Abstract
Attachment theory provides a framework for understanding individual differences in chronic interpersonal stress. Attachment anxiety, a type of relationship insecurity characterized by worry about rejection and abandonment, is a chronic interpersonal stressor. Stress impacts cellular immunity, including herpes-virus reactivation. We investigated whether attachment anxiety was related to the expression of a latent herpesvirus, Epstein–Barr virus (EBV), when individuals were being tested for breast or colon cancer and approximately 1 year later. Participants (N = 183) completed a standard attachment questionnaire and provided blood to assess EBV viral capsid antigen (VCA) IgG antibody titers. Individuals with more attachment anxiety had higher EBV VCA IgG antibody titers than those with less attachment anxiety. The strength of the association between attachment anxiety and antibody titers was the same at both assessments. This study is the first to show an association between latent herpesvirus reactivation and attachment anxiety. Because elevated herpesvirus antibody titers reflect poorer cellular immune system control over the latent virus, these data suggest that high attachment anxiety is associated with cellular immune dysregulation.
Keywords: Psychoneuroimmunology, Close relationships, Attachment theory, Attachment anxiety, Stress, Herpesvirus, Immune system
1. Introduction
There are well-documented links between close relationships and physical health. People who have supportive close relationships have lower rates of morbidity and mortality than those who have unsupportive and conflict-ridden relationships (Berkman and Syme, 1979; Brummett et al., 2001; Gilbert et al., 2009; House, 1988; Orth-Gomer and Johnson, 1987; Repetti et al., 2002; Tomaka et al., 2006; Uchino et al., 1996). Attachment theory provides a framework for understanding individual differences in chronic interpersonal stress and thus may offer insight into the associations between close relationships and health (Diamond and Hicks, 2004; Shaver and Mikulincer, 2007; Uchino, 2009).
Attachment theory suggests that people who have responsive and supportive parents during childhood develop a sense of emotional security that lasts into adulthood, while those who have unresponsive and unsupportive parents develop a sense of emotional insecurity (Mikulincer and Shaver, 2009; Thompson, 1999). Different academic and theoretical traditions conceptualize attachment insecurity in slightly different ways (Fraley and Waller, 1998); according to adult attachment theory, there are two patterns or dimensions of attachment insecurity: attachment anxiety and attachment avoidance (Mikulincer and Shaver, 2007). These patterns have the potential to change throughout one's lifespan but are thought to be relatively stable (Mikulincer and Shaver, 2007).
People with high attachment anxiety constantly worry about rejection and abandonment and use “hyperactivating” coping strategies, such as excessive rumination and preoccupation about stressful events (Brennan et al., 1998; Diamond and Fagundes, 2010; Diamond, 2001; Fraley and Shaver, 2000a). Indeed, people with higher attachment anxiety tend to have a more negative self-image and intense negative emotions than those with lower attachment anxiety (Mikulincer et al., 2006; Pietromonaco and Barrett, 1997).
People with high attachment avoidance are uncomfortable depending on others for support and use “deactivating” coping strategies that inhibit, exclude, or suppress distressing relational experiences (Brennan et al., 1998; Fraley and Shaver, 2000b). Individuals with higher attachment avoidance tend to denigrate and distrust support providers more than those with lower attachment avoidance (Mikulincer and Shaver, 2003; Shaver and Mikulincer, 2005). Furthermore, they keep negative emotions alive internally, while attempting not to express them externally (Mikulincer and Shaver, 2003; Shaver and Mikulincer, 2005).
Attachment anxiety has been reliably linked to many age-related health problems, while attachment avoidance has not (McWilliams and Bailey, 2010; Puig et al., 2012). The chronic social stress that is a feature of attachment anxiety may be an important mechanism underlying this link; chronic stress can impair vaccine responses, slow wound healing, promote inflammation, and dysregulate cellular immunity (Glaser and Kiecolt-Glaser, 2005a). Consistent with this argument, recent work in the field of psychoneuroimmunology demonstrated that people who had higher attachment anxiety had fewer numbers of CD3+ T-cells, CD45+ T-cells, CD3+CD4+ helper T-cells, and CD3+CD8+ cytotoxic T-cells compared with those who had lower attachment anxiety (Jaremka et al., 2013).
Chronic interpersonal stress can drive herpesvirus reactivation and replication by impairing cellular immune system control over viral latency through both autonomic and glucocorticoid pathways (Cacioppo et al., 2002; Glaser and Kiecolt-Glaser, 1994; Yang et al., 2010). Maladaptive alterations in cellular immune function can enhance herpesvirus reactivation and replication, resulting in elevated herpesvirus antibody titers (Glaser and Kiecolt-Glaser, 1994, 2005b; Glaser et al., 2005; Steptoe et al., 2007). For example, organ transplant patients have dysregulated cellular immunity and elevated herpesvirus antibody titers (Gray et al., 1995). Although usually asymptomatic, elevated herpesvirus antibody titers reflect poorer cellular immune system control over viral latency (Glaser and Kiecolt-Glaser, 1994). Accordingly, people who are anxiously attached may be vulnerable to latent herpesvirus reactivation and replication due to their chronic interpersonal stress and corresponding cellular immune dysregulation.
Based on the argument that attachment anxiety is a chronic interpersonal stressor, we hypothesized that those who were more anxiously attached would have higher EBV VCA IgG antibody titers than those who were less anxiously attached. Because most studies have not shown a consistent association between attachment avoidance and health or stress sensitivity, we made no a priori hypothesis about attachment avoidance (Dewitte et al., 2010; Jaremka et al., 2013). However, we examined the possibility that those who were more avoidantly attached would have higher EBV VCA IgG antibody titers than those who were less avoidantly attached. We also explored whether the association between attachment anxiety/avoidance and herpesvirus reactivation differed over time.
2. Methods
2.1. Participants and procedure overview
Study participants (N = 183) were recruited from oncology clinics as they were being tested for breast or colon cancer as part of an ongoing longitudinal observational study investigating potential links between fatigue and immune dysregulation. Participants were being tested for breast or colon cancer because of a suspicious initial test; all participants completed their first visit after this initial suspicious test. Participants eventually received a benign diagnosis as the result of one or more follow-up tests. On average, participants received a benign diagnosis from their final test 9.8 days (SD = 15.08) after their first study visit. Approximately one year later, participants completed a follow-up assessment. We did not have data for 12% of participants at the second assessment. Participants who did not complete the second visit were contacted by phone and email multiple times and (a) stated they did not want to continue due to lack of time or interest, or (b) never returned our messages. Screening exclusions included a prior history of cancer except basal or squamous cell skin cancers and severe cognitive impairment (e.g., Alzheimer's disease). Out of the 183 participants enrolled at the first visit, eight were EBV sero-negative (i.e., they never contracted EBV); therefore, they were not included in the analyses. All participants who were EBV seropositive at the first visit were also seropositive at the second visit; once contracted, herpesvirus seropositivity does not change. The average time between study visits was 365 days (SD = 124 days). The Institutional Review Board approved the project; all subjects gave written informed consent prior to participation.
2.2. Determination of EBV VCA IgG antibody titers in plasma
EBV VCA IgG represents the antibody response to the combination of multiple viral proteins that make up the virus coat. We assessed antibody against EBV VCA IgG in plasma to assess control over viral latency. Plasma was stored at −80 °C until assayed with Euroimmun EBV ELISA plates (Boonton Township, NJ). This ELISA's antigen, a cell lysate of human B-cells infected with EBV strain P3HR-1, comprises various viral capsid proteins, including p22, gp33, gp40, gp41, gp42, gp116. EBV-VCA IgG antibody titers were assessed following instructions, with kit controls (one positive sample, one negative sample, and three calibrators) run in duplicate. After the initial 1:101 dilution, six serial two-fold dilutions of each sample were assayed, and the last positive value was the IgG antibody titer. Calculated viral titers for each sample were plotted and samples were rerun if the end point did not fall within the linear range (±15%).
2.3. Self-report measures
2.3.1. Attachment insecurity
Attachment insecurity was assessed using a modified version of the Experiences in Close Relationships (ECR-M16) scale (Lo et al., 2009). The ECR-M16 was designed to assess attachment insecurity in patients of diverse ages. The 16-item self-report measure assesses general attachment insecurity in close relationships; it contains two 8-item subscales, one assessing attachment anxiety and the other assessing attachment avoidance. The anxiety sub-scale includes items such as “I worry about being abandoned” and “I need a lot of reassurance that I am loved by people with whom I feel close to.” The following items are representative of the avoidance scale: “I get uncomfortable when other people want to be very close to me,” and “I don't feel comfortable opening up to other people.” Both scales have high internal and test-retest reliability (Lo et al., 2009).
2.3.2. Beck Anxiety Inventory
The Beck Anxiety Inventory (BAI) assesses general anxiety symptoms. The BAI can discriminate between clinically anxious and non-clinically anxious people and has good test-retest reliability and internal consistency. The BAI provided a way to disentangle general anxiety from attachment anxiety (Steer and Beck, 1997).
2.3.3. Depression
The Center for Epidemiological Studies Depression Scale (CES-D) has been used extensively as a brief measure of depressive symptomatology (Basco et al., 1997; Radloff, 1977). Studies have shown acceptable test-retest reliability and excellent construct validity (Basco et al., 1997). Because the CES-D can distinguish depressed from non-depressed participants in community and clinical samples, discriminative validity appears acceptable as well (Basco et al., 1997). Population norms provide cutoffs for varying levels of depression (Basco et al., 1997), and it has been widely used in cancer studies (Demark-Wahnefried et al., 2003). The CES-D was included to disentangle the links among depression, attachment anxiety, and herpesvirus reactivation.
2.3.4. Comorbidities
The Charlson index is one of the most widely used comorbidity indices (Charlson et al., 1994). Originally developed for predicting mortality, it has now been widely used in cancer and non-cancer populations (Dobnig et al., 2008) The measure assigns weights to 19 comorbid conditions based on their potential to influence one-year mortality.
2.3.5. Sleep
The Insomnia Severity Index (Morin, 1993) reliably estimates insomnia severity (Bastien et al., 2001). Scores range from 0 to 28 with higher numbers indicating greater insomnia severity. The measure helped establish the link between attachment anxiety and herpesvirus reactivation independent of sleep problems.
2.3.6. Demographic and clinical variables
Participants answered questions about their age, race, highest level of education, marital status, smoking status, weekly average alcohol consumption, and current medication use. Following participants’ authorization, electronic medical records were reviewed to obtain height and weight data to calculate their body mass index (BMI; kg/m2).
2.4. Analytic method
Using linear mixed models, we addressed the question of whether attachment anxiety was associated with EBV VCA IgG antibody titers across both visits. We utilized restricted information maximum likelihood (REML) estimation to fit all models. REML is superior to listwise deletion for handling attrition (Jeličić et al., 2009). It also performs well when data are missing at random and improves nonrandom circumstances over ignoring cases entirely (Schafer and Graham, 2002). We employed an unstructured within-subjects covariance matrix and examined the model residuals to confirm that they were distributed normally. EBV VCA IgG antibody titers were log transformed prior to analyses because the original distribution was skewed. The log transformation normalized the distribution and alleviated concerns about ceiling effects. We adjusted for age, BMI, sleep, alcohol consumption, comorbidities, sex, and smoking status based on work showing relationships between these factors and cellular immune function; we also adjusted for general anxiety, which allowed us to focus our results on people who were anxious about their close relationships rather than simply anxious in general (Fraley and Shaver, 2000a; Messingham et al., 2002; Sopori, 2002; Stowe et al., 2007). Both attachment styles were included in the models simultaneously based on recommendations for utilizing the Experiences in Close Relationships Scale (Fraley et al., 2000; Lo et al., 2009). Smoking status, BMI, weekly alcohol use, sleep problems, comorbidities, age, and general anxiety were included as time-varying predictors. Attachment anxiety, attachment avoidance, and gender were time invariant predictors because they were only measured at the first assessment.
We conducted a series of ancillary analyses. First, we investigated whether the relationship between attachment style and EBV VCA IgG antibody titers differed between the first and second visit because the period surrounding the benign cancer diagnosis (i.e., visit 1) could potentially be stressful. To accomplish this goal, we added the attachment anxiety/attachment avoidance by visit interactions to the model. We also replaced the covariate of general anxiety with depressive symptoms because attachment anxiety is also consistently related to depression (Rholes et al., 2011) and depression and anxiety are closely related. Finally, we adjusted for cancer screening site (i.e. breast or colon) rather than sex (i.e. male or female) because all of the participants with benign breast cancer screening results were women.
3. Results
3.1. Preliminary analyses
Table 1 reports descriptive sample information for all individuals who were EBV seropositive (i.e., the individuals included in the analysis). There was considerable variability in both attachment anxiety and EBV VCA IgG antibody titers. In addition, people who were higher on attachment anxiety were above the normal range for patient populations based on the extant literature using the ECRM16 (Neel et al., 2013; Sockalingam et al., 2012, 2013). Individuals who were lost to attrition did not significantly differ on any of the study variables compared with those who completed both visits; however, substantive differences may be biased by uneven group sizes. Attachment anxiety was positively associated with attachment avoidance (b = .37, p < .001). Attachment anxiety was also associated with more general anxiety symptoms (b = .40, p < .001), but attachment avoidance was not (b = .08, p = .25). General anxiety was not statistically different while participants’ were awaiting diagnosis compared with a year later (b = .08, p = .25). Furthermore, the association between attachment anxiety and general anxiety did not differ across visits (b = .03, p = .55). Accordingly, the association between attachment anxiety and general anxiety was not more pronounced while participants were awaiting their diagnosis compared with a year after learning their diagnosis was benign. Attachment anxiety was not associated with any other study variable besides EBV VCA antibody titers (as described below). Females had lower attachment avoidance than males (b = −4.7, p < .001).
Table 1.
Sample characteristics of EBV seropositive individuals.
| Characteristic | % | Mean (SD) | Range |
|---|---|---|---|
| Attachment anxiety | - | 21.31 (9.72) | 8-45 |
| Attachment avoidance | - | 24.35 (8.89) | - |
| 8-50 | |||
| EBV VCA IgG antibody titers | |||
| Visit 1 | - | 1988.25 (18114.7) | 101-6464 |
| Visit 2 | - | 2090.39 (1788.90) | 101-6464 |
| Marital status | |||
| Single | 10 | - | 10 |
| Married | 63.3 | - | 63.3 |
| Domestic partner | 1.7 | - | 1.7 |
| Separated/ divorced | 21.1 | - | 21.1 |
| Widowed | 3.9 | - | 3.9 |
| Sex | |||
| Female | 80 | - | 80 |
| Male | 20 | - | 20 |
| Cancer screening site | |||
| Breast | 51.6 | - | 51.6 |
| Colorectal | 48.4 | - | 48.4 |
| Body mass index (BMI) | |||
| Visit 1 | - | 29.03 (6.59) | 17.9-49.6 |
| Visit 2 | - | 28.38(6.32) | 17.0-50.0 |
| Sleep quality | |||
| Visit 1 | - | 8.28 (5.69) | 0-26 |
| Visit 2 | - | 8.57 (5.24) | 0-24 |
| General anxiety symptoms | |||
| Visit 1 | - | 8.94 (8.18) | 0-51 |
| Visit 2 | - | 8.37 (8.29) | 0-51 |
| Depressive symptoms | |||
| Visit 1 | - | 13.92 (11.54) | 0-49 |
| Visit 2 | - | 12.46 (10.80) | 0-53 |
| Age, years | |||
| Visit 1 | - | 55.93 (10.36) | 32-83 |
| Visit 2 | - | 57.03 (10.51) | 34-84 |
| Education | |||
| High school or less | 22.0 | - | 22.0 |
| Some college | 17.7 | - | 17.7 |
| College or university graduate | 31.4 | - | 31.4 |
| Graduate or professional training | 28.0 | - | 28.0 |
| Comorbidities | |||
| Visit 1 | - | .49 (.85) | 0-5 |
| Visit 2 | - | .66 (1.07) | 0-6 |
| Race | |||
| Asian | 2.3 | - | 2.3 |
| Black | 16.6 | - | 16.6 |
| White | 80.6 | - | 80.6 |
| Other | .6 | - | .6 |
| Method(s) of diagnosis - breast* | |||
| Biopsy | 100 | - | 100 |
| Fine needle aspiration | 3.1 | - | 3.1 |
| MRI | 2.0 | - | 2.0 |
| Ultrasound | 64.3 | - | 64.3 |
| Mammogram | 82.4 | 82.4 | |
| Method(s) of diagnosis - colon* | |||
| Biopsy | 26.4 | - | 26.4 |
| Colonoscopy | 82.4 | - | 82.4 |
All participants completed their first visit after an initial suspicious test. Participants eventually received a benign diagnosis as the result of one or more follow-up tests; these follow-up tests are reported under method(s) of diagnosis.
3.2. Primary analysis
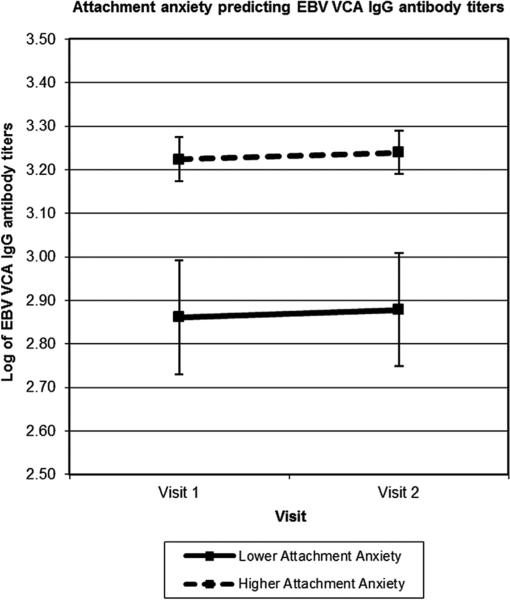
As can be seen in Table 2, participants with higher attachment anxiety had higher EBV VCA IgG antibody titers than those with lower attachment anxiety. Attachment avoidance was not associated with EBV VCA IgG antibody titers. EBV VCA IgG antibody titers did not differ across study visits. To estimate the magnitude of the EBV VCA titers by attachment status between participants lower and higher in attachment anxiety, we used the covariate-adjusted means at 1 standard deviation above and below the mean of attachment anxiety (see Fig. 1). Participants with higher attachment anxiety (+1 SD) had 11.8% higher EBV antibody titers than those with lower attachment anxiety (−1 SD).
Table 2.
Summary of mixed models analysis predicting EBV VCA IgG antibody titers.
| Variable | EBV VCA IgG (log10) |
|||
|---|---|---|---|---|
| B | SE | p | 95% CI | |
| Attachment anxiety | .008 | .004 | .036 | .001, .016 |
| Attachment avoidance | –.004 | .004 | .264 | –.013, .004 |
| Visit | .017 | .018 | .373 | –.020, .053 |
| Gender (Female = 0, Male = 1) | .021 | .090 | .808 | –.148, .190 |
| BMI | .002 | .005 | .633 | –.007, .012 |
| Smoker (No = 0, Yes = 1) | –.070 | .080 | .358 | –.220, .080 |
| Weekly alcohol use | –.002 | .002 | .356 | –.010, .002 |
| Sleep problems | .001 | .004 | .764 | –.008, .010 |
| Comorbidities | .032 | .026 | .210 | –.018, .083 |
| Age | .002 | .003 | .534 | –.004, .008 |
| General anxiety | .001 | .003 | .670 | –.004, .006 |
Fig. 1.
Mean EBV antibody titers across time in high and low anxiously attached individuals based on attachment anxiety scores 1 SD above and below the mean level of attachment anxiety. Those with more attachment anxiety had higher EBV antibody titers compared with those with less attachment anxiety reflecting poorer cellular immunity.
3.3. Secondary analyses
In additional analyses, we tested for the interaction between attachment anxiety and visit. The interaction was not significant (b = .01, p = .40) demonstrating that the strength of the association between attachment anxiety and EBV VCA IgG antibody titers did not differ across visits. Likewise, attachment avoidance did not interact with visit (b = .01, p = .55). We also adjusted for cancer screening site (i.e. breast or colon) rather than sex (i.e. male or female); neither the p-values nor the point estimates substantively changed. We also tested for the interaction between attachment anxiety and attachment avoidance predicting EBV VCA IgG and it was not significant (b = .00, p = .92). We then examined the association between attachment insecurity and EBV VCA IgG antibody titers adjusting for depressive symptoms rather than anxiety. The association between attachment anxiety and EBV VCA IgG antibody titers remained significant in the adjusted model (b = .008, p = .03), while there was still no significant association between attachment avoidance and EBV VCA IgG antibody titers (b = −.005, p = .25). Finally, we examined the association between attachment anxiety and EBV VCA IgG antibody titers without any of the covariates in the model. The association between attachment anxiety and EBV VCA IgG antibody titers remained significant in the unadjusted model (b = .008, p = .03), while there was still no significant association between attachment avoidance and EBV VCA IgG antibody titers (b = −.005, p = .20).
4. Discussion
Individuals who were more anxiously attached had higher EBV VCA IgG antibody titers, reflecting poorer cellular immune system control of the latent virus, than their less anxiously attached counterparts. They also had higher levels of general anxiety than those who had lower attachment anxiety. Attachment avoidance was not associated with EBV VCA IgG antibody titers or general anxiety. This study is the first to show an association between latent herpesvirus reactivation and attachment anxiety.
These results complement recent work showing a relationship between high attachment anxiety and other biologically relevant health outcomes. Indeed, other work from our lab demonstrated that higher attachment anxiety is associated with cell-mediated immunity, specifically fewer T-cells (Jaremka et al., 2013). Cortisol, which can dysregulate cellular immune function, was also higher among those who were more anxiously attached compared with those who were less anxiously attached (Jaremka et al, 2013).
The association between attachment anxiety and EBV VCA IgG antibody titers remained significant over and above both general anxiety and depressive symptoms. Accordingly, these findings suggest that attachment anxiety is a potent social stressor with unique physiological costs that are independent of general anxiety and depression (Diamond and Fagundes, 2010; Diamond, 2001) Indeed, attachment processes are critically important for people's ability to regulate their emotions when experiencing stress (Pietromonaco et al., 2013). Those with more attachment anxiety appraise adversities as threatening, irreversible, and uncontrollable. Researchers have argued that the sense of helplessness and lack of perceived support that characterizes individuals with more attachment anxiety impairs peoples’ ability to put both past and current stressors behind them (Mikulincer and Florian, 1998). It will be important for future studies to examine what aspects of attachment anxiety promote immune dysregulation.
The current study extends prior work on close relationships and physical health in new and important directions. Prior work from our lab demonstrated that attachment anxiety is related to differences in T-cell counts (Jaremka et al., 2013). This study is the first to link attachment anxiety to cellular immune competence by demonstrating that attachment anxiety is related to viral reactivation. Attachment anxiety is characterized by constant vigilance and hyper-sensitivity to cues of rejection, which in turn leads those with high attachment anxiety to easily perceive threats in their environment and frequently experience social interactions as stressful and worrisome (Mikulincer et al., 2003; Shaver and Mikulincer, 2002). This chronic relationship stress may be a key psychological feature driving the link between attachment anxiety and EBV reactivation that is independent of generalized anxiety and depressive symptoms.
We did not find an association between attachment avoidance and EBV VCA IgG antibody titers. As previously mentioned, attachment avoidance is not reliably linked to chronic stress or physical health (Dewitte et al., 2010; Jaremka et al., 2013). Indeed, most of the work relating attachment avoidance to stress-related physiological outcomes has focused on acute stress reactivity (Diamond and Fagundes, 2010; Gouin et al., 2009). Because chronic and acute stress reactivity are differentially related at both the psychological and physiological levels (Segerstrom and Miller, 2004), it may not be surprising that EBV VCA IgG antibody titers were related to higher levels of attachment anxiety, but not higher levels of attachment avoidance.
The period in which individuals were awaiting a diagnostic test did not elicit more self-reported anxiety than the follow-up assessment 1 year later. This is likely why we did not find a difference between antibody titers at the first visit compared with the second. Likewise, this may explain why the strength of the association between attachment anxiety and EBV VCA IgG antibody titers did not differ across visits. Indeed, it is possible that even when overall stress levels differ, the strength of the relationship between attachment anxiety and EBV reactivity would remain the same. This is consistent with the argument that attachment anxiety functions as a chronic interpersonal stressor, which may be the key psychological mechanism driving our results.
Although herpesvirus reactivation is asymptomatic and typically benign, latent herpesvirus reactivation may promote increased inflammation (Fagundes et al., 2012; Glaser et al., 2006; Harris et al., 1999; Roberts et al., 2010; Simanek et al., 2011; Stassen et al., 2006; Trzonkowski et al., 2003). Elevated inflammation has been linked to a number of age-related diseases (Steptoe et al., 2007). Indeed, older adults who have chronically elevated proinflammatory cytokines are at greater risk for cancer, cardiovascular disease, type II diabetes, osteoporosis, periodontal disease, and rheumatoid arthritis (Ershler and Keller, 2000; Libby, 2007). Accordingly, the findings of the current study may tap into one possible mechanism linking attachment anxiety to a number of chronic diseases.
Our sample was predominately white; it will be important for future studies to investigate if these findings exist among a more racially diverse sample. Furthermore, a large proportion of our sample was older. Normal aging is already marked by dysregulated immune function, including elevated herpesvirus antibody titers (Aw et al., 2007; Glaser and Kiecolt-Glaser, 2005a). Accordingly, it is noteworthy that our findings were detectable in this group of mostly older adults. Indeed, chronic interpersonal stress may be particularly detrimental to older adults because they already have maladaptive age-related immune changes (Glaser and Kiecolt-Glaser, 2005b). We conceptualized attachment anxiety as a stable construct and only measured it at the first visit; however, it would be interesting for future studies to examine how attachment insecurity may change as a result of a stressful life event such as a potential cancer diagnosis. Finally, as with most measures in the field of psychoneuroimmunology, there are currently no recommendations for clinically meaningful differences or clinical cut-offs in relation to EBV antibody titers. However, the percentage difference in antibody titers between those who were low on attachment anxiety compared with those high on attachment anxiety was 11.8%. This percentage is similar to percent differences found in other studies linking psychosocial and behavior factors to EBV VCA IgG antibody titers (Esterling et al., 1993; Glaser et al., 1999, 1985; Lutgendorf et al., 2001).
In conclusion, the current study demonstrated that people who have higher attachment anxiety have elevated EBV VCA IgG antibody titers compared with those who have lower attachment anxiety. Because elevated herpesvirus antibody titers reflect poorer cellular immune system control over the latent virus, these data suggest that high attachment anxiety is associated with cellular immune dysregulation. These findings extend attachment theory in an important direction by demonstrating the utility of a psycho-neuroimmunological approach to the study of attachment insecurity. The current results also add to our growing understanding of how interpersonal relationships and chronic interpersonal stress influence immune function.
Acknowledgments
Work on this project was supported in part by American Cancer Society Postdoctoral Fellowship Grants PF-11-007-01-CPPB and PF-12-040-01-CPPB and NIH grants CA131029, CA126857, and CA16058. Finally, the project was also supported by Award Number UL1TR000090 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
References
- Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basco MR, Krebaum SR, Rush AJ. Outcome measures of depression. In: Strupp HH, Horowitz LM, Lambert MJ, editors. Measuring Patient Changes in Mood, Anxiety, and Personality Disorders. American Psychological Association; Washington D.C.: 1997. pp. 207–245. [Google Scholar]
- Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. Rev. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Berkman L, Syme S. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am. J. Epidemiol. 1979;109(2):186. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Clark CL, Shaver PR. Self-report measurement of adult attachment: an integrative overview. In: Simpson JA, Rholes WS, editors. Attachment Theory and Close Relationships. Guilford press; New York: 1998. pp. 46–76. [Google Scholar]
- Brummett B, Barefoot J, Siegler I, Clapp-Channing N, Lytle B, Bosworth H, Mark D. Characteristics of socially isolated patients with coronary artery disease who are at elevated risk for mortality. Psychosom. Med. 2001;63(2):267. doi: 10.1097/00006842-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Kiecolt-Glaser JK, Malarkey WB, Laskowski BF, Rozlog LA, Poehlmann KM, Glaser R. Autonomic and glucocorticoid associations with the steady-state expression of latent Epstein–Barr virus. Horm. Behav. 2002;42(1):32–41. doi: 10.1006/hbeh.2002.1801. [DOI] [PubMed] [Google Scholar]
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. doi: 0895-4356(94)90129-5 [pii] [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Morey MC, Clipp EC, Pieper CF, Snyder DC, Sloane R, Cohen HJ. Leading the way in exercise and diet (Project LEAD): intervening to improve function among older breast and prostate cancer survivors. Control. Clin. Trials. 2003;24(2):206–223. doi: 10.1016/s0197-2456(02)00266-0. [DOI] [PubMed] [Google Scholar]
- Dewitte M, De Houwer J, Goubert L, Buysse A. A multi-modal approach to the study of attachment-related distress. Biol. Psychol. 2010;85(1):149–162. doi: 10.1016/j.biopsycho.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Diamond L, Fagundes C. Psychobiological research on attachment. J. Soc. Pers. Relat. 2010;27(2):218. [Google Scholar]
- Diamond LM. Contributions of psychophysiology to research on adult attachment: review and recommendations. Personal. Soc. Psychol. Rev. 2001;5:276–295. [Google Scholar]
- Diamond LM, Hicks AM. Psychobiological perspectives on attachment: implications for health over the lifespan. In: Simpson JA, Rholes WS, editors. Adult Attachment: New Directions and Emerging Issues. Guilford; New York: 2004. pp. 240–263. [Google Scholar]
- Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Maerz W. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch. Intern. Med. 2008;168(12):1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Esterling BA, Antoni MH, Kumar M, Schneiderman N. Defensiveness, trait anxiety, and Epstein–Barr viral capsid antigen antibody titers in healthy college students. Health Psychol. 1993;12(2):132. doi: 10.1037//0278-6133.12.2.132. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Alfano CM, Bennett JM, Povoski SP, Lipari AM, Farrar WB. Fatigue and herpesvirus latency in women newly diagnosed with breast cancer. Brain Behav. Immun. 2012;26(3):394–400. doi: 10.1016/j.bbi.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley RC, Shaver PR. Adult romantic attachment: theoretical developments, emerging controversies, and unanswered questions. Rev. General Psychol. 2000a;4(2):132. [Google Scholar]
- Fraley RC, Shaver PR. Adult romantic attachment: theoretical developments, emerging controversies, and unanswered questions. Rev. General Psychol. 2000b;4:132–154. [Google Scholar]
- Fraley RC, Waller NG. Adult attachment patterns. Attach. Theo. Close Relat. 1998:77–114. [Google Scholar]
- Fraley RC, Waller NG, Brennan KA. An item response theory analysis of self-report measures of adult attachment. J. Pers. Soc. Psychol. 2000;78(2):350–365. doi: 10.1037//0022-3514.78.2.350. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Widom C, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. The Lancet. 2009;373(9657):68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- Glaser R, Friedman SB, Smyth J, Ader R, Bijur P, Brunell P, Stone A. The differential impact of training stress and final examination stress on herpesvirus latency at the United States Military Academy at West Point. Brain Behav. Immun. 1999;13(3):240–251. doi: 10.1006/brbi.1999.0566. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-associated immune modulation and its implications for reactivation of latent herpesviruses. In: Glaser R, Jones J, editors. Human Herpesvirus Infections. Dekker; New York: 1994. pp. 245–270. [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005a;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005b;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. Stress, loneliness, and changes in herpesvirus latency. J. Behav. Med. 1985;8(3):249–260. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- Glaser R, Litsky ML, Padgett DA, Baiocchic RA, Yang EV, Chen M, Williams MV. EBV-encoded dUTPase induces immune dysregulation: implications for the pathophysiology of EBV-associated disease. Virology. 2006;346:205–218. doi: 10.1016/j.virol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Glaser R, Padgett D, Litsky M, Baiocchi R, Yang E, Chen M, Marshall G. Stress-associated changes in the steady-state expression of latent Epstein–Barr virus: implications for chronic fatigue syndrome and cancer. Brain Behav. Immun. 2005;19(2):91–103. doi: 10.1016/j.bbi.2004.09.001. doi: 10.1016/j.bbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Gouin J-P, Glaser R, Loving TJ, Malarkey WB, Stowell J, Houts C, Kiecolt-Glaser JK. Attachment avoidance predicts inflammatory responses to marital conflict. Brain Behav. Immun. 2009;23(7):898–904. doi: 10.1016/j.bbi.2008.09.016. doi: 10.1016/j.bbi.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, Wreghitt T, Pavel P, Smyth R, Parameshwar J, Stewart S, Wallwork J. Epstein–Barr virus infection in heart and heart–lung transplant recipients: incidence and clinical impact. J. Heart Lung Transpl. 1995;14(4):640–646. [PubMed] [Google Scholar]
- Harris T, Ferrucci L, Tracy R, Corti M, Wacholder S, Ettinger WJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- House JS. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Jaremka LM, Glaser R, Loving TJ, Malarkey WB, Stowell JR, Kiecolt-Glaser JK. Attachment anxiety is linked to alterations in cortisol production and cellular immunity. Psychol. Sci. 2013 doi: 10.1177/0956797612452571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeličić H, Phelps E, Lerner RM. Use of missing data methods in longitudinal studies: the persistence of bad practices in developmental psychology. Dev. Psychol. 2009;45(4):1195–1199. doi: 10.1037/a0015665. http://dx.doi.org/10.1037/a0015665. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr. Rev. 2007;65(12):S140–S146. doi: 10.1111/j.1753-4887.2007.tb00352.x. doi: 10.1301/nr.2007.dec.S140-S146. [DOI] [PubMed] [Google Scholar]
- Lo C, Walsh A, Mikulincer M, Gagliese L, Zimmermann C, Rodin G. Measuring attachment security in patients with advanced cancer: psychometric properties of a modified and brief experiences in Close Relationships Scale. Psycho-Oncology. 2009;18(5):490–499. doi: 10.1002/pon.1417. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Reimer TT, Harvey JH, Marks G, Hong S-Y, Hillis SL, Lubaroff DM. Effects of housing relocation on immunocompetence and psychosocial functioning in older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001;56(2):M97–M105. doi: 10.1093/gerona/56.2.m97. [DOI] [PubMed] [Google Scholar]
- McWilliams LA, Bailey SJ. Associations between adult attachment ratings and health conditions: evidence from the National Comorbidity Survey Replication. Health Psychol. 2010;29(4):446. doi: 10.1037/a0020061. [DOI] [PubMed] [Google Scholar]
- Messingham KAN, Faunce DE, Kovacs EJ. Review article: alcohol, injury, and cellular immunity. Alcohol. 2002;28(3):137–149. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- Mikulincer M, Florian V. The relationship between adult attachment styles and emotional and cognitive reactions to stressful events. Attach. Theo. Close Relat. 1998;143:165. [Google Scholar]
- Mikulincer M, Shaver PR. The attachment behavioral system in adulthood: activation, psychodynamics, and interpersonal processes. In: Zanna M, editor. Adv. Exp. Soc. Psychol. Vol. 35. Academic Press; New York: 2003. pp. 53–152. [Google Scholar]
- Mikulincer M, Shaver PR. Attachment in Adulthood: Structure, Dynamics, and Change. Guilford Press; New York, NY, US.: 2007. [Google Scholar]
- Mikulincer M, Shaver PR. An attachment and behavioral systems perspective on social support. J. Soc. Pers. Relat. 2009;26(1):7–19. doi: 10.1177/0265407509105518. [Google Scholar]
- Mikulincer M, Shaver PR, Horesh N. Attachment bases of emotion regulation and posttraumatic adjustment. In: Snyder DK, Simpson J, Hughes JN, editors. Emotion Regulation in Couples and Families: Pathways to Dysfunction and Health. American Psychological Association; Washington, DC: 2006. pp. 77–99. [Google Scholar]
- Mikulincer M, Shaver PR, Pereg D. Attachment theory and affect regulation: the dynamics, development, and cognitive consequences of attachment-related strategies. Motivat. Emot. 2003;27(2):77–102. [Google Scholar]
- Morin CM. Insomnia: Psychological Assessment and Management. Guilford Press; New York: 1993. [Google Scholar]
- Neel C, Lo C, Rydall A, Hales S, Rodin G. Determinants of death anxiety in patients with advanced cancer. BMJ Supp. Palliat. Care. 2013 doi: 10.1136/bmjspcare-2012-000420. bmjspcare-2012-000420. [DOI] [PubMed] [Google Scholar]
- Orth-Gomer K, Johnson J. Social network interaction and mortality: a six year follow-up study of a random sample of the Swedish population. J. Chron. Dis. 1987;40(10):949–957. doi: 10.1016/0021-9681(87)90145-7. [DOI] [PubMed] [Google Scholar]
- Pietromonaco PR, Barrett LF. Working models of attachment and daily social interactions. J. Pers. Soc. Psychol. 1997;73(6):1409. doi: 10.1037//0022-3514.73.6.1409. [DOI] [PubMed] [Google Scholar]
- Pietromonaco PR, Uchino B, Dunkel Schetter C. Close relationship processes and health: implications of attachment theory for health and disease. Health Psychol. 2013;32(5):499. doi: 10.1037/a0029349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig J, Englund MM, Simpson JA, Collins WA. Predicting adult physical illness from infant attachment: a prospective longitudinal study. 2012 doi: 10.1037/a0028889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol. Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- Rholes WS, Simpson JA, Kohn JL, Wilson CL, Martin AM, III, Tran S, Kashy DA. Attachment orientations and depression: a longitudinal study of new parents. J. Pers. Soc. Psychol. 2011;100(4):567. doi: 10.1037/a0022802. [DOI] [PubMed] [Google Scholar]
- Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am. J. Epidemiol. 2010;172(4):363. doi: 10.1093/aje/kwq177. doi: 10.1093/aje/kwq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol. Methods. 2002;7(2):147–177. doi: 10.1037/1082-989x.7.2.147. [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004;130(4):601. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaver PR, Mikulincer M. Attachment-related psychodynamics. Attach. Hum. Dev. 2002;4:133–161. doi: 10.1080/14616730210154171. [DOI] [PubMed] [Google Scholar]
- Shaver PR, Mikulincer M. Attachment theory and research: resurrection of the psychodynamic approach to personality. J. Res. Pers. 2005;39(1):22–45. [Google Scholar]
- Shaver PR, Mikulincer M. Adult attachment strategies and the regulation of emotion. In: Gross JJ, editor. Handbook of Emotion Regulation. Guilford Press; New York, NY, US: 2007. pp. 446–465. [Google Scholar]
- Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One 6. 2011;(2) doi: 10.1371/journal.pone.0016103. doi: e1610310.1371/journal.pone.0016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockalingam S, Blank D, Abdelhamid N, Abbey SE, Hirschfield GM. Identifying opportunities to improve management of autoimmune hepatitis: evaluation of drug adherence and psychosocial factors. J. Hepatol. 2012;57(6):1299–1304. doi: 10.1016/j.jhep.2012.07.032. [DOI] [PubMed] [Google Scholar]
- Sockalingam S, Cassin S, Hawa R, Khan A, Wnuk S, Jackson T, Okrainec A. Predictors of post-bariatric surgery appointment attendance: the role of relationship style. Obes. Surg. 2013;23(12):2026–2032. doi: 10.1007/s11695-013-1009-9. [DOI] [PubMed] [Google Scholar]
- Sopori M. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2002;2(5):372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- Stassen FR, Vega-Córdova X, Vliegen I, Bruggeman CA. Immune activation following cytomegalovirus infection: more important than direct viral effects in cardiovascular disease? J. Clin. Virol. 2006;35(3):349–353. doi: 10.1016/j.jcv.2005.11.007. http://dx.doi.org/10.1016/j.jcv.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Steer RA, Beck AT. Beck Anxiety Inventory. 1997 [Google Scholar]
- Steptoe A, Shamaei-Tousi A, Gylfe A, Henderson B, Bergstrom S, Marmot M. Socioeconomic status, pathogen burden and cardiovascular disease risk. Heart. 2007;93(12):1567–1570. doi: 10.1136/hrt.2006.113993. 10.1136/hrt.2006.113993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. Chronic herpesvirus reactivation occurs in aging. Exp. Gerontol. 2007;42(6):563–570. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RA. Early attachment and later development. In: Cassidy J, Shaver PR, editors. Handbook of Attachment: Theory, Research, and Clinical Applications. Guilford; New York, NY: 1999. pp. 265–286. [Google Scholar]
- Tomaka J, Thompson S, Palacios R. The relation of social isolation, loneliness, and social support to disease outcomes among the elderly. J. Aging Health. 2006;18(3):359. doi: 10.1177/0898264305280993. [DOI] [PubMed] [Google Scholar]
- Trzonkowski P, Myliwska J, Szmit E, Wickiewicz J. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination –an impact of immunosenescence. Vaccine. 2003;21(25–26):3826–3836. doi: 10.1016/s0264-410x(03)00309-8. doi: 10.1016/S0264-410X(03)00309-8. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Understanding the links between social support and physical health: a life-span perspective with emphasis on the separability of perceived and received support. Perspect. Psychol. Sci. 2009;4(3):236–255. doi: 10.1111/j.1745-6924.2009.01122.x. http://dx.doi.org/10.1111/j.1745-6924.2009.01122.x. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol. Bull. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Yang EV, Webster Marketon JI, Chen M, Lo KW, Kim S-J, Glaser R. Glucocorticoids activate Epstein Barr virus lytic replication through the upregulation of immediate early BZLF1 gene expression. Brain Behav. Immun. 2010;24(7):1089–1096. doi: 10.1016/j.bbi.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]