Abstract
For many long-lived mammalian species, extended maternal investment has a profound effect on offspring integration in complex social environments. One component of this investment may be aiding young in aggressive interactions, which can set the stage for offspring social position later in life. Here we examined maternal effects on dyadic aggressive interactions between immature (<12 years) chimpanzees. Specifically, we tested whether relative maternal rank predicted the probability of winning an aggressive interaction. We also examined maternal responses to aggressive interactions to determine whether maternal interventions explain interaction outcomes. Using a 12-year behavioural data set (2000–2011) from Gombe National Park, Tanzania, we found that relative maternal rank predicted the probability of winning aggressive interactions in male–male and male–female aggressive interactions: offspring were more likely to win if their mother outranked their opponent’s mother. Female–female aggressive interactions occurred infrequently (two interactions), so could not be analysed. The probability of winning was also higher for relatively older individuals in male–male interactions, and for males in male–female interactions. Maternal interventions were rare (7.3% of 137 interactions), suggesting that direct involvement does not explain the outcome for the vast majority of aggressive interactions. These findings provide important insight into the ontogeny of aggressive behaviour and early dominance relationships in wild apes and highlight a potential social advantage for offspring of higher-ranking mothers. This advantage may be particularly pronounced for sons, given male philopatry in chimpanzees and the potential for social status early in life to translate more directly to adult rank.
Keywords: aggression, maternal effect, ontogeny, Pan troglodytes schweinfurthii, wild chimpanzee
For many animals, high rank affords priority of access to resources and ultimately influences increased survivorship and reproduction (reviewed in: Clutton-Brock, 1988; Ellis, 1995; Majolo et al., 2012). While these benefits are well characterized for adults, the adaptive value of dominance status among immature individuals is poorly understood. One hypothesis is that the formation of dominant–subordinate relationships in early life sets the stage for social status and reproductive success in adulthood. Mothers may therefore increase their inclusive fitness by aiding young offspring in agonistic encounters.
Indeed, maternal intervention on behalf of an offspring is well documented in several species (reviewed in: Maestripieri, 2009; Mateo, 2009) and often results in the offspring outranking his or her competitor (e.g. vervet monkeys, Cercopithecus aethiops: Fairbanks & McGuire, 1985; rhesus monkeys, Macaca mulatta: Datta 1988; spotted hyaenas, Crocuta crocuta: Engh et al., 2000). When mothers aid their offspring in aggressive interactions, maternal intervention can be a mechanism by which matrilineal hierarchies are maintained within social groups (e.g. Harcourt & Stewart, 1987; Pereira, 1995). Maternal interventions may thus be particularly influential for the philopatric sex because social status early in life can translate more directly to adult rank. However, maternal support can also afford immediate benefits to offspring of the dispersing sex via protection and/or access to resources. More broadly, long-term fitness benefits associated with maternal effects in early life have been documented for offspring of the dispersing sex in several social species including spotted hyaenas (Hofer & East, 2003; Honer et al., 2010) and yellow baboons, Papio cynocephalus (Altmann & Alberts, 2005; Onyango et al., 2008).
Chimpanzees, Pan troglodytes, live in multimale, multifemale communities characterized by male philopatry (Goodall, 1986; Nishida & Hiraiwa-Hasegawa, 1987; Pusey, 1979) and a fission–fusion social organization (Goodall, 1986; Nishida, 1968). Despite nutritional independence at weaning between the ages of 3 and 5 years, offspring continue to travel with their mother for several years thereafter (Pusey, 1983, 1990). Maternal effects can be realized across this long period of dependency. For example, a recent study demonstrated lower survivorship for offspring that were orphaned after weaning (Nakamura et al., 2014). Similar patterns have been observed in other long-lived mammals, including killer whales, Orcinus orca (Foster et al., 2012) and red deer, Cervus elaphus (Andres et al., 2013).
Although both male and female chimpanzees rely upon prolonged maternal investment, emerging evidence has highlighted sex biases in some components of maternal care. For instance, mothers with sons are more gregarious than mothers with daughters (Murray et al., in press). Male infants take advantage of these social opportunities by playing and grooming with significantly more individuals than female infants (Lonsdorf, Anderson, et al., 2014; Murray et al., in press). Together, these studies emphasize the interaction between maternal effects and sex differences in offspring prosocial development. The extent to which mothers influence aggressive interactions in immature offspring, particularly with peers, has not yet been quantified. Furthermore, it is unknown whether maternal effects on aggressive outcomes are more influential for chimpanzee sons or daughters, despite adult sex differences in dispersal and propensity for social interaction (see below).
For many primates, peer interactions provide opportunities to learn essential social and behavioural skills (Pagel & Harvey, 2002; Pereira & Fairbanks, 2002). Early establishment of play and grooming partners may be especially important for male chimpanzees, which remain in their natal communities and form long-lasting bonds that function in dominance rank acquisition, cooperative defence and communal hunting (Gilby & Wrangham, 2008; Mitani, 2009). Adult male dominance rank is correlated with reproductive success, with several studies demonstrating that alpha males sire a disproportionate number of offspring (Boesch et al., 2006; Newton-Fisher et al., 2010; Wroblewski et al., 2009). In comparison to males, overt female–female aggression is rare in chimpanzees (Goodall 1986; reviewed in Murray 2007). Nevertheless, female dominance rank likewise correlates with reproductive success (Emery Thompson et al., 2007; Pusey et al., 1997). Despite the importance of dominance rank later in life, relatively little is known about dominance interactions among immature chimpanzees. However, earlier reports indicate that mothers sometimes support their offspring in aggressive interactions (Goodall, 1968, 1971; Pusey, 1983).
Here, we examined the influence of maternal rank on aggressive interactions between immature chimpanzees to test our overarching hypothesis that high maternal rank benefits immature offspring in competitive contests. Specifically, we tested whether relative maternal rank predicts the probability of an immature offspring winning an aggressive interaction. In addition, we evaluated the behavioural response of mothers following aggressive interactions involving their offspring to determine whether direct interventions explain interaction outcomes. Recognizing the importance of maternal help in offspring contests and its potential implications for rank ‘inheritance’ (e.g. Harcourt & Stewart, 1987), we predicted that higher-ranking mothers would be more likely than lower-ranking mothers to intervene in aggressive interactions involving their offspring.
METHODS
This study was part of long-term research on wild eastern chimpanzees (P. t. schweinfurthii) living in the Kasekela community of Gombe National Park, Tanzania. All chimpanzees within the study population were individually identifiable by field researchers of the Gombe Stream Research Centre, and each mother with her youngest dependent offspring (i.e. ‘family group’) in the community was targeted for detailed observations at least once per month. Each observation day, researchers recorded detailed behavioural observations on activity type and social partners for the focal mother and her youngest dependent offspring using 1 min scans (Altmann, 1974). Observation began when the focal mother descended from her sleeping nest or was first located, and continued (at maximum) until the mother ascended into a sleeping site in the evening. The data presented here were collected from January 2000 through December 2011, a time period for which details on aggressive interactions have been extracted from field notes originally recorded in Swahili. During the study period, the community contained 48–62 total individuals, 19–26 of which were immatures. We defined immatures as individuals younger than 12 years, consistent with previous research on our study population (e.g. Markham et al., 2014; Murray et al., 2006; Murray et al., 2009). This age criterion coincides with current, unpublished records for the youngest age of female emigration (9.9 years), first birth for a female (11.1 years) and first fathering of an offspring by a male (12.9 years).
Aggressive Interactions and Maternal Interventions
Data on aggressive interactions among all chimpanzees were recorded ad libitum. Aggressive interactions are typically noisy and conspicuous events, and thus the vast majority of interactions are readily noticeable to field observers; our analytical emphasis on the outcome of observed aggressive interactions (see below) minimizes the effect of any undetected subtle (and therefore atypical) agonistic encounters. Only decided, dyadic aggressive interactions between immature individuals were included in analyses (resulting in the exclusion of two interactions that had undecided outcomes). Extending Goodall (1986), decided aggressive interactions were defined as contests in which only one individual (the ‘winner’) expressed aggressive behaviour (directed threat, display, chase or attack) towards another individual and/or in which only one individual (the ‘loser’) expressed clear submissive behaviour (flee, scream or cry) in response to another individual. We did not include play fighting, which can be distinguished on the basis of facial expressions (e.g. relaxed jaw versus bared teeth). Field assistants regularly record play fighting for the focal of a follow on the 1 min scan but not on an ad libitum basis for all immatures in the party. We therefore did not include play fighting in our analyses, although future work should investigate its relevance to dominance interactions (Paquette, 1994).
When a dyad interacted aggressively more than once on a single observation day, the average ± SE duration between consecutive aggressive interactions was 51 ± 10.9 min (range 1–335 min). We considered successive aggressive interactions as a single, ongoing event when they occurred within 10 min of each other. Only one aggressive interaction occurring within 10 min was a ‘reversal’ (i.e. aggressor–recipient roles were inconsistent in the consecutive aggressive interactions); in this case, we included only the outcome of the final interaction in our analyses. Researchers recorded descriptive notes of maternal intervention in the immediate aftermath of immature aggressive interactions. Because data on aggressive interactions were collected on all immatures in the party (i.e. not limited to members of the focal family group), detailed observations of participant behaviour prior to the interaction were not available for analysis.
Maternal Rank
We categorized mothers as high, middle or low ranking based upon their position in the female dominance hierarchy. The dominance hierarchy was calculated from the directionality of female–female pant-grunts observed during 2-year time periods (e.g. 2000–2001–2002– 2003– 2004–2005). Pant-grunt vocalizations are formal indicators of subordination in chimpanzees (Bygott, 1979). All pant-grunts recorded during follows of adults as part of the long-term study (Goodall, 1986; Wilson, 2012), as well as follows of mothers and infants (described above) and student projects conducted during our study period were consolidated and provided to us by the Jane Goodall Institute Research Center at Duke University (Durham, NC, U.S.A.).
We used dyadic pant-grunts to calculate the modified David’s score (MDS) (de Vries et al., 2006). Only pant-grunts between females that were sexually mature adults were included. For analyses, high-ranking females were categorized as individuals with an MDS greater than 0.5 SD above the mean score; low-ranking females were categorized as individuals with an MDS less than 0.5 SD below the mean score. All other females were considered middle ranking. Given the relationship between age and relative rank (Goodall, 1986; Murray et al., 2006; Nishida, 1989; Pusey et al., 1997), female categorical rank is usually stable over long periods. Therefore, if no pant-grunts involving a particular female were observed in a given 2-year period, we assigned the female the same categorical rank held in the previous period.
Ethical Note
The observational protocol adhered to all welfare and legal requirements of the host country (Tanzania) and was approved and permitted through the Tanzania Wildlife Research Institute, Tanzania National Parks and Tanzania’s Commission on Science and Technology, and adhered to the ASAB/ABS Guidelines for the Use of Animals in Research.
Statistical Analyses
Rates of aggressive interaction were calculated as the number of decided, dyadic aggressive interactions involving a given individual divided by observation time of that individual. Rates of winning aggressive interaction were calculated using the subset of aggressive interactions that a given individual won. Data were pooled for an individual over all observations from birth through 12 years. However, only 2-year age classes prior to adulthood (e.g. 0–2, 2–4, 4–6 years) when a given individual was observed for at least 1000 min (~16.6 h) were included in calculations, a criterion employed to minimize zero values given the overall rarity of aggressive interactions. To test for determinants of winning aggressive interactions, we randomly selected one individual from each aggressive interaction as the focal subject for analysis. We used generalized estimating equations (GEEs) with an independent correlation structure to test whether relative age and difference in maternal rank predicted the interaction’s outcome (i.e. whether the focal individual won or lost). GEEs can control for repeated sampling of subjects and/or time periods (Diggle et al., 2002), and are a suitable methodology for analysing discrete and correlated data. Dyad identity was entered as a repeated measure in the model to account for multiple interactions between the same two individuals. Relative age was calculated as the age of the focal minus the age of the opponent on the interaction date. Differences in maternal ranks were defined as (1) the focal’s mother was higher ranking than the opponent’s mother, (2) the mothers had the same rank, or (3) the focal’s mother was lower ranking than the opponent’s mother. Aggressive interactions between maternal siblings (N = 30) and involving individual(s) whose mother’s rank was unknown (N = 10) were excluded from this analysis. For the remaining interactions, we analysed male–male (N = 53) and male–female interactions (N = 42) in two separate models, and included focal sex as an additional predictor variable in our model for mixed-sex interactions; female–female aggressive interactions were observed too rarely to permit statistical analyses (see below). Both mothers were present in the same party as their offspring for the majority of the analysed aggressive interactions (89%; N = 85), prohibiting the analytical opportunity to test whether maternal presence influenced the outcome of aggressive interactions. All statistical tests were performed in SPSS 22.0 (SPSS Inc., Chicago, IL, U.S.A.). The alpha value for statistical significance was set to 0.05 for all analyses.
RESULTS
We observed 137 aggressive interactions involving immature chimpanzees (N = 17 males, N = 16 females, N = 1 unsexed individual). Seventy-three (53.3%) of these aggressive interactions were between an immature male and immature female, 61 (44.5%) aggressive interactions were between two immature males, and two (1.5%) aggressive interactions were between two immature females. One aggressive interaction (0.7%) was observed between an immature male and an immature infant who died before its sex was positively determined. Males (N = 44) and females (N = 30) had the same mean rate of aggressive interactions per 100 h (0.5 ± 0.16 SE). However, the mean ± SE rate of winning aggressive interactions/100 h for males (0.3 ± 0.11) was three-fold higher than that observed for females (0.1 ± 0.04).
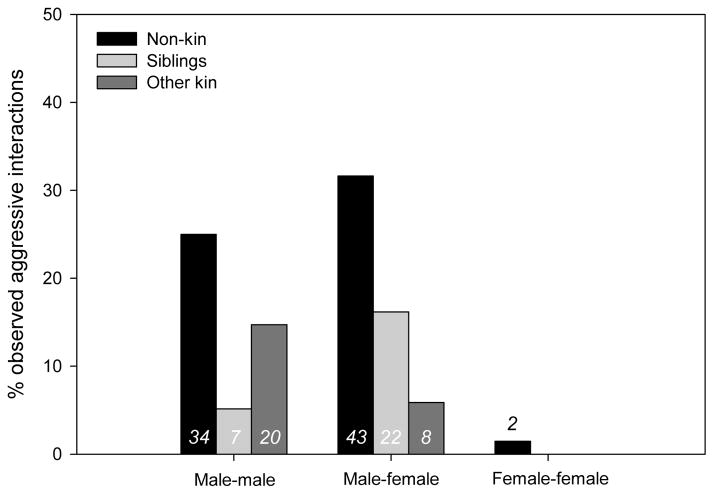
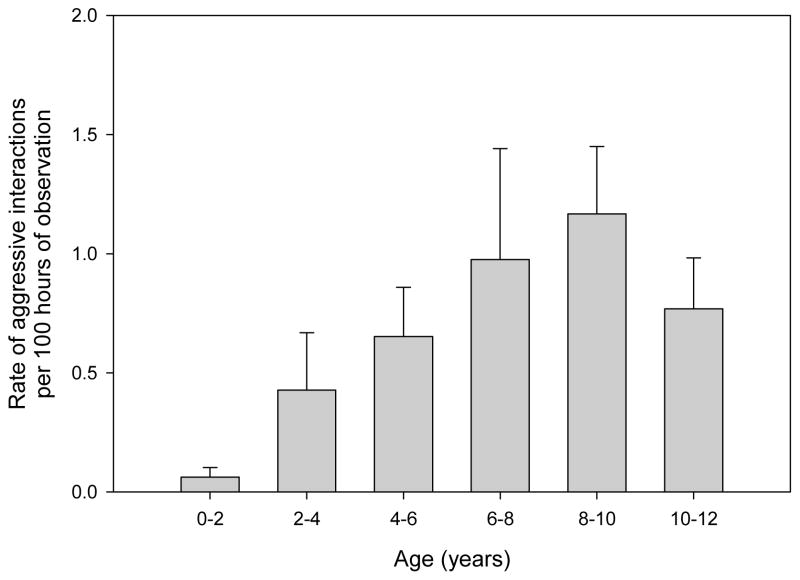
The majority of aggressive interactions (N = 79; 57.7%), regardless of participant’s sex, were between individuals that were not maternal kin (Fig. 1). The average ± SE age of an immature involved in an aggressive interaction was 6.7 ± 0.17 years (range 0.02–11.78 years), but aggression rates increased through age 10 years; see Fig. 2 for the rates of aggressive interaction by age class. The average ± SE age difference between the participants was 3.4 ± 0.16 years (range 0.04–9.20 years). For additional details on the nature of aggressive interactions, Table 1 provides a summary of the percentage of aggressive interactions involving direct contact (e.g. hitting, kicking, biting) by the aggressor; examples of noncontact aggression include chases, displays and threats.
Figure 1.
Percentage of total observed aggressive interactions between immature (<12 years) chimpanzees by dyad sex and kinship. Note that kinship is traced only through maternal line. Numbers near bars indicate the number of observations. One aggressive interaction between a brother and his sibling of unknown sex was not included.
Figure 2.
Mean ± SE rates of aggressive interactions between immature (<12 years) chimpanzees by age. Rates calculated only for immatures observed for more than 1000 min in a given age range.
Table 1.
Percentage of aggressive interactions by offspring of different maternal rank involving direct contact (e.g. hitting, kicking, biting) by the aggressor
| Maternal rank of loser
|
|||
|---|---|---|---|
| High | Middle | Low | |
| Maternal rank of winner | |||
| High | 53.8% (13) | 25% (20) | 34.2% (38) |
| Middle | 30% (10) | 50% (4) | 50% (4) |
| Low | 25% (4) | 50% (2) | NR |
The number of total observed aggressive interactions is provided in parentheses. Only male–male and male–female nonsibling aggressive interactions included.
Probability of Winning
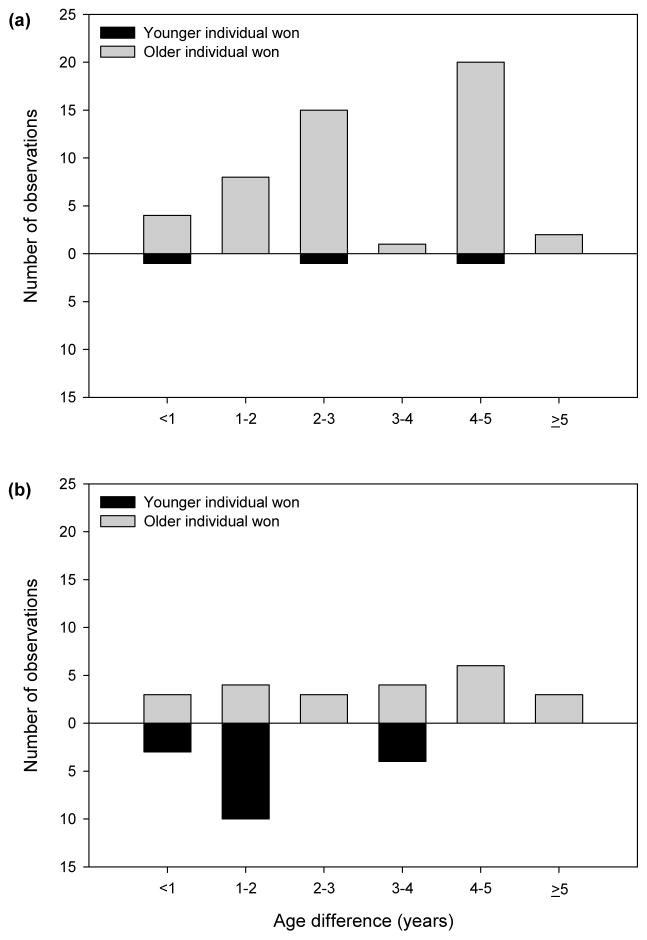
In male–male aggressive interactions, age difference (Fig. 3a) and relative maternal rank significantly predicted the probability of winning. Relatively older individuals (GEE: Wald χ21 = 6.938, P = 0.008) and individuals whose mother outranked their opponent’s mother were more likely to win (GEE: Wald χ22 = 6.479, P = 0.039). In male–female aggressive interactions, males were significantly more likely to win an aggressive encounter than were females (GEE: Wald χ21 = 6.459, P = 0.011). Age difference did not significantly predict probability of winning (GEE: Wald χ21 = 1.998, P = 0.158; Fig. 3b). Maternal rank was again a significant predictor of winning such that an individual was more likely to win an aggressive interaction if his/her mother was higher ranking than the opponent’s mother (GEE: Wald χ22 = 7.464, P = 0.024). See Table 2 for full statistical results.
Figure 3.
Effects of age asymmetries on the outcome of (a) male–male and (b) male–female aggressive interactions. Only nonsibling aggressive interactions included.
Table 2.
Results from generalized estimating equation (GEEs) testing the effect of relative age and relative maternal rank on the probability of winning an aggressive interaction between immature (< 12 years) chimpanzees
| B | Wald χ2 | df | P | |
|---|---|---|---|---|
| Male–male aggressive interactions | ||||
| Intercept | −0.402 | 3.288 | 1 | 0.070 |
| Relative age (focal–opponent) | 1.075 | 6.938 | 1 | 0.008 |
| Relative maternal ranka | 0.745 | 6.479 | 2 | 0.039 |
| Male–female aggressive interactions | ||||
| Intercept | 0.965 | 0.893 | 1 | 0.345 |
| Relative age (focal–opponent) | 0.200 | 1.998 | 1 | 0.158 |
| Relative maternal rankb | 0.367 | 7.464 | 2 | 0.024 |
| Focal’s sexc | −1.674 | 6.459 | 1 | 0.011 |
Probability of winning in male–male and male–female aggressive interactions was tested in separate models. The model for male–female aggressive interactions also included focal’s sex as a predictor variable. Statistically significant results are shown in bold.
For higher relative rank; B = −2.016 for lower relative rank; same relative rank reference category.
For higher relative rank; B = −1.756 for lower relative rank; same relative rank reference category.
For females.
Maternal Response to Aggressive Interactions
We observed only 10 instances (7.3% of all observed aggressive interactions) when a mother directly intervened in an aggressive interaction involving her immature offspring (i.e. attempted to influence the outcome of the interaction by being aggressive and/or retrieving her offspring). Three of these 10 interventions were following disputes between siblings. See Table 3 for details on maternal interventions (N = 7) in nonsibling aggressive interactions.
Table 3.
Details on maternal interventions (N = 7) in nonsibling aggressive interactions
| Dyad sex | Intervening mother
|
Did offspring of intervening mother win? | ||
|---|---|---|---|---|
| Relative rank | Offspring absolute age (years); relative agea (years) | Offspring sex | ||
| Male–female | Lower ranking | 3.8 (−5.9) | Male | No |
| Male–female | Higher ranking | 3.3 (−1.6) | Female | Yes |
| Male–female | NA | 10.9 (−0.1) | Female | Yes |
| Male–male | Lower ranking | 3.7 (−4.3) | Male | No |
| Male–male | Lower ranking | 3.8 (−2.4) | Male | No |
| Male–male | Lower ranking | 2.5 (−1.4) | Male | No |
| Male–male | NA | 5.0 (−6.0) | Male | Yes |
NA: not available.
Calculated as the age of the intervening mother’s offspring minus the age of the opponent; negative value indicates that the intervening mother’s offspring was younger than his/her opponent.
DISCUSSION
We found that the probability of winning an aggressive interaction with another immature individual was significantly predicted by relative maternal rank, such that offspring were more likely to win if their mother outranked their opponent’s mother. However, contrary to patterns observed in several other primate species (e.g. Cheney, 1977; Fairbanks & McGuire, 1985; Walters, 1980; but see Horrocks & Hunte, 1983), mothers rarely intervened on behalf of their immature offspring. The effect of maternal rank on the probability of winning is thus not clearly attributable to direct involvement. One hypothesis for the mechanism underlying this pattern is that the threat of intervention from higher-ranking mothers can influence the interaction’s outcome. This effect may be particularly strong when mother and offspring are together, suggesting that further insight into the distinction between direct and indirect maternal effects can be gained by separately analysing aggressive interaction outcomes when immatures are away from their mothers. Such analyses, however, were beyond the scope of the data currently available. To address this topic, future studies would benefit from increased monitoring effort focused on immature chimpanzees while they travel independently from their mothers.
Because of the low frequency of female–female aggression in this study, we were unable to test whether mothers have more effect on the within-sex interactions of their philopatric (male) offspring than their dispersing (female) offspring. We note, however, that female dispersal patterns are variable and that approximately 50% of Kasekela females remain in their natal community (Pusey et al., 1997). There is some indication that daughters of high-ranking mothers are more likely to remain natal when their mothers are alive (Pusey, 1983; Kahlenberg et al., 2008), perhaps because of maternal support in agonistic interactions.
Alternative hypotheses explaining the effect of maternal rank on interaction outcome are that offspring of higher-ranking mothers are in better condition due to nutritional advantages and/or prenatal conditions that affect offspring success in aggressive encounters. It is possible that offspring of higher-ranking mothers are larger for their age and that this discrepancy alone may explain their greater likelihood of winning. The link between maternal rank and offspring condition, growth and dominance is well known in other species (reviewed in Maestripieri, 2009). Another possibility is that prenatal maternal effects may influence an offspring’s rate of aggression and ability to dominate others (reviewed in Mateo, 2009). In spotted hyaenas, androgen concentrations during late pregnancy are higher in dominant females than in subordinate females, and offspring exposed to higher prenatal androgen levels show higher rates of aggressive behaviour (Dloniak et al., 2006).
Comparable to other reports on the early emergence of behavioural sex differences in chimpanzees (Lonsdorf, Anderson, et al., 2014; Lonsdorf, Markham, et al., 2014), our findings indicate that sex differences in aggressive behaviour are apparent before adulthood. The patterns reported here mirror those observed later in life when male–male aggressive interactions are more prevalent (reviewed in: Boesch et al., 2006; Muller, 2002; Muller et al., 2011; Newton-Fisher et al., 2010; Wroblewski et al., 2009) and overt female–female aggressive interactions occur less frequently (Goodall, 1986; reviewed in Murray, 2007). Changes in propensity for aggressive interaction associated with increasing testosterone levels may in part explain why we observed considerably lower rates of aggressive interaction among immatures compared to previously published rates among adults (e.g. Muller et al., 2007; Muller & Wrangham, 2004). We cannot rule out the possibility that these differences may also be due to methodological differences in rate calculations (discussed in Wrangham et al., 2006; but see Muller et al., 2007) and/or lower detectability of immature aggressive interactions if interactions between immatures are less conspicuous than adult encounters.
We also found that after controlling for age and differences in maternal rank, immature males were significantly more likely to win aggressive interactions with immature females, a result comparable to patterns in adulthood when males dominate females (Feldblum et al., in press; Muller et al., 2011). Interestingly, the sex differences in the probability of winning aggressive interactions reported here are evident prior to the development of sexual dimorphism in size and prior to puberty in many cases (Pusey et al., 2005). This suggests that intersexual dominance among immatures is not simply attributable to overall weight disparities, although nevertheless may reflect differences in muscle mass and/or hormone levels. For example, in captive chimpanzees, Behringer et al. (2014) found that urinary testosterone levels markedly increase in both males and females around 8 years, and Anestis (2006) found a positive correlation between an immature male’s urinary testosterone levels and his rate of aggression directed towards peers.
Previous accounts found that offspring protection is a common context for adult female aggressive interaction (Goodall, 1986). The rarity of maternal intervention we report is therefore noteworthy. One possible reason for this discrepancy is that we focused on intervention exclusively in the context of aggressive interactions between immatures, whereas earlier reports additionally included instances when offspring received aggression from adults (Goodall, 1968, 1971; Pusey, 1983). Aggression from adults, particularly when directed at very young infants, can pose a severe and potentially fatal threat to young chimpanzees (Arcadi & Wrangham, 1999; Pusey et al., 2008; Wilson et al., 2014). That mothers may be less likely to intervene when offspring engage aggressively with other immatures could reflect a lower risk of injury in these encounters. It is unclear whether the mothers are more relaxed or the infants less solicitous of help during relatively low-risk encounters between immatures. Nevertheless, a competitive advantage for offspring of higher-ranking females despite low rates of intervention highlights the importance of indirect maternal effects on offspring social development.
Conclusions
Our results contribute novel evidence for the importance of indirect maternal effects in Pan species. In a previous study, Williams et al. (2002) found that chimpanzee males whose mothers were more social during their immaturity tended to attain higher rank as adults. Among chimpanzees (Boesch, 2009) and especially bonobos (Pan paniscus: Furuichi, 1989; Ihobe, 1992), maternal presence has been found to influence dominance relationships among adult males. It has also been reported that the presence of bonobo mothers increases the mating success of adult sons, mediated in part by direct interventions in aggressive interactions (Surbeck et al., 2011). The stronger evidence for prolonged maternal influence into adulthood in male bonobos may be linked to increased paedomorphic behavioural traits in adult bonobos relative to adult chimpanzees (Hare et al., 2012). Alternatively, adult female bonobos, unlike chimpanzees (Feldblum et al., in press; Muller et al., 2011), are dominant over males and therefore may be more influential when intervening in agonistic encounters on behalf of their adult sons. Our findings complement this body of literature, providing important insight into the ontogeny of aggressive behaviour in wild apes and highlighting a potential social advantage for both male and female offspring of higher-ranking mothers. Given human’s shared evolutionary history with chimpanzees, these findings raise intriguing questions about maternal effects on the ontogeny of aggressive behaviour in our own species.
Highlights.
We examined the outcome of aggressive interactions between wild immature chimpanzees.
Offspring were more likely to win if their mother outranked their opponent’s mother.
Direct maternal interventions were rare in aggressive interactions.
This suggests that maternal interventions do not often explain interaction outcome.
Acknowledgments
We are grateful to Tanzania National Parks, the Tanzania Wildlife Research Institute and the Tanzanian Council for Science and Technology for granting us permission to work in Gombe National Park. We also thank Jane Goodall and the Jane Goodall Institute for supporting long-term chimpanzee research at Gombe National Park, and the Gombe Stream Research Centre staff for maintaining data collection, which was supplemented by M. Heintz, E. Greengrass and M. Lucasik during the study period. The Jane Goodall Institute Research Center at Duke University (supported by grants from the National Science Foundation (LTREB-1052693), the National Institutes of Health (R01 AI058715) and Duke University) provided data on female ranks based on pant-grunt data that were consolidated from the long-term data and graduate student projects through the efforts of I. Gilby and J. Feldblum. Collection, digitization and analysis of behavioural data were supported by grants from the National Institutes of Health (R00 HD057992) and the Leo S. Guthman Foundation. Finally, we thank K. Anderson, V. Fiorentino and S. Reji for data management, H. Gaynor, G. Harper, J. Miller, A. Mustapha and many others for data entry, and M. Stanton and two anonymous referees for their valuable insights.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Altmann J, Alberts SC. Growth rates in a wild primate population: ecological influences and maternal effects. Behavioral Ecology and Sociobiology. 2005;57:490–501. [Google Scholar]
- Andres D, Clutton-Brock TH, Kruuk LEB, Pemberton JM, Stopher KV, Ruckstuhl KE. Sex differences in the consequences of maternal loss in a long-lived mammal, the red deer (Cervus elaphus) Behavioral Ecology and Sociobiology. 2013;67:1249–1258. [Google Scholar]
- Anestis SF. Testosterone in juvenile and adolescent male chimpanzees (Pan troglodytes): effects of dominance rank, aggression, and behavioral style. American Journal of Physical Anthropology. 2006;130:536–545. doi: 10.1002/ajpa.20387. [DOI] [PubMed] [Google Scholar]
- Arcadi AC, Wrangham RW. Infanticide in chimpanzees: review of cases and a new within-group observation from the Kanyawara study group in Kibale National Park. Primates. 1999;40:337–351. [Google Scholar]
- Behringer V, Deschner T, Deimel C, Stevens JMG, Hohmann G. Age-related changes in urinary testosterone levels suggest differences in puberty onset and divergent life history strategies in bonobos and chimpanzees. Hormones and Behavior. 2014;66:525–533. doi: 10.1016/j.yhbeh.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Boesch C. The real chimpanzee: Sex strategies in the forest. Cambridge, U.K: Cambridge University Press; 2009. [Google Scholar]
- Boesch C, Kohou G, Nene H, Vigilant L. Male competition and paternity in wild chimpanzees of the Tai forest. American Journal of Physical Anthropology. 2006;130:103–115. doi: 10.1002/ajpa.20341. [DOI] [PubMed] [Google Scholar]
- Bygott JD. Agonistic behaviour, dominance and social structure in wild chimpanzees of the Gombe National Park. In: Hamburg DA, McCown ER, editors. The great apes. Menlo Park, CA: Benjamin/Cummings; 1979. pp. 405–427. [Google Scholar]
- Cheney DL. The acquisition of rank and the development of reciprocal alliances among free-ranging immature baboons. Behavioral Ecology and Sociobiology. 1977;2:303–318. [Google Scholar]
- Clutton-Brock TH. Reproductive success. In: Clutton-Brock TH, editor. Reproductive success: Studies of individual variation in contrasting breeding systems. Chicago, IL: University of Chicago Press; 1988. pp. 472–486. [Google Scholar]
- Datta S. The acquisition of dominance among free-ranging rhesus monkey siblings. Animal Behaviour. 1988;36:754–772. [Google Scholar]
- Diggle PJ, Heagerty PJ, Liang K-Y, Zeger SL. Analysis of longitudinal data. 2. New York, NY: Oxford University Press; 2002. [Google Scholar]
- Dloniak SM, French JA, Holekamp KE. Rank-related maternal effects of androgens on behaviour in wild spotted hyaenas. Nature. 2006;440:1190–1193. doi: 10.1038/nature04540. [DOI] [PubMed] [Google Scholar]
- Ellis L. Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethology and Sociobiology. 1995;16:257–333. [Google Scholar]
- Engh AL, Esch K, Smale L, Holekamp KE. Mechanisms of maternal rank ‘inheritance’ in the spotted hyaena, Crocuta crocuta. Animal Behaviour. 2000;60:323–332. doi: 10.1006/anbe.2000.1502. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, McGuire MT. Relationships of vervet mothers with sons and daughters from one through three years of age. Animal Behaviour. 1985;33:40–50. [Google Scholar]
- Feldblum JT, Wroblewski EE, Rudicell RS, Hahn BH, Paiva T, Cetinkaya-Rundel M, et al. Male chimpanzee aggression toward females: long-term intimidation increases likelihood of paternity. Current Biology In press. [Google Scholar]
- Foster EA, Franks DW, Mazzi S, Darden SK, Balcomb KC, Ford JKB, et al. Adaptive prolonged postreproductive life span in killer whales. Science. 2012;337:1313. doi: 10.1126/science.1224198. [DOI] [PubMed] [Google Scholar]
- Furuichi T. Social interactions and the life history of female Pan paniscus in Wamba, Zaire. International Journal of Primatology. 1989;10:173–197. [Google Scholar]
- Gilby IC, Wrangham RW. Association patterns among wild chimpanzees (Pan troglodytes schweinfurthii) reflect sex differences in cooperation. Behavioral Ecology and Sociobiology. 2008;62:1831–1842. [Google Scholar]
- Goodall J. The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Animal Behaviour Monograph. 1968;1:161–311. [Google Scholar]
- Goodall J. In the shadow of man. London, U.K: William Collins; 1971. [Google Scholar]
- Goodall J. The chimpanzees of Gombe. Cambridge, MA: Harvard University Press; 1986. [Google Scholar]
- Harcourt AH, Stewart KJ. The influence of help in contests on dominance rank in primates: hints from gorillas. Animal Behaviour. 1987;35:182–190. [Google Scholar]
- Hare B, Wobber V, Wrangham R. The self-domestication hypothesis: evolution of bonobo psychology is due to selection against aggression. Animal Behaviour. 2012;83:573–585. [Google Scholar]
- Hofer H, East ML. Behavioral processes and costs of co-existence in female spotted hyenas: a life history perspective. Evolutionary Ecology. 2003;17:315–331. [Google Scholar]
- Honer OP, Wachter B, Hofer H, Wilhelm K, Thierer D, Trillmich F, et al. The fitness of dispersing spotted hyaena sons is influenced by maternal social status. Nature Communications. 2010;1:1–7. doi: 10.1038/ncomms1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrocks J, Hunte W. Maternal rank and offspring rank in vervet monkeys: an appraisal of the mechanisms of rank acquisition. Animal Behaviour. 1983;31:772–782. [Google Scholar]
- Ihobe H. Male–male relationships among wild bonobos (Pan paniscus) at Wamba, Republic of Zaire. Primates. 1992;33:163–179. [Google Scholar]
- Kahlenberg SM, Emery Thompson M, Muller MN, Wrangham RW. Immigration costs for female chimpanzees and male protection as an immigrant counterstragey to intrasexual aggression. Animal Behaviour. 2008;76:1497–1509. [Google Scholar]
- Lonsdorf EV, Anderson KE, Stanton MA, Shender M, Heintz MR, Goodall J, et al. Boys will be boys: sex differences in wild infant chimpanzee social interactions. Animal Behaviour. 2014;88:79–83. doi: 10.1016/j.anbehav.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf EV, Markham AC, Heintz MR, Anderson KE, Ciuk DJ, Goodall J, et al. Sex differences in wild chimpanzee behavior emerge during infancy. PLoS One. 2014;9:e99099. doi: 10.1371/journal.pone.0099099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D. Maternal influences on offspring growth, reproduction, and behavior in primates. In: Maestripieri D, Mateo JM, editors. Maternal effects in mammals. Chicago, IL: Chicago University Press; 2009. pp. 256–291. [Google Scholar]
- Majolo B, Lehmann J, de Bortoli Vizioli A, Schino G. Fitness-related benefits of dominance in primates. American Journal of Physical Anthropology. 2012;147:652–660. doi: 10.1002/ajpa.22031. [DOI] [PubMed] [Google Scholar]
- Mateo JM. Maternal influences on development, social relationships, and survival behaviors. In: Maestripieri D, Mateo JM, editors. Maternal effects in mammals. Chicago, IL: Chicago University Press; 2009. pp. 133–158. [Google Scholar]
- Mitani JC. Male chimpanzees form enduring and equitable social bonds. Animal Behaviour. 2009;77:633–640. [Google Scholar]
- Muller MN. Agonistic relations among Kanyawara chimpanzees. In: Boesch C, Hohmann G, Marchant L, editors. Behavioural diversity in chimpanzees and bonobos. Cambridge, U.K: Cambridge University Press; 2002. pp. 112–124. [Google Scholar]
- Muller MN, Kahlenberg SM, Emery Thompson M, Wrangham RW. Male coercion and the costs of promiscuous mating for female chimpanzees. Proceedings of the Royal Society B: Biological Sciences. 2007;274:1009–1014. doi: 10.1098/rspb.2006.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Emery Thompson M, Kahlenberg SM, Wrangham RW. Sexual coercion by male chimpanzees shows that female choice may be more apparent than real. Behavioral Ecology and Sociobiology. 2011;65:921–933. [Google Scholar]
- Muller MN, Wrangham RW. Dominance, aggression and testosterone in wild chimpanzees: a test of the ‘challenge hypothesis’. Animal Behaviour. 2004;67:113–123. [Google Scholar]
- Murray CM. A method for assigning categorical rank in female chimpanzees (Pan troglodytes) via the frequency of approaches. International Journal of Primatology. 2007;28:853– 864. [Google Scholar]
- Murray CM, Eberly LE, Pusey AE. Foraging strategies as a function of season and rank among wild female chimpanzees (Pan troglodytes) Behavioral Ecology. 2006;17:1020–1028. [Google Scholar]
- Murray CM, Lonsdorf EV, Eberly LE, Pusey AE. Reproductive energetics in free-living female chimpanzees (Pan troglodytes schweinfurthii) Behavioral Ecology. 2009;20:1211–1216. [Google Scholar]
- Murray CM, Lonsdorf EV, Stanton MA, Wellens KR, Miller JA, Goodall J, et al. Early socialization in wild chimpanzees: mothers socialize sons more than daughters. Proceedings of the National Academy of Sciences of the United States of America.; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Hayaki H, Hosaka K, Itoh N, Zamma K. Brief communication: orphaned male chimpanzees die young even after weaning. American Journal of Physical Anthropology. 2014;153:139–143. doi: 10.1002/ajpa.22411. [DOI] [PubMed] [Google Scholar]
- Newton-Fisher NE, Emery Thompson M, Reynolds V, Boesch C, Vigilant L. Paternity and social rank in wild chimpanzees (Pan troglodytes) from the Budongo Forest, Uganda. American Journal of Physical Anthropology. 2010;142:417–428. doi: 10.1002/ajpa.21241. [DOI] [PubMed] [Google Scholar]
- Nishida T. The social group of wild chimpanzees in the Mahali Mountains. Primates. 1968;9:167–224. [Google Scholar]
- Nishida T. Social interactions between resident and immigrant female chimpanzees. In: Heltne PG, Marquardt LF, editors. Understanding chimpanzees. Cambridge, MA: Harvard University Press; 1989. pp. 68–89. [Google Scholar]
- Nishida T, Hiraiwa-Hasegawa M. Chimpanzees and bonobos: cooperative relationships among males. In: Smuts BB, Cheney DL, Seyfarth RM, Struhsaker TT, Wrangham RW, editors. Primate societies. Chicago, IL: University of Chicago Press; 1987. pp. 165–177. [Google Scholar]
- Onyango PO, Gesquiere LR, Wango EO, Alberts SC, Altmann J. Persistence of maternal effects in baboons: mother’s dominance rank at son’s conception predicts stress hormone levels in subadult males. Hormones and Behavior. 2008;54:319–324. doi: 10.1016/j.yhbeh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel MD, Harvey PH. Evolution of the juvenile period in mammals. In: Pereira ME, Fairbanks LA, editors. Juvenile primates: Life history, development, and behavior. New York, NY: Oxford University Press; 2002. pp. 28–37. [Google Scholar]
- Paquette D. Fighting and playfighting in captive adolescent chimpanzees. Aggressive Behavior. 1994;20:49–65. [Google Scholar]
- Pereira ME. Development and social dominance among group-living primates. American Journal of Primatology. 1995;37:143–175. doi: 10.1002/ajp.1350370207. [DOI] [PubMed] [Google Scholar]
- Pereira ME, Fairbanks LA. Foreword. In: Pereira ME, Fairbanks LA, editors. Juvenile primates: Life history, development, and behavior. New York, NY: Oxford University Press; 2002. pp. VII–XXIII. [Google Scholar]
- Pusey AE. Intercommunity transfer of chimpanzees in Gombe National Park. In: Hamburg DA, McCown ER, editors. The great apes. Menlo Park, CA: Benjamin/Cummings; 1979. pp. 465–479. [Google Scholar]
- Pusey AE. Mother–offspring relationships in chimpanzees after weaning. Animal Behaviour. 1983;31:363–377. [Google Scholar]
- Pusey AE. Behavioural changes at adolescence in chimpanzees. Behaviour. 1990;115:203–246. [Google Scholar]
- Pusey A, Murray C, Wallauer W, Wilson M, Wroblewski E, Goodall J. Severe aggression among female Pan troglodytes schweinfurthii at Gombe National Park, Tanzania. International Journal of Primatology. 2008;29:949–973. [Google Scholar]
- Pusey AE, Oehlert GW, Williams JM, Goodall J. Influence of ecological and social factors on body mass of wild chimpanzees. International Journal of Primatology. 2005;26:3–31. [Google Scholar]
- Pusey AE, Williams J, Goodall J. The influence of dominance rank on reproductive success of female chimpanzees. Science. 1997;277:828–831. doi: 10.1126/science.277.5327.828. [DOI] [PubMed] [Google Scholar]
- Surbeck M, Mundry R, Hohmann G. Mothers matter! Maternal support, dominance status and mating success in male bonobos (Pan paniscus) Proceedings of the Royal Society B: Biological Sciences. 2011;278:590–598. doi: 10.1098/rspb.2010.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries H, Stevens JMG, Vervaecke H. Measuring and testing the steepness of dominance hierarchies. Animal Behaviour. 2006;71:585–592. [Google Scholar]
- Walters J. Intervention and the development of dominance relationships in female baboons. Folia Primatologica. 1980;34:61–89. doi: 10.1159/000155948. [DOI] [PubMed] [Google Scholar]
- Williams JM, Liu H-Y, Pusey AE. Costs and benefits of grouping for female chimpanzees at Gombe. In: Boesch C, Hohmann G, Marchant LF, editors. Behavioural diversity in chimpanzees and bonobos. Cambridge, U.K: Cambridge University Press; 2002. pp. 192–203. [Google Scholar]
- Wilson ML. Long-term studies of the chimpanzees of Gombe National Park, Tanzania. In: Kappeler PM, Watts DP, editors. Long-term field studies of primates. Heidelberg, Germany: Springer-Verlag; 2012. pp. 357–384. [Google Scholar]
- Wrangham RW, Wilson ML, Muller MN. Comparative rates of violence in chimpanzees and humans. Primates. 2006;47:14–26. doi: 10.1007/s10329-005-0140-1. [DOI] [PubMed] [Google Scholar]
- Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Animal Behaviour. 2009;77:873–885. doi: 10.1016/j.anbehav.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]