Abstract
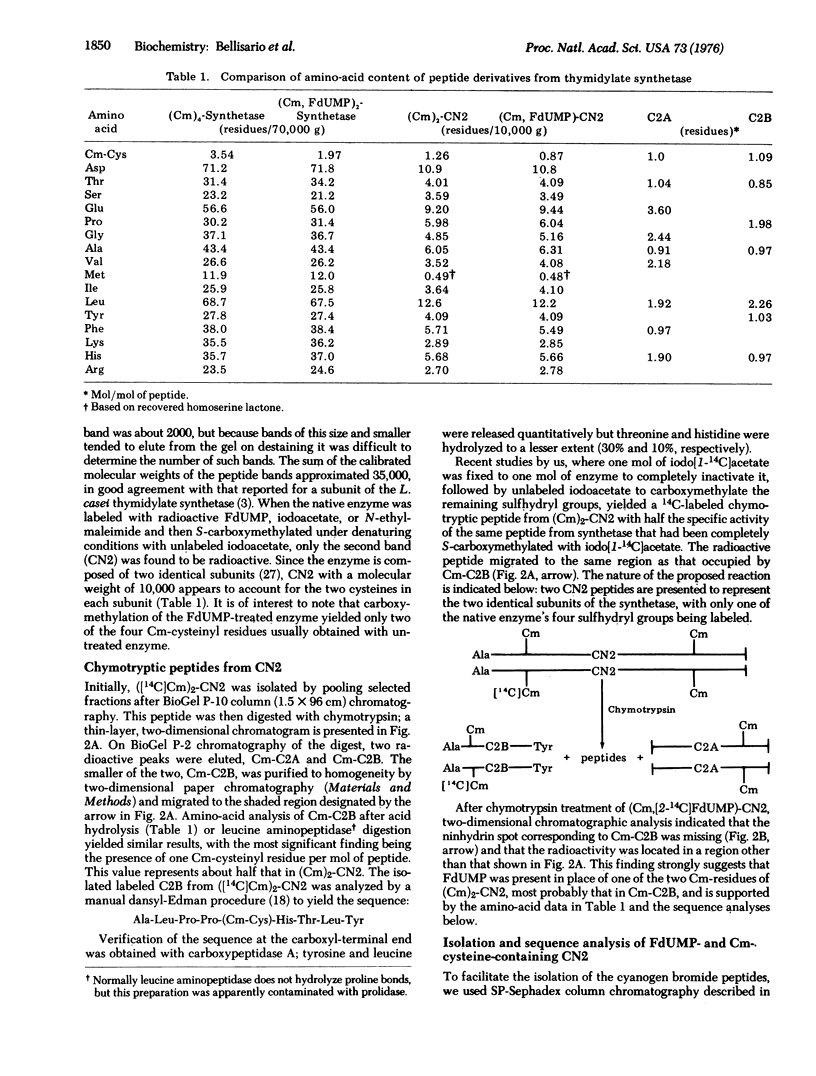
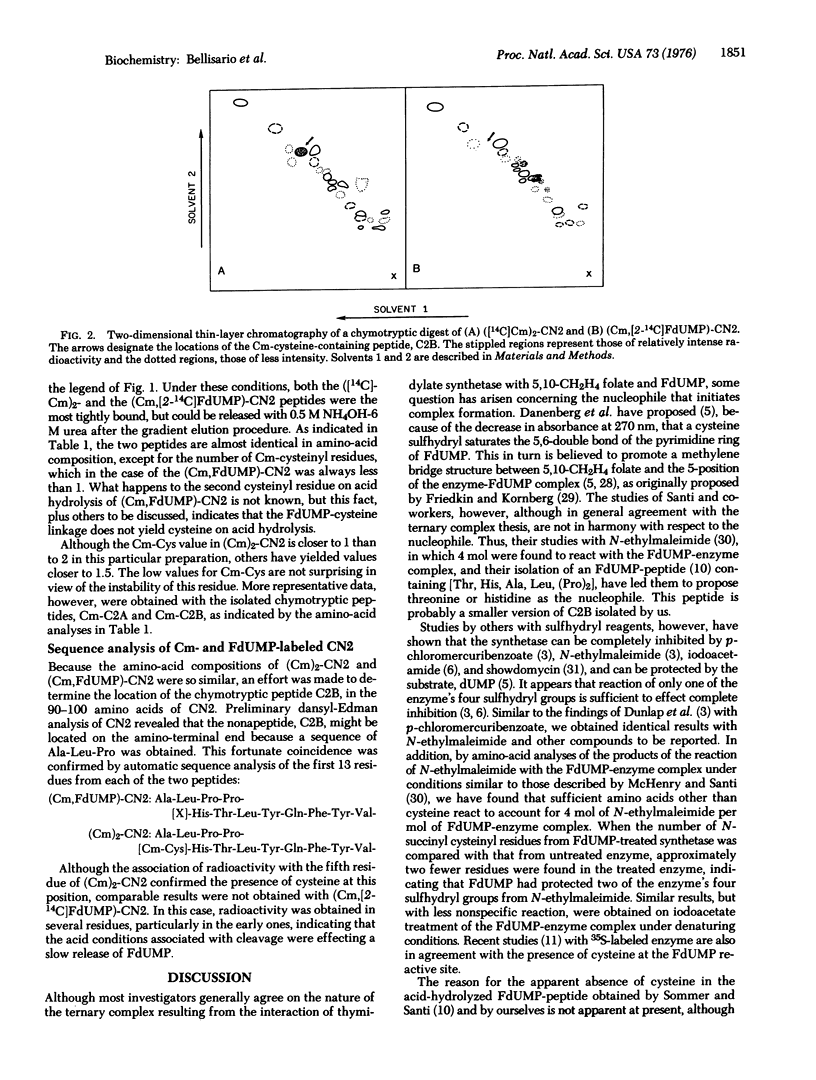
Cyanogen bromide treatment of thymidylate synthetase of Lactobacillus casei, which had been converted to a ternary complex with [2-14c] FdUMP and 5,10-methylene-tetrahydrofolate followed by S-carboxymethylation, yielded at least four visible peptide bands, the largest with a molecular weight of about 13,000, on polyacrylamide gel electrophoresis in sodium dodecyl sulfate-urea. Identical results were obtained with enzyme that had all four of its cysteinyl residues S-carboxymethylated with iodo [I-14C] acetate in the absence of FdUMP and cofactor. In each case, only the second band from the top of the gel (CN2), with an approximate molecular weight of 10,000= was labeled. Analysis of CN2 that had been labeled with [2-14C] FdUMP and nonradioactive iodoacetate and of that labeled only with iodo[1-14C] acetate revealed that their amino-acid contents were almost identical except for the presence of two S-carboxymethyl (Cm)-cysteinyl residues in the latter peptide and only one in FdUMP-CN2. A nonapeptide was isolated from (Cm)2-CN2 after chymotrypsin digestion that contained the following sequence by dansyl-Edman analysis: Ala-Leu-Pro-Pro-[Cm-Cys]-His-Thr-Leu-Tyr. This peptide was found to be located on the NH2-terminal end of CN2. Automatic sequence analysis of the first 13 residues of (Cm)2-CN2 and of the FdUMP-containing CN2 yielded identical results except for the fifth, or cysteinyl, residue, which could not be identified in the latter peptide. These findings strongly suggest that FdUMP is linked to a cysteinyl residue in thymidylate synthetase that has been inactivated irreversibly by this nucleotide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellisario R., Carlsen R. B., Bahl O. P. Human chorionic gonadotropin. Linear amino acid sequence of the alpha subunit. J Biol Chem. 1973 Oct 10;248(19):6796–6809. [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Bruton C. J., Hartley B. S. Chemical studies on methionyl-tRNA synthetase from Escherichia coli. J Mol Biol. 1970 Sep 14;52(2):165–178. doi: 10.1016/0022-2836(70)90023-9. [DOI] [PubMed] [Google Scholar]
- Danenberg P. V., Langenbach R. J., Heidelberger C. Structures of reversible and irreversible complexes of thymidylate synthetase and fluorinated pyrimidine nucleotides. Biochemistry. 1974 Feb 26;13(5):926–933. doi: 10.1021/bi00702a016. [DOI] [PubMed] [Google Scholar]
- Dunlap R. B., Harding N. G., Huennekens F. M. Thymidylate synthetase from amethopterin-resistant Lactobacillus casei. Biochemistry. 1971 Jan 5;10(1):88–97. doi: 10.1021/bi00777a014. [DOI] [PubMed] [Google Scholar]
- Galivan J. H., Maley G. F., Maley F. Factors affecting substrate binding in Lactobacillus casei thymidylate synthetase as studied by equilibrium dialysis. Biochemistry. 1976 Jan 27;15(2):356–362. doi: 10.1021/bi00647a018. [DOI] [PubMed] [Google Scholar]
- Galivan J. H., Maley G. F., Maley F. The effect of substrate analogs on the circular dichroic spectra of thymidylate synthetase from Lactobacillus casei. Biochemistry. 1975 Jul 29;14(15):3338–3344. doi: 10.1021/bi00686a008. [DOI] [PubMed] [Google Scholar]
- Howard S. M., Pierce J. G. The tryptic glycopeptides of bovine thyrotropin. Their composition and similarities to those of luteinizing hormone. J Biol Chem. 1969 Dec 10;244(23):6468–6476. [PubMed] [Google Scholar]
- Inglis A. S., Nicholls P. W. Identification of phenylthiohydantoins of amino acids by thin-layer chromatography. J Chromatogr. 1973 May 16;79:344–346. doi: 10.1016/s0021-9673(01)85310-3. [DOI] [PubMed] [Google Scholar]
- Kalman T. I. Glutathione-catalyzed hydrogen isotope exchange at position 5 of uridine. A model for enzymic carbon alkylation reactions of pyrimidines. Biochemistry. 1971 Jun 22;10(13):2567–2573. doi: 10.1021/bi00789a024. [DOI] [PubMed] [Google Scholar]
- Kalman T. I. Inhibition of thymidylate synthetase by showdomycin and its 5'-phosphate. Biochem Biophys Res Commun. 1972 Nov 15;49(4):1007–1013. doi: 10.1016/0006-291x(72)90312-9. [DOI] [PubMed] [Google Scholar]
- Kuhn R. W., Walsh K. A., Neurath H. Isolation and partial characterization of an acid carboxypeptidase from yeast. Biochemistry. 1974 Sep 10;13(19):3871–3877. doi: 10.1021/bi00716a008. [DOI] [PubMed] [Google Scholar]
- Langenbach R. J., Danenberg P. V., Heidelberger C. Thymidylate synthetase: mechanism of inhibition by 5-fluoro-2'-deoxyuridylate. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1565–1571. doi: 10.1016/0006-291x(72)90892-3. [DOI] [PubMed] [Google Scholar]
- Langley K. E., Fowler A. V., Zabin I. Amino acid sequence of beta-galactosidase. IV. Sequence of an alpha-complementing cyanogen bromide peptide, residues 3 to 92. J Biol Chem. 1975 Apr 10;250(7):2587–2592. [PubMed] [Google Scholar]
- Leary R. P., Beaudette N., Kisliuk R. L. Interaction of deoxyuridylate with thymidylate synthetase. J Biol Chem. 1975 Jul 10;250(13):4864–4868. [PubMed] [Google Scholar]
- Leary R. P., Kisliuk R. L. Crystalline thymidylate synthetase from dichloromethotrexate resistant Lactobacillus casei. Prep Biochem. 1971 Jan;1(1):47–54. doi: 10.1080/00327487108081929. [DOI] [PubMed] [Google Scholar]
- Loeble R. B., Dunlap R. B. Characterization of the subunits of thymidylate synthetase. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1671–1677. doi: 10.1016/0006-291x(72)90535-9. [DOI] [PubMed] [Google Scholar]
- Lorenson M. Y., Maley G. F., Maley F. The purification and properties of thymidylate synthetase from chick embryo extracts. J Biol Chem. 1967 Jul 25;242(14):3332–3344. [PubMed] [Google Scholar]
- McHenry C. S., Santi D. V. A sulfhydryl group is not the covalent catalyst in the thymidylate synthetase reaction. Biochem Biophys Res Commun. 1974 Mar 15;57(1):204–208. doi: 10.1016/s0006-291x(74)80377-3. [DOI] [PubMed] [Google Scholar]
- STEERS E., Jr, CRAVEN G. R., ANFINSEN C. B., BETHUNE J. L. EVIDENCE FOR NONIDENTICAL CHAINS IN THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2478–2484. [PubMed] [Google Scholar]
- Santi D. V., Brewer C. F. Model studies of thymidylate synthetase. Intramolecular catalysis of 5-hydrogen exchange and 5-hydroxymethylation of 1-substituted uracils. Biochemistry. 1973 Jun 19;12(13):2416–2424. doi: 10.1021/bi00737a008. [DOI] [PubMed] [Google Scholar]
- Santi D. V., Brewer C. F. Model studies of thymidylate synthetase. Neighboring-group facilitation of electrophilic substitution reactions of uracil furanosides. J Am Chem Soc. 1968 Oct 23;90(22):6236–6238. doi: 10.1021/ja01024a063. [DOI] [PubMed] [Google Scholar]
- Santi D. V., McHenry C. S. 5-Fluoro-2'-deoxyuridylate: covalent complex with thymidylate synthetase. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1855–1857. doi: 10.1073/pnas.69.7.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi D. V., McHenry C. S., Sommer H. Mechanism of interaction of thymidylate synthetase with 5-fluorodeoxyuridylate. Biochemistry. 1974 Jan 29;13(3):471–481. doi: 10.1021/bi00700a012. [DOI] [PubMed] [Google Scholar]
- Schachner M., Zillig W. Fingerprint maps of tryptic peptides from subunits of Escherichia coli and T 4 -modified DNA-dependent RNA polymerases. Eur J Biochem. 1971 Oct 26;22(4):513–519. doi: 10.1111/j.1432-1033.1971.tb01571.x. [DOI] [PubMed] [Google Scholar]
- Sommer H., Santi D. V. Purification and amino acid analysis of an active site peptide from thymidylate synthetase containing covalently bound 5-fluoro-2'-deoxyuridylate and methylenetetrahydrofolate. Biochem Biophys Res Commun. 1974 Apr 8;57(3):689–695. doi: 10.1016/0006-291x(74)90601-9. [DOI] [PubMed] [Google Scholar]
- Summers M. R., Smythers G. W., Oroszlan S. Thin-layer chromatography of sub-nanomole amounts of phenylthiohydantoin (PTH) amino acids on polyamide sheets. Anal Biochem. 1973 Jun;53(2):624–628. doi: 10.1016/0003-2697(73)90114-0. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Kato T., Takenishi T. A novel method for phosphorylation of nucleosides to 5'-nucleotides. Tetrahedron Lett. 1967 Dec;50:5065–5068. doi: 10.1016/s0040-4039(01)89915-9. [DOI] [PubMed] [Google Scholar]



