg diet); and 4) an HSFD supplemented with lycopene (HSFD+Ly; 100 mg lycopene/kg diet) for 12 wk. We included both male and female mice in our experiments because there are no previously published studies to our knowledge that examined the biological effects of APO10LA in BCO2-KO mice. All mice in the experiments were given fresh diets every 2–3 d and maintained on their respective diets until the experiment was completed. Mice were weighed weekly and killed at 18 wk of age by exsanguinations under deep isoflurane (Isothesia; Butler Schein) anesthesia without food deprivation.
Dietary supplementation.
The APO10LA used in the experiments of this study was provided by Dr. Hansgeorg Ernst (BASF, Ludwigshafen, Germany) with 99% purity, analyzed by HPLC as previously described (Supplemental Figure 3) (13, 14). The lycopene supplement was in the form of a 10% lycopene beadlet (BASF). The composition of the lycopene placebo beadlet is listed in Supplemental Table 1. APO10LA, lycopene, or placebo beadlet without lycopene was incorporated directly into the HSFD to achieve a homogenous diet mixture and stored as previously described (18). We selected the same APO10LA dosage (10 mg/kg diet) as in our published study (18), where APO10LA supplementation for 24 wk reduced HSFD-promoted hepatic inflammation in C57Bl/6J male mice. This APO10LA-supplemented dose (10 mg/kg diet) is approximately equivalent to 0.36 mg/d APO10LA in a 60-kg adult man (22, 23). A/J mice supplemented with the same APO10LA dose for 14 wk had a plasma APO10LA concentration of ∼1.0 nmol/L (14). This plasma concentration is comparable to the sum of apo-lycopenals (1.9 nmol/L) found in human plasma of individuals who had consumed tomato juice (with 21.8 mg/d lycopene) for 8 wk (24). Based on the assumption that BCO2-KO mice have similar carotenoid absorption as other strains of mice on a vitamin-supplemented, semipurified diet (∼1/10 of human absorption) (14, 15, 25), our lycopene-supplemented dose (100 mg/kg diet) is approximately equivalent to 8.1 mg/d lycopene in a 60-kg adult man (22, 23). The mean human dietary lycopene was ∼8 mg/d (26, 27). In addition, our recently published study (28) showed that BCO2-KO mice supplemented with the identical lycopene dose for 24 wk had mean hepatic lycopene concentrations that were within human ranges (0.1–20.7 nmol/g tissue) (29).
Liver and adipose tissue collection and processing.
After the mice were killed, whole livers and mesenteric adipose tissue (MAT) were removed from experimental mice and processed for biochemical and histologic analyses as described previously (18).
Histopathologic evaluation.
Five-μm sections of formalin-fixed, paraffin-embedded liver left lobes were processed and stained with hematoxylin and eosin. Two independent investigators unaware of treatment groups evaluated the liver sections under light microscopy for steatosis magnitude and inflammation severity, as described previously (18), in 20 random fields at 100× magnification per sample. Briefly, the degree of steatosis was graded 0–4 (grading 0 = <5%, 1 = 5–25%, 2 = 26–50%, 3 = 51–75%, 4 = >75%), based on the mean percentage of fat-accumulated hepatocytes per field. Hepatic steatosis incidence was evaluated by the percentage of mice in each group with a steatosis grading >0. Inflammatory foci were evaluated by the number of inflammatory-cell clusters, which mainly constitute mononuclear inflammatory cells as described previously (18). Hepatic inflammatory foci incidence was evaluated by the percentage of mice in each group with ≥1 liver inflammatory foci.
Plasma lipid and lipoprotein profile.
Blood from cardiac puncture was collected into EDTA-coated tubes, and plasma was separated from RBCs by centrifugation at 1500 × g for 20 min at 4°C. Plasma total cholesterol (TC), HDL cholesterol, and TG concentrations were determined on a Cobas Mira automated analyzer with use of enzymatic reagents (Roche Diagnostics). Non–HDL cholesterol was calculated as the difference between TC and HDL cholesterol.
Hepatic cholesterol and TG contents.
Hepatic TC and free cholesterol (FC) contents of extracted liver lipids were analyzed by GC as described previously (30). Cholesteryl ester (CE) was computed as the difference between TC and FC (30). Liver tissue (25 mg of wet weight) was used to determine hepatic TG content as previously described (15). Hepatic cholesterol and TG were corrected for the wet liver weight used in the analysis.
RNA extraction and qRT-PCR.
Total RNA extraction from frozen liver and MAT tissue and subsequent qRT-PCR were performed as described previously (18). Primer sequences are listed in Supplemental Table 2.
Protein isolation and Western blotting.
Protein isolation from frozen liver tissue and subsequent Western blotting analysis of specific proteins were executed as described previously (18). The following antibodies were used for western blotting: acetyl-CoA carboxylase (ACC), AMP-activated protein kinase (AMPK) α, phosphorylated-ACC (Ser79), phosphorylated-AMPKα (Thr172), stearoyl-CoA desaturase (SCD1) (Cell Signaling), IL-6 (R&D), acetylated-Foxhead box protein O1 (FoxO1), heme-oxygenase (HO)-1, and sirtuin 1 (SIRT1). Horseradish peroxidase–conjugated secondary antibody (Bio-Rad) was used to detect specific proteins, and the specific bands were visualized by a SuperSignal West Pico Chemiluminescent Substrate Kit (Pierce) according to the manufacturer’s instructions. Dilution series and calibration curve were performed for each of the antibodies used to quantify protein. β-Actin protein was used for loading normalization of some proteins unless specified otherwise, which was detected by an anti-actin antibody (Sigma-Aldrich). A GS-710 Calibrated Imaging Densitometer (Bio-Rad) was used to quantify the intensities of protein bands.
Statistical analysis.
SAS 9.3 software was used to perform the statistical analysis. Values in the text are means ± SEMs or medians (IQRs). Two-factor ANOVA was used to examine the overall, diet, sex, or diet × sex interaction effects on experimental outcomes modified by APO10LA or lycopene supplementation. Student’s two-sample unequal variance t test, Wilcoxon signed-rank test, and χ-square test were used to examine the differences for the following comparisons: 1) HSFD and HSFD+APO10LA, and 2) HSFD+LyP and HSFD+Ly. Spearman’s rank correlation was used to perform nonparametric measures of statistical dependence between 2 variables. The P value was set at 0.05 for the comparisons to reach statistical significance.
Results
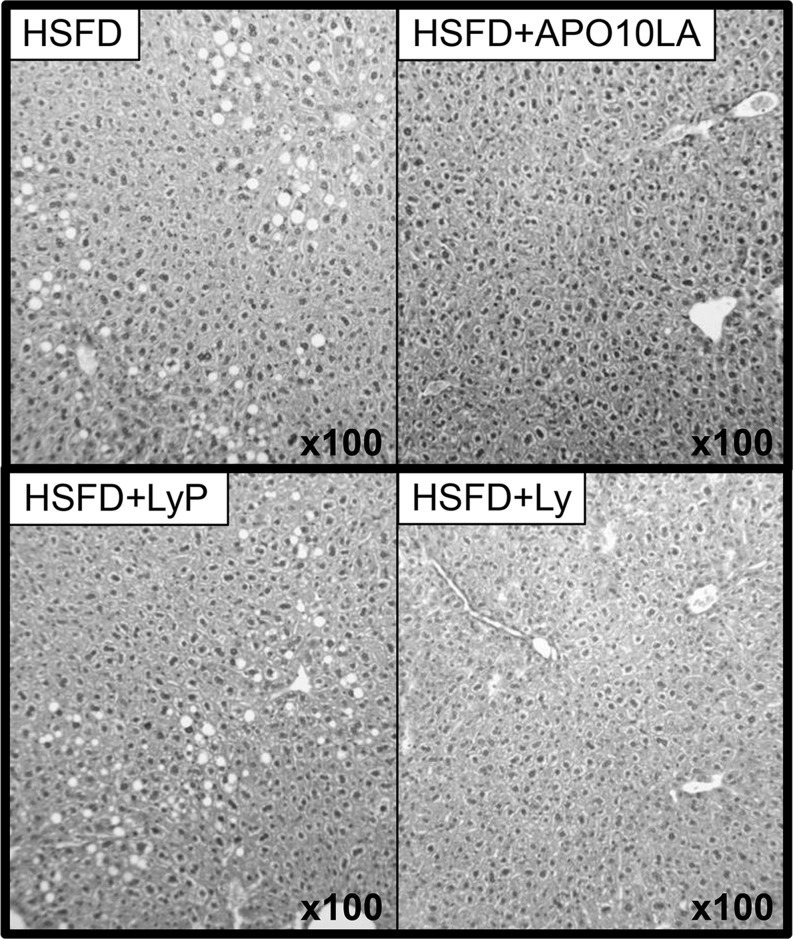
APO10LA and lycopene supplementation ameliorated HSFD-induced hepatic steatosis in BCO2-KO male mice.
Final mean body and liver weights did not differ significantly between supplemented and nonsupplemented BCO2-KO mice (Tables 1 and 2). There was a marked difference in the response of female and male mice to the HSFD for liver steatosis, cholesterol accumulation, and plasma lipid concentrations. Male mice weighed significantly more than female mice at the start and end of the experiments (Tables 1 and 2). The percentage weight gain was 63.4 (10.3 g) in the female mice and 83.3 (16.2 g) in the male mice. In male mice, APO10LA and lycopene supplementation significantly decreased HSFD-induced liver steatosis incidence and score (Figure 1, Tables 1 and 2). APO10LA or lycopene supplementation tended to decrease hepatic TGs (Tables 1 and 2). In female mice, the HSFD did not induce steatosis, and APO10LA or lycopene supplementation did not alter hepatic steatosis outcome or TGs (Tables 1 and 2). In both male and female mice, APO10LA, but not lycopene supplementation, significantly reduced plasma TG and hepatic FC and CE concentrations (Tables 1 and 2). Final body weight (BW), BW gain, and hepatic FC and TC were correlated with steatosis score in both female and male mice (R2 = 0.52, 0.52, 0.33, 0.35; P = 0.001, 0.001, 0.030, 0.020, respectively). Female mice had significantly greater hepatic TC and CE than males, but FC was not significantly different between males and females (Tables 1 and 2). Female mice had less weight gain, and plasma TGs, TC, and non–HDL cholesterol than males (Tables 1 and 2). Female mice had lower plasma TGs than males only within the HSFD and HSFD+APO10LA groups (Table 1). These results suggest that lycopene and APO10LA had biological effects in the absence of BCO2. Sex disparity exists on diet-induced hepatic steatosis and lipid metabolism in BCO2-KO mice.
TABLE 1.
Primary outcomes of BCO2-KO mice fed an HSFD with or without APO10LA supplementation for 12 wk1
| Experimental groups |
||||||||
| Males |
Females |
Two-factor ANOVA |
||||||
| HSFD | HSFD+APO10LA | HSFD | HSFD+APO10LA | Overall | Diet × sex | Diet | Sex | |
| Mice, n | 10 | 12 | 10 | 12 | ||||
| Liver weight, g | 1.02 ± 0.03 | 1.02 ± 0.03 | 0.92 ± 0.04 | 0.97 ± 0.02 | 0.01 | 0.92 | 0.8 | <0.01 |
| Initial BW, g | 19.6 ± 0.55 | 19.5 ± 1.46 | 16.3 ± 0.50 | 16.2 ± 0.31 | <0.01 | 0.95 | 0.80 | <0.01 |
| Final BW, g | 36.9 ± 1.62 | 36.3 ± 0.93 | 26.7 ± 1.37 | 27.4 ± 0.94 | <0.01 | 0.59 | 0.99 | <0.01 |
| BW gain, g | 17.3 ± 1.39 | 16.8 ± 0.87 | 10.3 ± 1.34 | 11.2 ± 0.73 | <0.01 | 0.52 | 0.91 | <0.01 |
| Hepatic steatosis incidence, % | 90 | 25* | 0 | 0 | NA | NA | 0.09 | <0.01 |
| Hepatic steatosis score, median (range) | 1 (0–2) | 0 (0–2)* | 0 (0–0) | 0 (0–0) | NA | NA | 0.06 | <0.01 |
| Hepatic inflammatory foci incidence, % of mice | 40 | 0* | 20 | 16 | NA | NA | 0.04 | 0.88 |
| Hepatic lipids, mg/g wet tissue | ||||||||
| TC | 2.46 ± 0.16 | 2.01 ± 0.09* | 3.50 ± 0.37 | 2.69 ± 0.16* | <0.01 | 0.4 | 0.01 | <0.01 |
| FC | 1.50 ± 0.05 | 1.36 ± 0.03* | 1.51 ± 0.05 | 1.41 ± 0.04* | 0.04 | 0.64 | 0.01 | 0.47 |
| CE | 0.96 ± 0.14 | 0.65 ± 0.08* | 1.98 ± 0.34 | 1.28 ± 0.15* | <0.01 | 0.31 | 0.01 | <0.01 |
| TG | 33.4 ± 1.89 | 29.2 ± 1.09† | 38.7 ± 5.09 | 33.4 ± 3.09 | 0.20 | 0.86 | 0.13 | 0.13 |
| Plasma lipids | ||||||||
| TG, mg/dL | 312 ± 22.1 | 252 ± 20.1 | 235 ± 19.0 | 222 ± 9.70 | <0.01 | 0.19 | 0.04 | <0.01 |
| TC, mg/dL | 117 ± 3.50 | 121 ± 5.21 | 97.4 ± 6.42 | 96.7 ± 4.67 | <0.01 | 0.64 | 0.83 | <0.01 |
| HDL-C:TC, % | 45.8 ± 0.89 | 47.4 ± 0.81 | 46.3 ± 0.98 | 47.2 ± 0.42 | 0.43 | 0.67 | 0.11 | 0.84 |
| Non–HDL-C, mg/dL | 63.7 ± 2.66 | 63.8 ± 2.89 | 52.7 ± 4.13 | 51.1 ± 2.60 | <0.01 | 0.78 | 0.74 | <0.01 |
Values are means ± SEMs or n (%) unless otherwise indicated. Two-factor ANOVA was used to examine the overall, diet, sex, and diet × sex effects. Symbols indicate different between HSFD and HSFD+APO10LA from HSFD+LyP within sex: *P < 0.05, †P = 0.07. APO10LA, apo-10′-lycopenoic acid; BCO2-KO, β-carotene-9′,10′-oxygenase-knockout; BW, body weight; CE, cholesteryl ester; FC, free cholesterol; HDL-C, HDL cholesterol; HSFD, high saturated fat diet; HSFD+APO10LA, high saturated fat diet supplemented with apo-10′-lycopenoic acid; NA, not applicable; TC, total cholesterol.
TABLE 2.
Primary outcomes of BCO2-KO mice fed an HSFD with or without lycopene supplementation for 12 wk1
| Experimental groups |
||||||||
| Males |
Females |
Two-factor ANOVA |
||||||
| HSFD+LyP | HSFD+Ly | HSFD+LyP | HSFD+Ly | Overall | Diet × sex | Diet | Sex | |
| Mice, n | 10 | 12 | 10 | 12 | ||||
| Liver weight, g | 1.04 ± 0.04 | 0.98 ± 0.03 | 0.89 ± 0.03 | 0.90 ± 0.02 | <0.01 | 0.26 | 0.37 | <0.01 |
| Initial BW, g | 19.5 ± 0.50 | 19.4 ± 0.40 | 16.2 ± 0.37 | 16.1 ± 0.28 | <0.01 | 0.99 | 0.75 | <0.01 |
| Final BW, g | 35.3 ± 1.27 | 34.5 ± 1.25 | 25.3 ± 1.13 | 26.7 ± 1.13 | <0.01 | 0.36 | 0.9 | <0.01 |
| BW gain, g | 15.8 ± 1.17 | 15.0 ± 1.34 | 9.04 ± 0.99 | 10.6 ± 0.92 | <0.01 | 0.31 | 0.79 | <0.01 |
| Hepatic steatosis incidence, % | 80 | 18* | 0 | 0 | NA | NA | 0.02 | <0.01 |
| Hepatic steatosis score, median (range) | 1 (0–2) | 0 (0–2)* | 0 (0–0) | 0 (0–0) | NA | NA | 0.02 | <0.01 |
| Hepatic inflammatory foci incidence, % of mice | 30 | 8 | 0 | 8 | NA | NA | 0.53 | 0.14 |
| Hepatic lipids, mg/g wet tissue | ||||||||
| TC | 2.47 ± 0.30 | 2.28 ± 0.20 | 3.01 ± 0.21 | 3.04 ± 0.17 | 0.04 | 0.62 | 0.73 | 0.01 |
| FC | 1.50 ± 0.05 | 1.43 ± 0.04 | 1.50 ± 0.03 | 1.49 ± 0.04 | 0.44 | 0.38 | 0.25 | 0.45 |
| CE | 0.96 ± 0.27 | 0.85 ± 0.14 | 1.51 ± 0.19 | 1.55 ± 0.14 | 0.02 | 0.7 | 0.87 | <0.01 |
| TG | 29.9 ± 2.32 | 24.2 ± 2.27† | 33.9 ± 2.44 | 30.0 ± 1.38 | 0.02 | 0.67 | 0.03 | 0.06 |
| Plasma lipids | ||||||||
| TG, mg/dL | 255 ± 17.1 | 249 ± 18.0 | 251 ± 18.7 | 234 ± 22.7 | 0.88 | 0.79 | 0.55 | 0.62 |
| TC, mg/dL | 109 ± 2.79 | 107.8 ± 4.04 | 96.5 ± 5.16 | 95.5 ± 5.79 | 0.08 | 0.96 | 0.79 | 0.01 |
| HDL-C:TC, % | 51.5 ± 1.11 | 46.8 ± 0.90 | 47.2 ± 0.76 | 47.5 ± 0.79 | 0.92 | 0.66 | 0.99 | 0.59 |
| Non–HDL-C, mg/dL | 57.9 ± 1.96 | 57.4 ± 2.51 | 51.2 ± 3.18 | 50.3 ± 3.56 | 0.15 | 0.94 | 0.79 | 0.02 |
Values are means ± SEMs or n (%) unless otherwise indicated. Two-factor ANOVA was used to examine the overall, diet, sex, and diet × sex effects. Symbols indicate different from HSFD+LyP within sex: *P < 0.05, †P = 0.09. BCO2-KO, β-carotene-9′,10′-oxygenase-knockout; BW, body weight; CE, cholesteryl ester; FC, free cholesterol; HDL-C, HDL cholesterol; HSFD, high saturated fat diet; HSFD+Ly, high saturated fat diet supplemented with lycopene; HSFD+LyP, high saturated fat diet supplemented with lycopene placebo beadlet; NA, not applicable; TC, total cholesterol.
FIGURE 1.
Histopathology of liver of BCO2-KO male mice that were or were not supplemented with lycopene or APO10LA for 12 wk. Representative hematoxylin-and-eosin–stained livers from HSFD, HSFD+APO10LA, HSFD+LyP, and HSFD+Ly mice. APO10LA, apo-10′-lycopenoic acid; BCO2-KO, β-carotene-9′,10′-oxygenase-knockout; HSFD, high saturated fat diet; HSFD+APO10LA, high saturated fat diet supplemented with apo-10′-lycopenoic acid; HSFD+Ly, high saturated fat diet supplemented with lycopene; HSFD+LyP, high saturated fat diet supplemented with lycopene placebo beadlet.
APO10LA, but not lycopene, decreased HSFD-induced hepatic inflammation in BCO2-KO male but not female mice.
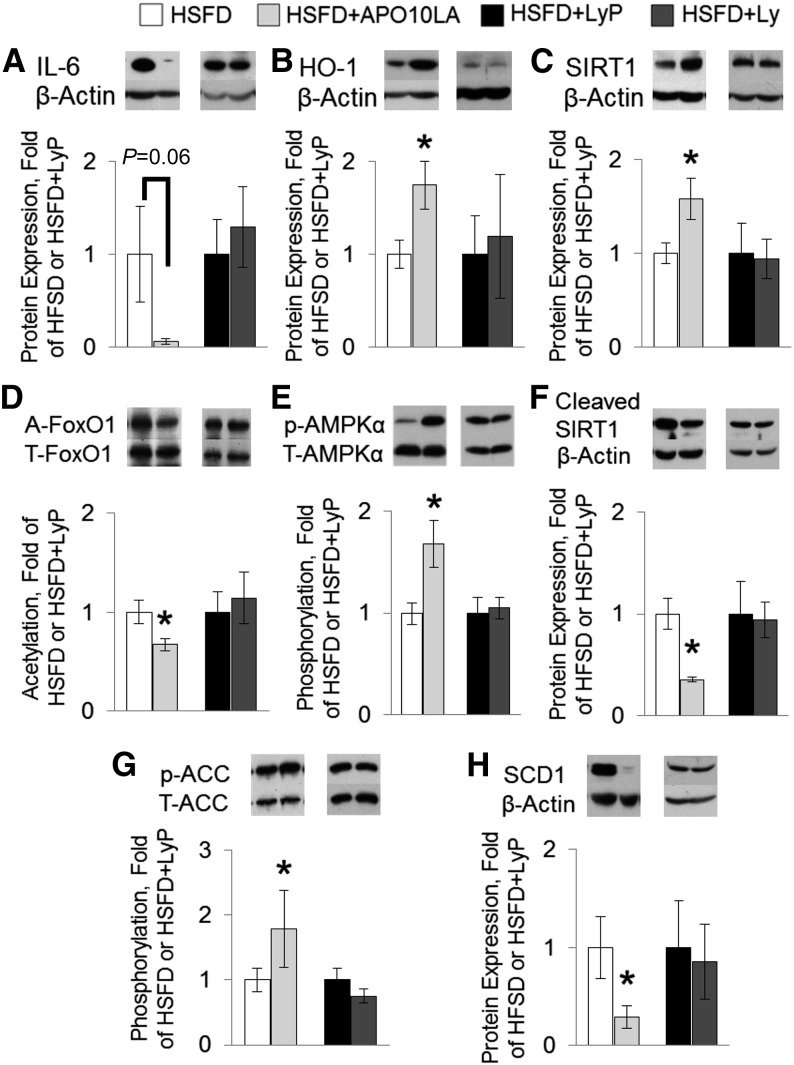
APO10LA, but not lycopene supplementation, significantly reduced hepatic inflammatory foci incidence in male but not female BCO2-KO mice (Table 1). This APO10LA-mediated inflammation suppression in male mice was associated with reduced hepatic IL-6 protein (94%, P = 0.06; Figure 2A) and with elevated anti-inflammatory HO-1 protein (74%; Figure 2B) expression in male mice.
FIGURE 2.
Hepatic molecular markers in BCO2-KO male mice that were or were not supplemented with lycopene or APO10LA for 12 wk. Protein expression in liver lysates (HSFD, HSFD+APO10LA, HSFD+LyP, and HSFD+Ly male BCO2-KO mice). β-Actin was used as loading control unless specified otherwise. Graphical representation of fold changes in (A) IL-6; (B) HO-1; (C) SIRT1; (D) FoxO1 acetylation (FoxO1 as loading control); (E) phosphorylated AMPKα (Thr172; AMPKα as loading control); (F) cleaved-SIRT1; (G) phosphorylated ACC (Ser79; ACC as loading control); and (H) SCD1. Representative western blots with 1 sample per group are shown. Fold changes normalized to HSFD or HSFD+LyP. Values are means ± SEMs, n = 10–12. *Different from HSFD, P < 0.05. A-, acetylated; ACC, acetyl-CoA carboxylase; AMPK, AMP-activated protein kinase; APO10LA, apo-10′-lycopenoic acid; BCO2-KO, β-carotene-9′,10′-oxygenase-knockout; FoxO, Foxhead box protein O; HO, heme-oxygenase; HSFD, high saturated fat diet; HSFD+APO10LA, high saturated fat diet supplemented with apo-10′-lycopenoic acid; HSFD+Ly, high saturated fat diet supplemented with lycopene; HSFD+LyP, high saturated fat diet supplemented with lycopene placebo beadlet; p-, phosphorylated; SCD1, stearoyl-CoA desaturase 1; SIRT1, sirtuin 1.
APO10LA increased hepatic SIRT1 protein, deacetylation of FoxO1, and AMPKα phosphorylation in male mice.
APO10LA, but not lycopene supplementation, significantly increased hepatic SIRT1 protein expression (58%; Figure 2C) and induced the deacetylation of SIRT1 direct target FoxO1 (33%; Figure 2D) in male mice compared to the HSFD mice. These observations were associated with a significant increase in AMPKα phosphorylation (Thr172, 67%; Figure 2E) and decreased SIRT1 protein cleavage (64%; Figure 2F). Hepatic SIRT1 protein cleavage was inversely correlated with SIRT1 protein (R2 = −0.50, P = 0.001) and HO-1 protein (R2 = −0.59, P < 0.001) in the HSFD and HSFD+APO10LA mice but not in the HSFD+Ly and HSFD+LyP mice. These data suggest that APO10LA mitigated HSFD-induced steatosis through activating SIRT1 signaling.
APO10LA-induced hepatic SIRT1 signaling was associated with decreased lipogenic biomarkers expression and with upregulated cholesterol efflux genes in male mice.
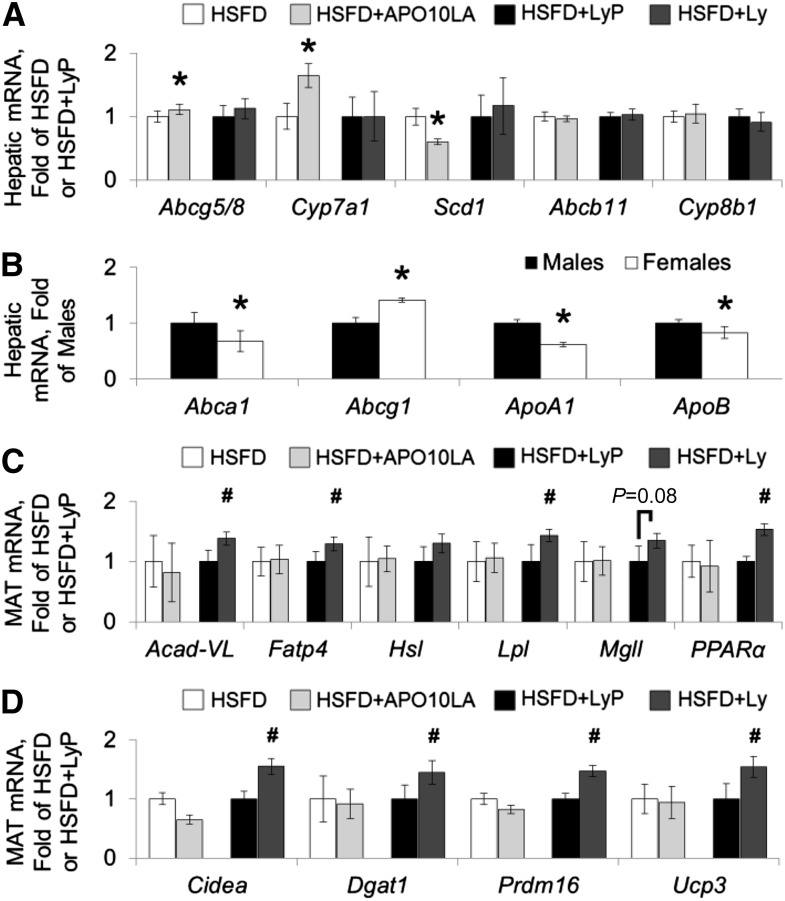
Supplementation of APO10LA, but not lycopene, significantly reduced expression/activation of proteins involved in hepatic lipogenesis, which included ACC inhibition by phosphorylation (Ser79, 79%; Figure 2G), reduced SCD1 protein expression (71%; Figure 2H), and decreased Scd1 gene expression (40%; Figure 3A). Hepatic Scd1 gene expression was significantly correlated with SCD1 protein (R2 = 0.64, P = 0.003), SIRT1 protein (R2 = −0.31, P = 0.044), and hepatic steatosis score (R2 = 0.45, P = 0.002) in HSFD and HSFD+APO10LA mice. Hepatic ACC phosphorylation was significantly correlated with SIRT1 protein expression (R2 = 0.43, P < 0.001) in all mice. APO10LA also significantly increased hepatic cholesterol efflux gene ATP-binding cassette transporter (Abc)g5/8 expression (11%; Figure 3A) and bile synthesis gene cytochrome P450 family (Cyp)7a1 expression (65%; Figure 3A), and had no significant effect on Abcb11 or Cyp8b1 gene expression (Figure 3A). In addition, hepatic SIRT1 protein expression was correlated with Abcg5/8 gene expression in all mice (R2 = 0.32, P = 0.005). APO10LA or lycopene supplementation did not alter other genes involved in hepatic cholesterol metabolism (Abca1, Abcg1, Apo-A1, and ApoB; data not shown). Female mice had lower expression of the Abca1, ApoA1, and ApoB genes but higher expression of the Abcg1 gene than males (Figure 3B). These data suggest that APO10LA reduced hepatic steatosis and cholesterol through inhibiting FA synthesis while promoting FA β-oxidation and cholesterol efflux. Female BCO2-KO mice exhibited differential hepatic cholesterol metabolism compared to male mice.
FIGURE 3.
Hepatic and mesenteric adipose mRNA expression in BCO2-KO male mice that were or were not supplemented with lycopene or APO10LA for 12 wk. mRNA expression in liver or mesenteric adipose tissue lysates [(A, C, D) HSFD, HSFD+APO10LA, HSFD+LyP, and HSFD+Ly male BCO2-KO mice, n = 10–12/group; (B) males or females, n = 42/group]. Graphical representation of fold changes in (A) hepatic mRNA of Abcg5/8, Cyp7a1, Scd1, Abcb11, Cyp8b1; (B) hepatic mRNA of Abca1, Abcg1, ApoA1, ApoB; (C) PPARα-associated genes: Acad-VL, Fatp4, Hsl, Lpl, Mgll, PPARα; and (D) PPARγ-associated genes: Dgat1, Cidea, Prdm16, Ucp3. Fold changes normalized to HSFD, HSFD+LyP, or males. Values are means ± SEMs. *Different from HSFD or males, #Different from HSFD+LyP, P < 0.05. Abc, ATP-binding cassette transporter; Acad, acyl-CoA dehydrogenase; APO10LA, apo-10′-lycopenoic acid; BCO2-KO, β-carotene-9′,10′-oxygenase-knockout; Cidea, cell death–inducing DNA fragmentation factor, α subunit-like effector a; Cyp, cytochrome P450 family; Dgat, diglyceride acyltransferase; Fatp, FA transport protein; HSFD, high saturated fat diet; HSFD+APO10LA, high saturated fat diet supplemented with apo-10′-lycopenoic acid; HSFD+Ly, high saturated fat diet supplemented with lycopene; HSFD+LyP, high saturated fat diet supplemented with lycopene placebo beadlet; Hsl, hormone-sensitive lipase; MAT, mesenteric adipose tissue; Mgll, monoacylglycerol lipase; Prdm16, PR domain containing 16; Scd1, stearoyl-CoA desaturase 1; Ucp3, uncoupling protein 3.
Lycopene upregulated PPARα-inducible genes in MAT but not in hepatic tissue in male mice.
PPARα is a transcription factor involved in lipid metabolism including FA β-oxidation, FA uptake, and lipolysis (31). We observed that lycopene supplementation, but not APO10LA, in BCO2-KO mice significantly induced PPARα genes (53%; Figure 3C) and PPARα-inducible genes including very-long-chain acyl-CoA dehydrogenase (Acad-VL, 38%; Figure 3C), FA transport protein 4 (Fatp4, 29%; Figure 3C), and lipoprotein lipase (43%; Figure 3C) in BCO2-KO male mice. Similar trends were observed in other PPARα-associated genes in MAT [including medium-chain Acad (Acad-M), acyl-CoA oxidase 1 (Acox1), Cd36, Fatp1, hormone-sensitive lipase (Hsl), monoacylglycerol lipase (Mgll), and patatin-like phospholipase domain containing 2 (Pnpla2); Figure 3C and Supplemental Figure 4]. In addition, PPARα gene expression in MAT was significantly correlated with a number of PPARα-inducible genes (Supplemental Table 3). Lycopene-induced Acad-M and Pnpla2 gene expression were inversely correlated with hepatic steatosis score (R2 = −0.31, P = 0.018; R2 = −0.23, P = 0.070, respectively). APO10LA or lycopene supplementation had no effects on hepatic PPARα-associated genes (Acad-M, Cd36, Cpt, Fatp2, Fatp5, and PPARα) in BCO2-KO male mice (data not shown). These data suggest that PPARα-associated gene induction by lycopene was tissue specific in the absence of BCO2.
Lycopene-mitigated steatosis in male mice was associated with elevated expression of PPARγ-associated genes in MAT.
Activation of the transcription factor PPARγ was shown to promote mitochondrial uncoupling and expression of FA utilizing genes (31). Lycopene, but not APO10LA, supplementation significantly increased MAT expression of PPARγ-associated genes including cell death–inducing DNA fragmentation factor, α subunit-like effector a (Cidea, 55%; Figure 3D), diglyceride acyltransferase 1 (Dgat1, 45%; Figure 3D), PR domain-containing 16 (Prdm16, 47%; Figure 3D), and uncoupling protein 3 (Ucp3, 55%; Figure 3D) in BCO2-KO male mice. Similar trends were observed in other PPARγ-associated genes [including Cd36, cytochrome c (Cysc), phosphoenolpyruvate carboxykinase (Pck1), PPARγ, PPARγ coactivator 1α (Pgc1α), solute carrier family 2 type 4 (Slc2a4), and Ucp1; Figure 3D and Supplemental Figure 4] in MAT. Lycopene-induced Cysc, Dgat1, Pgc1α, PPARγ, Prdm16, Slc2a4, Ucp1, and Ucp3 gene expression were inversely correlated with hepatic steatosis score (R2 = −0.46, −0.24, −0.48, −0.42, −0.55, −0.35, −0.26, and −0.55, respectively; P = 0.001, 0.056, 0.007, 0.001, 0.001, 0.011, 0.029, and 0.035, respectively). PPARy gene and PPARγ-associated genes were significantly correlated with each other (Supplemental Table 4). These data suggest that lycopene induced MAT PPARγ-associated genes in the absence of BCO2 and inhibited steatosis by upregulating MAT FA utilization.
Discussion
The present study evaluated the effects of lycopene and APO10LA supplementation on hepatic steatosis in male and female mice that were deficient in BCO2 expression. BCO2-KO male mice developed hepatic steatosis as examined by histology after 12 wk of being fed an HSFD, suggesting that this in vivo model can be useful in evaluating dietary effects on liver steatosis. Supplementation with either lycopene or APO10LA inhibited high-saturated-fat–induced hepatic steatosis incidence and severity in BCO2-KO male mice. Dietary lycopene supplementation mitigated steatosis despite the absence of BCO2 enzyme expression, suggesting that lycopene itself exhibits biological functions. It should be noted that APO10LA or lycopene supplementation tended to decrease hepatic TGs in BCO2-KO male mice, but the effects were not significant (P = 0.07 and 0.09, respectively). This discrepancy between our histopathologic steatosis grading and hepatic TGs was likely because of the regional distribution of steatosis in the liver and the small sample size that led to high variability within each experimental group. Therefore, we believe that the histopathologic steatosis grading of left liver lobes was more valid in evaluating hepatic steatosis.
In the present study, the biochemical and molecular analyses of liver and MAT suggest that lycopene and APO10LA have differential mechanisms of biological action in the BCO2-KO mice. APO10LA-mitigated steatosis and inflammation in BCO2-KO male mice coincided with induced SIRT1 protein, deacetylation of SIRT1 direct downstream target, and FoxO1, as well as with reduced SIRT1 protein cleavage. These results suggest that APO10LA modulated SIRT1 protein in a post-translational manner, which is similar to our previous findings (18). APO10LA-mediated steatosis reduction and SIRT1-signaling activation in BCO2-KO mice were associated with decreased lipogenic SCD1 gene and protein as well as with increased AMPK activation and ACC phosphorylation. Importantly, Scd1 gene expression in APO10LA-supplemented BCO2-KO mice correlated with hepatic steatosis score and negatively correlated with SIRT1 protein. SIRT1-signaling activation protected mice from HSFD-induced steatosis and inflammation through inhibiting FA biosynthesis (32, 33), promoting FA β-oxidation (32, 33), inhibiting Scd gene induction (34), and activating AMPK (35). AMPK is an enzyme that inhibits ACC by phosphorylation to the inactive form of the enzyme (36). Hepatic ACC activation promotes FA biosynthesis and steatosis while inhibiting FA β-oxidation (34–36). Therefore, these data suggest that APO10LA reduced steatosis through SIRT1-signaling activation. Moreover, the APO10LA biological effects in the present study are in agreement with our previous findings using mouse strains with BCO2 expression (15, 18), indicating that APO10LA may not be further metabolized by the BCO2 enzyme despite the presence of a 9′,10′ double bond in its structure.
Hepatic FC accumulation was shown to sensitize rats to cytokine-mediated steatohepatitis (37). In the present study, the decreased hepatic FC in APO10LA-fed BCO2-KO male mice coincided with reduced hepatic inflammation and IL-6 expression (P = 0.06) as well as elevated HO-1 expression. APO10LA also induced hepatic cholesterol efflux and bile synthesis genes, without altering other genes involved in hepatic cholesterol metabolism. APO10LA supplementation stimulated HO-1 in the lungs of A/J mice (14). The SIRT1-activator resveratrol induced hepatic HO-1 (38), cholesterol efflux, and bile synthesis genes (39). In contrast, hepatocyte-specific deletion of SIRT1 in mice suppressed hepatic Cyp7a1 and promoted hepatic cholesterol accumulation, steatosis, and inflammation (33). Notably, APO10LA-induced hepatic Abcg5/8 in the present study was correlated with SIRT1 protein. Abcg5/8 and Cyp7a1 are also targets of the transcription factor farnesoid X receptor (40), but APO10LA did not modulate 2 other farnesoid X receptor–modifiable genes, Abcb11 and Cyp8b1. Therefore, these results suggest that APO10LA modulated Abcg5/8 and Cyp7a1 genes through SIRT1 activation.
In the present study, lycopene protected BCO2-KO male mice against HSFD-induced hepatic steatosis despite the absence of hepatic SIRT1-signaling modulations. Lycopene can accumulate in adipose tissue (10) and modulate adipose lipid/inflammation-associated signaling cascades (41, 42). Evidence showed that the portal vein directly connects the liver with the visceral MAT (43, 44), and visceral fat content is highly correlated with the degree of liver steatosis in humans (45, 46). In the present study, the hepatic steatosis reduction by lycopene was associated and inversely correlated with induced PPARα- and PPARγ-associated genes in MAT. PPARα activation can result in the net effect of reducing FA synthesis and secretion from cells (47). PPARγ activation in adipose tissue increases adipocyte differentiation, FA sequestration, and FA utilization (48, 49). Therefore, lycopene-mediated upregulation in MAT PPARα- and PPARγ-associated genes may have promoted MAT FA utilization, and subsequently reduced lipid delivery from MAT to the liver. Nevertheless, it should be noted that lycopene effects on PPARα- and PPARγ-mediated signaling were relatively modest; thus, this might explain why lycopene did not reduce final BW. Lycopene modulations in PPAR-associated signaling were observed in the adrenal glands, liver, and kidney of rodents in previous studies (16, 50, 51). During the preparation of this manuscript, a recent publication by Tan et al. (50) showed that 3-wk-old BCO2-KO male mice on a lycopene-supplemented (250 mg/kg diet) AIN-93G semipurified diet for 3 wk modulated hepatic PPAR-associated signaling that could potentially affect hepatic responses to metabolic, infectious, or chemical stress. However, the present study showed that lycopene in HSFD-fed BCO2-KO mice only modified PPARα-associated genes in MAT and not in the liver. This discrepancy between the present study and the findings published by Tan et al. may be the result of the differential experimental designs, diet composition, and/or lycopene-supplemented dosage/duration. Future research is needed to examine whether BCO2 expression can modify tissue specificity of lycopene modulations in PPARα-associated signaling.
The sex difference in BW gain and hepatic steatosis outcomes have previously been demonstrated in a number of mouse and rat models (52–55). Indeed, the present study showed that HSFD feeding induced hepatic steatosis in male but not in female BCO2-KO mice. BCO2-KO females gained less BW and exhibited differential hepatic lipid metabolism compared to males. NAFLD patients are more likely to be males and postmenopausal women (56), and the difference in NAFLD prevalence may be attributed to the putative protective effects of estrogen. Estrogen treatment inhibited hepatic lipid storage in estrogen-deficient mice (57, 58) and decreased BW in HSFD-fed C57Bl/6J female mice (59). Therefore, the sex disparity on HSFD-induced steatosis and BW in the present study may be the difference in circulatory estrogen between male and female BCO2-KO mice. Other factors including the elevated Abcg1 gene in BCO2-KO females compared to males may also protect females from hepatic lipid accumulation. This is because targeted disruption of Abcg1 in mice resulted in accumulation of neutral lipids and phospholipid in hepatocytes (60).
Female BCO2-KO mice in the present study had greater hepatic TC and CE and lower plasma TGs, TC, and non–HDL cholesterol but no differences in HDL cholesterol compared to male mice. These observations in BCO2-KO females were associated with lower Abca1, ApoA1, and ApoB gene expression, but greater Abcg1 gene expression than male mice. ApoB is required for the assembly and secretion of intestinal and hepatic apolipoproteins that transport CE and TG (61). Therapeutic inhibition of ApoB synthesis reduced plasma TC and TG in humans (61). Therefore, it is plausible that the reduced ApoB expression in BCO2-KO females compared to males may contribute to the sex disparity in plasma lipids. Hepatic cholesterol efflux to HDL requires ABCG1, whereas efflux to the HDL precursor ApoA1 requires ABCA1 (60). Because female BCO2-KO mice had elevated Abcg1 but reduced Abca1 and ApoA1 genes compared to males, these results may explain why male and female BCO2-KO mice had similar HDL cholesterol.
Because we aimed to isolate the differential effects of lycopene and APO10LA by using mice without BCO2 expression, we did not include wild-type mice in the present study. However, we acknowledge that the scope of the present study would be broadened with the inclusion of wild-type mice. In addition, BCO2 is believed to be the key enzyme involved in lycopene eccentric metabolism (12, 13), and BCO2-KO mice had elevated lycopene accumulation in tissue and serum compared to wild-type mice (16, 17). Lycopene-supplemented mice without carotene-15,15′-oxygenase (BCO1) expression did not have elevated lycopene accumulation in tissue and serum compared to wild-type mice (16, 17). BCO1 also did not cleave lycopene in vitro in previous studies (10, 62). However, recent studies suggested that lycopene could be metabolized to acyclo-retinal by purified recombinant human BCO1 (63) and by recombinant murine BCO1 (64). Further investigations with BCO1/BCO2–double KO mice are currently ongoing in our laboratory to examine if lycopene-mediated functions are the effects of lycopene metabolites generated by BCO1 cleavage.
Taken together, the results from the present study suggest that both lycopene and APO10LA supplementation were effective in reducing high saturated fat–induced liver steatosis in mice where BCO2 expression was absent. The APO10LA-mediated ability to prevent hepatic steatosis and inflammation in male mice was associated with SIRT1-signaling activation in the liver, whereas the lycopene-mediated ability to prevent hepatic steatosis was associated with modulations in MAT lipid metabolism. Female BCO2-KO mice were less prone to HSFD-induced liver steatosis, weight gain, and elevated plasma TGs and TC than male mice.
Supplementary Material
Acknowledgments
We thank Dr. Hansgeorg Ernst (Fine Chemicals and Biocatalysis Research, BASF, Ludwigshafen, Germany) for providing APO10LA; Drs. Lynne M Ausman, Stefania Lamon-Fava, and Martin S Obin for their valuable comments; and Dr. Nirupa R Matthan and Ms. Sarah Gibeley for their assistance with the hepatic cholesterol measurements. In addition, we thank Dr. Donald E Smith of the Comparative Biology Unit as well as Ms. Shahin Sarkarati Smith and Ms. Stephanie Thea Leon Valliere of the Nutrition Evaluation Laboratory (Human Nutrition Research Center on Aging, Tufts University) for their assistance and support. BCI and X-DW designed the research; BCI, CL, and X-DW conducted the research; BCI analyzed the data; BCI, AHL, JvL, and X-DW wrote the paper; and X-DW had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: Abc, ATP-binding cassette transporter; Acad, acyl-CoA dehydrogenase; ACC, acetyl-CoA carboxylase; Acox1, acyl-CoA oxidase 1; AMPK, AMP-activated protein kinase; APO10LA, apo-10′-lycopenoic acid; BCO2, β-carotene-9′,10′-oxygenase; BCO2-KO, β-carotene-9′,10′-oxygenase-knockout; BW, body weight; CE, cholesteryl ester; Cidea, cell death–inducing DNA fragmentation factor, α subunit-like effector a; CVD, cardiovascular disease; Cyp, cytochrome P450 family; Cysc, cytochrome c; Dgat, diglyceride acyltransferase; Fatp4, FA transport protein 4; FC, free cholesterol; FoxO, Foxhead box protein O; HO, heme-oxygenase; HSFD, high saturated fat diet; HSFD+APO10LA, high saturated fat diet supplemented with apo-10′-lycopenoic acid; HSFD+Ly, high saturated fat diet supplemented with lycopene; HSFD+LyP, high saturated fat diet supplemented with lycopene placebo beadlet; Hsl, hormone-sensitive lipase; MAT, mesenteric adipose tissue; Mgll, monoacylglycerol lipase; NAFLD, nonalcoholic fatty liver disease; Pck, phosphoenolpyruvate carboxykinase; Pgc1α, PPARγ coactivator 1α; Pnpla2, patatin-like phospholipase domain containing 2; Prdm16, PR domain-containing 16; SCD1, stearoyl-CoA desaturase 1; SIRT1, sirtuin 1; Slc2a4, solute carrier family 2 type 4; TC, total cholesterol; Ucp3, uncoupling protein 3.
References
- 1.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol 2012;56:1384–91. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science 2011;332:1519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 2013;5:1544–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341–50. [DOI] [PubMed] [Google Scholar]
- 5.DeFilippis AP, Blaha MJ, Martin SS, Reed RM, Jones SR, Nasir K, Blumenthal RS, Budoff MJ. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2013;227:429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rissanen TH, Voutilainen S, Nyyssonen K, Salonen R, Kaplan GA, Salonen JT. Serum lycopene concentrations and carotid atherosclerosis: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 2003;77:133–8. [DOI] [PubMed] [Google Scholar]
- 7.Sesso HD, Liu S, Gaziano JM, Buring JE. Dietary lycopene, tomato-based food products and cardiovascular disease in women. J Nutr 2003;133:2336–41. [DOI] [PubMed] [Google Scholar]
- 8.Story EN, Kopec RE, Schwartz SJ, Harris GK. An update on the health effects of tomato lycopene. Annu Rev Food Sci Technol 2010;1:189–210. [DOI] [PMC free article] [PubMed]
- 9.Wang XD. Lycopene metabolism and its biological significance. Am J Clin Nutr 2012;96:1214S–22S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ip BC, Wang X-D. Non-alcoholic steatohepatitis and hepatocellular carcinoma: implications for lycopene intervention. Nutrients 2014;6:124–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erhardt A, Stahl W, Sies H, Lirussi F, Donner A, Haussinger D. Plasma levels of vitamin E and carotenoids are decreased in patients with nonalcoholic steatohepatitis (NASH). Eur J Med Res 2011;16:76–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem 2001;276:14110–6. [DOI] [PubMed] [Google Scholar]
- 13.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem 2006;281:19327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Apo-10'-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis 2007;28:1567–74. [DOI] [PubMed] [Google Scholar]
- 15.Chung J, Koo K, Lian F, Hu KQ, Ernst H, Wang XD. Apo-10'-lycopenoic acid, a lycopene metabolite, increases sirtuin 1 mRNA and protein levels and decreases hepatic fat accumulation in ob/ob mice. J Nutr 2012;142:405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford NA, Elsen AC, Erdman JW., Jr Genetic ablation of carotene oxygenases and consumption of lycopene or tomato powder diets modulate carotenoid and lipid metabolism in mice. Nutr Res 2013;33:733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford NA, Clinton SK, von Lintig J, Wyss A, Erdman JW. Loss of carotene-9′,10′-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice. J Nutr 2010;140:2134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ip BC, Hu KQ, Liu C, Smith DE, Obin MS, Ausman LM, Wang XD. Lycopene metabolite, apo-10′-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev Res (Phila) 2013;6:1304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lietz G, Oxley A, Boesch-Saadatmandi C, Kobayashi D. Importance of beta-carotene 15,15'-monooxygenase 1 (BCMO1) and beta-carotene 9′,10′-dioxygenase 2 (BCDO2) in nutrition and health. Mol Nutr Food Res 2012;56:241–50. [DOI] [PubMed] [Google Scholar]
- 20.Amengual J, Lobo GP, Golczak M, Li HNM, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J 2011;25:948–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ip BC, Liu C, Smith DE, Ausman LM, Wang X-D. High-refined-carbohydrate and high-fat diets induce comparable hepatic tumorigenesis in male mice. J Nutr 2014;144:647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma V, McNeill JH. To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol 2009;157:907–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008;22:659–61. [DOI] [PubMed] [Google Scholar]
- 24.Kopec RE, Riedl KM, Harrison EH, Curley RW, Jr, Hruszkewycz DP, Clinton SK, Schwartz SJ. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J Agric Food Chem 2010;58:3290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C-S, Chuang C-H, Hu M-L. Effects of lycopene supplementation on plasma and tissue lycopene levels in various rodent strains. Int J Vitam Nutr Res 2006;76:377–84. [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst 1999;91:317–31. [DOI] [PubMed] [Google Scholar]
- 27.Riso P, Visioli F, Grande S, Guarnieri S, Gardana C, Simonetti P, Porrini M. Effect of a tomato-based drink on markers of inflammation, immunomodulation, and oxidative stress. J Agric Food Chem 2006;54:2563–6. [DOI] [PubMed] [Google Scholar]
- 28.Ip BC, Liu C, Ausman LM, von Lintig J, Wang XD. Lycopene attenuated hepatic tumorigenesis via differential mechanisms depending on carotenoid cleavage enzyme in mice. Cancer Prev Res (Phila) 2014 Oct 7 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz HH, Poor CL, Wellman R, Erdman JW., Jr Concentrations of selected carotenoids and vitamin A in human liver, kidney and lung tissue. J Nutr 1991;121:1613–21. [DOI] [PubMed] [Google Scholar]
- 30.Matthan NR, Dillard A, Lecker JL, Ip B, Lichtenstein AH. Effects of dietary palmitoleic acid on plasma lipoprotein profile and aortic cholesterol accumulation are similar to those of other unsaturated fatty acids in the F1B golden Syrian hamster. J Nutr 2009;139:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts LD, Palma MJ, Calhoun S, Georgiadi A, Chen M-H, Ramachandran VS, Larson MG, Bouchard C, Rankinen T, Souza AL. b-aminoisobutyric acid induces browning of white fat and hepatic b-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 2014;19:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA 2008;105:9793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab 2009;9:327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponugoti B, Kim D-H, Xiao Z, Smith Z, Miao J, Zang M, Wu S-Y, Chiang C-M, Veenstra TD, Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem 2010;285:33959–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 2008;283:20015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen Z-P, O’Neill HM, Ford RJ, Palanivel R, O’Brien M. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med 2013;19:1649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marí M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, Fernandez-Checa JC, García-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF-and Fas-mediated steatohepatitis. Cell Metab 2006;4:185–98. [DOI] [PubMed] [Google Scholar]
- 38.Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK, Maulik N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med 2007;43:720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell 2007;28:91–106. [DOI] [PubMed] [Google Scholar]
- 40.Lu TT, Repa JJ, Mangelsdorf DJ. Orphan nuclear receptors as eLiXiRs and FiXeRs of sterol metabolism. J Biol Chem 2001;276:37735–8. [DOI] [PubMed] [Google Scholar]
- 41.Gouranton E, Thabuis C, Riollet C, Malezet-Desmoulins C, El Yazidi C, Amiot MJ, Borel P, Landrier JF. Lycopene inhibits proinflammatory cytokine and chemokine expression in adipose tissue. J Nutr Biochem 2011;22:642–8. [DOI] [PubMed] [Google Scholar]
- 42.Luvizotto RA, Nascimento AF, Imaizumi E, Pierine DT, Conde SJ, Correa CR, Yeum K-J, Ferreira ALA. Lycopene supplementation modulates plasma concentrations and epididymal adipose tissue mRNA of leptin, resistin and IL-6 in diet-induced obese rats. Br J Nutr 2013;110:1803–9. [DOI] [PubMed]
- 43.Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK, Bergman RN. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab 2005;288:E454–61. [DOI] [PubMed] [Google Scholar]
- 44.Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage–selective fat transplantation. Diabetes 2011;60:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, Buzzigoli E, Sironi AM, Cersosimo E, Ferrannini E. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007;133:496–506. [DOI] [PubMed] [Google Scholar]
- 46.Kotronen A, Yki-Järvinen H, Sevastianova K, Bergholm R, Hakkarainen A, Pietiläinen KH, Juurinen L, Lundbom N, Sørensen TI. Comparison of the relative contributions of intra-abdominal and liver fat to components of the metabolic syndrome. Obesity (Silver Spring) 2011;19:23–8. [DOI] [PubMed] [Google Scholar]
- 47.Martin G, Schoonjans K, Lefebvre A-M, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARα and PPARγ activators. J Biol Chem 1997;272:28210–7. [DOI] [PubMed] [Google Scholar]
- 48.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPAR [gamma] signaling and metabolism: the good, the bad and the future. Nat Med 2013;19:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010;285:7153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan H-L, Moran NE, Cichon MJ, Riedl KM, Schwartz SJ, Erdman JW, Pearl DK, Thomas-Ahner JM, Clinton SK. β-carotene-9′, 10′-oxygenase status modulates the impact of dietary tomato and lycopene on hepatic nuclear receptor, stress-, and metabolism-related gene expression in mice. J Nutr 2014;144:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaripheh S, Nara TY, Nakamura MT, Erdman JW. Dietary lycopene downregulates carotenoid 15, 15′-monooxygenase and PPAR-γ in selected rat tissues. J Nutr 2006;136:932–8. [DOI] [PubMed] [Google Scholar]
- 52.Kirsch R, Clarkson V, Shephard EG, Marais DA, Jaffer MA, Woodburne VE, Kirsch RE, Hall PDLM. Rodent nutritional model of non-alcoholic steatohepatitis: species, strain and sex difference studies. J Gastroenterol Hepatol 2003;18:1272–82. [DOI] [PubMed] [Google Scholar]
- 53.Djouadi F, Weinheimer CJ, Saffitz JE, Pitchford C, Bastin J, Gonzalez FJ, Kelly DP. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor alpha-deficient mice. J Clin Invest 1998;102:1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anezaki Y, Ohshima S, Ishii H, Kinoshita N, Dohmen T, Kataoka E, Sato W, Iizuka M, Goto T, Sasaki J. Sex difference in the liver of hepatocyte-specific Pten-deficient mice: a model of nonalcoholic steatohepatitis. Hepatol Res 2009;39:609–18. [DOI] [PubMed] [Google Scholar]
- 55.Medrikova D, Jilkova Z, Bardova K, Janovska P, Rossmeisl M, Kopecky J. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int J Obes (Lond) 2012;36:262–72. [DOI] [PubMed] [Google Scholar]
- 56.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–31. [DOI] [PubMed] [Google Scholar]
- 57.Zhu L, Brown WC, Cai Q, Krust A, Chambon P, McGuinness OP, Stafford JM. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes 2013;62:424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hewitt KN, Pratis K, Jones ME, Simpson ER. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology 2004;145:1842–8. [DOI] [PubMed] [Google Scholar]
- 59.Bryzgalova G, Lundholm L, Portwood N, Gustafsson J-Å, Khan A, Efendic S, Dahlman-Wright K. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab 2008;295:E904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab 2005;1:121–31. [DOI] [PubMed] [Google Scholar]
- 61.Stein EA, Dufour R, Gagne C, Gaudet D, East C, Donovan JM, Chin W, Tribble DL, McGowan M. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation 2012;126:2283–92. [DOI] [PubMed] [Google Scholar]
- 62.Lindqvist A, Andersson S. Biochemical properties of purified recombinant human β-carotene 15, 15′-monooxygenase. J Biol Chem 2002;277:23942–8. [DOI] [PubMed] [Google Scholar]
- 63.dela Seña C, Narayanasamy S, Riedl KM, Curley RW, Schwartz SJ, Harrison EH. Substrate specificity of purified recombinant human β-carotene 15, 15′-oxygenase (BCO1). J Biol Chem 2013;288:37094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX. Identification, expression, and substrate specificity of a mammalian β-carotene 15, 15′-dioxygenase. J Biol Chem 2001;276:6560–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.