Abstract
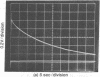
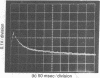
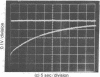
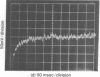
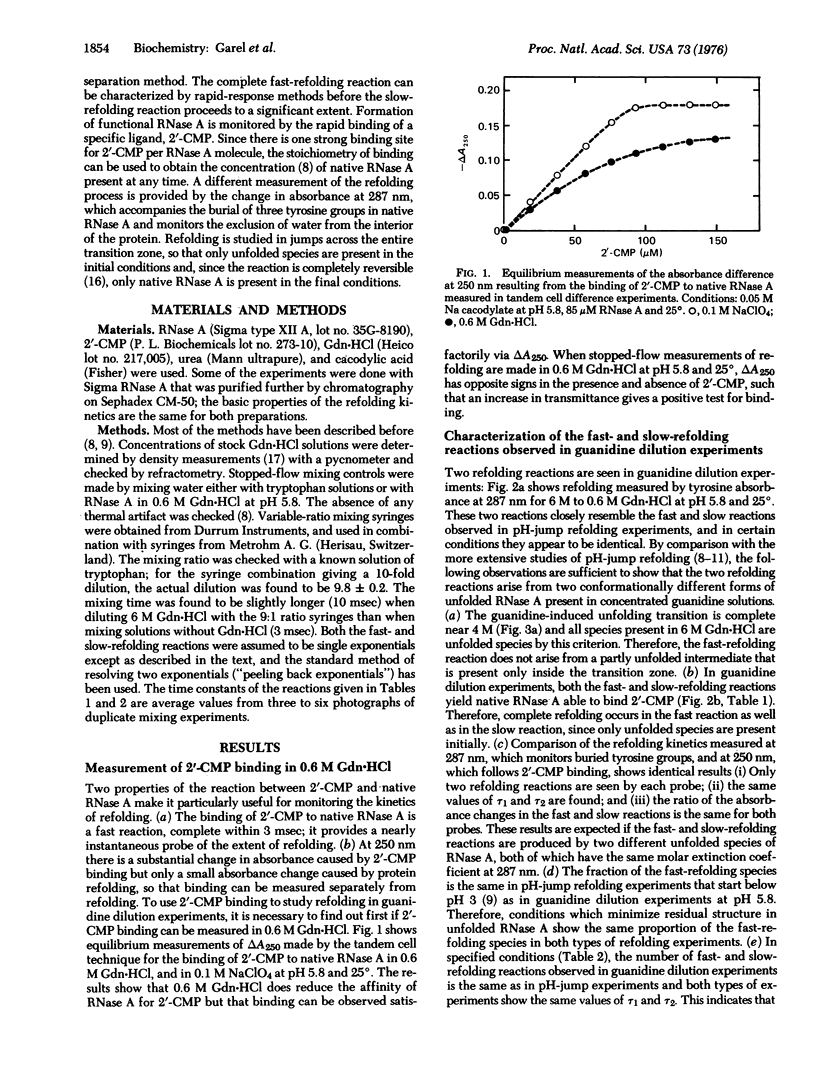
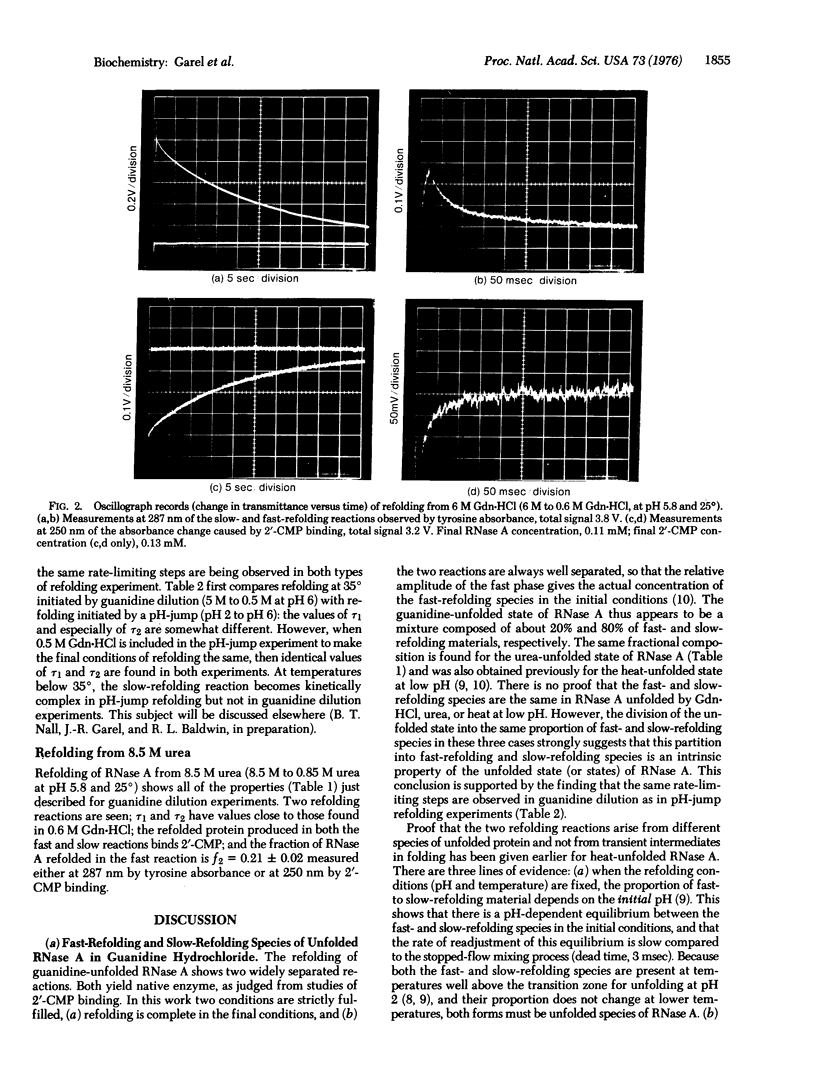
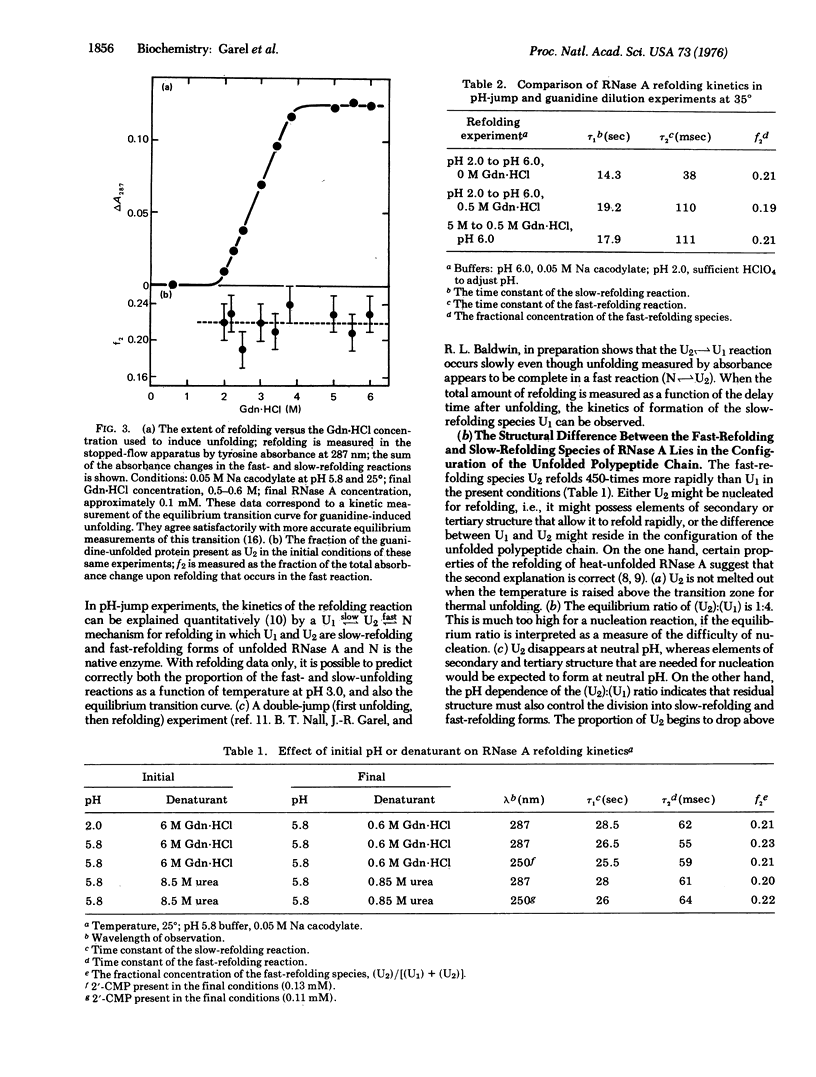
The kinetics of the refolding reaction of ribonuclease A from high concentrations of guanidine hydrochloride or urea are biphasic, and show two refolding reactions whose rates differ 450-fold at pH 5.8 and 25 degrees. Measurements of cytidine 2'-phosphate binding during refolding, after stopped-flow dilution of guanidine hydrochloride (Gdn.HCl) or urea, show that functional bovine pancreatic ribonuclease A (RNase A; ribonucleate 3'-pyrimidino-oligonucleotidohydrolase, EC 3.1.4.22) is formed in both the fast and slow phases of the refolding process. We conclude that the guanidine-unfolded state of RNase A is an equilibrium mixture of fast- and slow-refolding species, as was found previously for the heat-unfolded state at low pH. The fraction of the fast-refolding species in guanidine or urea-unfolded RNase A is the same as that in the heat-unfolded protein at pH 2. Previous work has shown that the fast-refolding species disappears as the pH is raised from 3 to 5 for heat-unfolded RNase A. This pH effect is not present in refolding from concentrated Gdn.HCl solutions: the same proportion of the fast-refolding species is found from pH 2 to pH 6, and also from 2 M to 6 M Gdn.HCl at pH 5.8. We conclude that the same proportion of the fast-refolding species is present at equilibrium whenever the residual structure in unfolded RNase A is reduced to a low level, and that the structural difference between the fast-refolding and slow-refolding species of RNase A lies in the configuration of the random coil polypeptide chain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aune K. C., Salahuddin A., Zarlengo M. H., Tanford C. Evidence for residual structure in acid- and heat-denatured proteins. J Biol Chem. 1967 Oct 10;242(19):4486–4489. [PubMed] [Google Scholar]
- Brandts J. F., Halvorson H. R., Brennan M. Consideration of the Possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry. 1975 Nov 4;14(22):4953–4963. doi: 10.1021/bi00693a026. [DOI] [PubMed] [Google Scholar]
- Fermandjian S., Tran-Dinh, Savrda J., Sala E., Mermet-Bouvier R., Bricas E., Fromageot P. 13C-nuclear magnetic resonance studies of 85% 13C-enriched amino acids and small peptides. pH effects on the chemical shifts, coupling constants, kinetics of cis-trans isomerisation and conformation aspects. Biochim Biophys Acta. 1975 Aug 13;399(2):313–338. doi: 10.1016/0304-4165(75)90261-5. [DOI] [PubMed] [Google Scholar]
- Garel J. R., Baldwin R. L. Both the fast and slow refolding reactions of ribonuclease A yield native enzyme. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3347–3351. doi: 10.1073/pnas.70.12.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel J. R., Baldwin R. L. The heat-unfolded state of ribonuclease A is an equilibrium mixture of fast and slow refolding species. J Mol Biol. 1975 Jun 5;94(4):611–620. doi: 10.1016/0022-2836(75)90325-3. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J., Baldwin R. L. A quantitative treatment of the kinetics of the folding transition of ribonuclease A. Biochemistry. 1976 Apr 6;15(7):1462–1473. doi: 10.1021/bi00652a017. [DOI] [PubMed] [Google Scholar]
- Henkens R. W., Turner S. R. Kinetics of refolding of guanidine hydrochloride denatured cytochrome c. Temperature dependence. Biochemistry. 1973 Apr 10;12(8):1618–1621. doi: 10.1021/bi00732a024. [DOI] [PubMed] [Google Scholar]
- Ikai A., Fish W. W., Tanford C. Kinetics of unfolding and refolding of proteins. II. Results for cytochrome c. J Mol Biol. 1973 Jan 10;73(2):165–184. doi: 10.1016/0022-2836(73)90321-5. [DOI] [PubMed] [Google Scholar]
- SCHWARZ G. ON THE KINETICS OF THE HELIX-COIL TRANSITION OF POLYPEPTIDES IN SOLUTION. J Mol Biol. 1965 Jan;11:64–77. doi: 10.1016/s0022-2836(65)80171-1. [DOI] [PubMed] [Google Scholar]
- Sachs D. H., Schechter A. N., Eastlake A., Anfinsen C. B. An immunologic approach to the conformational equilibria of polypeptides. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3790–3794. doi: 10.1073/pnas.69.12.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin A., Tanford C. Thermodynamics of the denaturation of ribonuclease by guanidine hydrochloride. Biochemistry. 1970 Mar 17;9(6):1342–1347. doi: 10.1021/bi00808a007. [DOI] [PubMed] [Google Scholar]
- Tanford C., Aune K. C., Ikai A. Kinetics of unfolding and refolding of proteins. 3. Results for lysozyme. J Mol Biol. 1973 Jan 10;73(2):185–197. doi: 10.1016/0022-2836(73)90322-7. [DOI] [PubMed] [Google Scholar]
- Tanford C., Kawahara K., Lapanje S., Hooker T. M., Jr, Zarlengo M. H., Salahuddin A., Aune K. C., Takagi T. Proteins as random coils. 3. Optical rotatory dispersion in 6 M guanidine hydrochloride. J Am Chem Soc. 1967 Sep 13;89(19):5023–5029. doi: 10.1021/ja00995a034. [DOI] [PubMed] [Google Scholar]
- Tanford C., Kawahara K., Lapanje S. Proteins in 6-M guanidine hydrochloride. Demonstration of random coil behavior. J Biol Chem. 1966 Apr 25;241(8):1921–1923. [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. An acid induced conformational transition of denatured cytochrome c in urea and guanidine hydrochloride solutions. Biochemistry. 1975 Apr 8;14(7):1542–1547. doi: 10.1021/bi00678a031. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y., Baldwin R. L., Elson E. L. Properties of the refolding and unfolding reactions of ribonuclease A. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1809–1812. doi: 10.1073/pnas.69.7.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazgan A., Henkens R. W. Role of zinc (II) in the refolding of guanidine hydrochloride denatured bovine carbonic anhydrase. Biochemistry. 1972 Mar 28;11(7):1314–1318. doi: 10.1021/bi00757a031. [DOI] [PubMed] [Google Scholar]