Abstract
We report non-invasive 3D imaging of capillary blood flow within human finger cuticle by the use of Doppler optical microangiography (DOMAG) and ultra-high sensitive optical microangiography (UHS-OMAG) techniques. Wide velocity range DOMAG method is applied to provide RBC axial velocity mapping in capillary loops with ranges of ±0.9 mm/s and ±0.3 mm/s. Additionally, UHS-OMAG technique is engineered to acquire high resolution image of capillary morphology. The presented results are promising to facilitate clinical trials of treatment and diagnosis of various diseases such as diabetes, Raynaud's phenomenon, and connective tissue diseases by quantifying cutaneous blood flow changes within human finger cuticle.
Maximum projection image of the bidirectional RBC axial velocity mapping in the human finger cuticle capillaries.
Keywords: Optical coherence tomography, Doppler optical microangiography, Ultra-high sensitive optical microangiography, Skin microcirculation, Capillary blood flow
1. Introduction
Red blood cell (RBC) dynamics in capillaries plays an essential role for living tissue beds by providing oxygen and nutritive substances and disposing waste products. Monitoring the RBC dynamics is an important tool to study and diagnose various diseases such as diabetes [1], Raynaud's phenomenon (RP) [2,3], and connective tissue diseases, e.g., Scleroderma spectrum disorder (SSD) [4] and systemic sclerosis (SSc) [5]. Typically, capillary abnormalities such as dilated vessel loops and/or change in RBC velocity occur as symptoms of these diseases. Hence, an ability to quantify these abnormalities and their inter-relationship would be essential in the clinical studies of these conditions.
Microvascular changes in morphology and function have been monitored in the skin, particularly in the finger nail-fold area. Laser Doppler imaging (LDI) [6], Laser Doppler flowmetry (LDF) [7], nail-fold capillaroscopy (NC) [8] and nail-fold video capillaroscopy (NVC) [9] are the main techniques, the use of which in clinical situation has accumulated a vast amount of data of dysfunctional cutaneous blood flow responses in patients. However, there are still several limitations and disadvantages of these methods. LDI and LDF techniques are capable of obtaining averaged changes in blood flow within a large tissue volume but cannot measure the velocity in absolute values [6]. On the other hand, NC and NVC methods can visualize only some of the capillaries under the nail-fold, and they fail to provide a comprehensive outlook of the microvasculature under the skin [8,9]. Additionally, melanin absorbs light strongly in the visible spectrum making highly pigmented skin difficult to image with capillaroscopy [8]. The recording takes ~2 min or longer depending on the patient’s condition [8], and only a limited number of capillaries can be examined. These lead to subjectivity and make it difficult to measure the same set of capillaries of patients at each visit, which is crucial for treatment of connective tissue diseases.
Optical coherence tomography (OCT) has become a popular technique in determining the microstructural composition of biological tissues in vivo and in real time since its introduction in the early 1990s [10]. It provides cross-sectional images of structure below the tissue surface with axial resolutions of 1–15 µm [11]. The maximum imaging depth is limited by optical attenuation and scattering to approximately 1–3 mm in most cases.
Optical micro-angiography (OMAG) is a label-free noninvasive imaging and processing method used to obtain 3-D blood perfusion map in microcirculatory tissue beds in vivo using Fourier domain OCT (FD-OCT) [12]. Ultrahigh-sensitive OMAG (UHS-OMAG) is a variation of OMAG technique, capable of imaging microvasculature down to capillary level by separating structural tissue from dynamic scatters (e.g., moving red blood cells within patent vessels) using magnitude subtraction, phase-compensated subtraction [13,14] or eigendecomposition (ED) based [15] OMAG algorithms. It has been applied to visualize micro-vasculature in various living tissue samples such as retina [14], cerebral [16], renal microcirculation [17] and skin [18].
Doppler optical coherence tomography (DOCT) [19] is based upon OCT combined with LDF. It permits the quantitative imaging of fluid flow in highly scattering media, for example blood flow beneath the skin. Doppler optical microangiography (DOMAG) [20] is a technological extension to DOCT, in which DOCT is combined with OMAG technique to provide highly sensitive velocity mapping of blood flows within tissue beds in vivo. Phase-resolved technique [21] is commonly used to calculate the axial flow velocity of the RBC,
| (1) |
where λ is the center wavelength of light source, n is the tissue refractive index, and φ and T are the phase difference and time interval between adjacent A-scans, respectively. The maximal and minimal detectable velocities are determined by the time interval T, the π-ambiguity and the system phase noise level [21]. Mapping of RBC velocities in human finger cuticle capillaries, which are typically from ~0.3 to 1 mm/s [22], requires relatively long T, i.e. very slow imaging speed (>20 min. for one 3D scan), which is not practical in actual clinical studies with patients. Moreover, using very slow imaging speed makes inevitable bulk tissue motion artifacts more apparent in the final image, leading to difficulty in interpreting the results. Hence, RBC velocity mapping in human finger cuticle capillaries using DOMAG remains as an important and challenging task.
Lately, Shi et al. have developed a wide velocity range DOMAG technique [23] which is capable of measuring multiple velocity ranges without requiring a long scanning time by skipping A-scans for DOCT processing [24] and benefiting from step-scan strategy [25]. However, this technique is currently optimized for mouse cerebral blood flow imaging where RBC velocity in capillaries is nearly an order of magnitude larger than the typical cutaneous capillaries in human finger.
This work presents a scanning protocol specifically devised for measuring the directional RBC velocity in human finger cuticle capillaries by utilizing a wide velocity range DOMAG technique [23]. To the best of authors’ knowledge, this is the first time RBC velocity mapping is demonstrated in human finger cuticle capillaries using optical microangiography. Moreover, UHS-OMAG with ED algorithm [15] is engineered to visualize the volumetric microvasculature in human finger cuticle with a resolution high enough to delineate capillary loops. The proposed imaging modality provides fast and accurate information about the RBC dynamics in the human finger cuticle along with a clear picture of microvasculature which might be a critical step in studying various diseases that lead to capillary abnormalities.
2. System and methods
A fiber-based SD-OCT system similar to previously described in [11] is used for the experiments. The system used a superluminescent diode as the light source, which has the central wavelength of 1340 nm and bandwidth of 110 nm that provided a ~7 µm axial resolution in the air. In the sample arm, a 10× objective lens with 18 mm focal length was used to achieve lateral resolution of ~7 µm. The output light from the interferometer was routed to a home-built spectrometer, which had a designed spectral resolution of ~0.141 nm that provided a detectable depth range of ~3 mm on each side of the zero delay line. The line rate of the camera was 92 kHz. The system had a measured dynamic range of 105 dB with the light power of 3.5 mW at sample surface. The operations for probe beam scanning, data acquisition, data storage and hand-shaking between them were controlled by a custom software package written in Labview.
Wide velocity range DOMAG method relies on a step-scanning protocol with repeated A-scans at each step [23]. Performing DOCT processing between adjacent A-scans in the data set provides axial velocity mapping of blood flows within tissue bed in vivo. In addition, selected numbers of A-scans can be skipped during processing to increase the time interval, T, between processed A-scans. This enables to obtain multiple velocity ranges without a significant impact on the scanning time (Eq. (1)). Slower velocity ranges can be acquired by skipping larger number of A-scans. However correlation degree (CD) between A-scans, which should be high for accurate imaging, diminishes as skipped A-scan number increases [23]. Considering this trade-off, skipping three A-scans is decided to be the most effective strategy to speed up the imaging without sacrificing from high correlation degree between A-scans. The imaging rate was adjusted at 0.5 frames (B-scan) per second (fps) which gives long enough T for capturing RBC axial velocities in capillaries with a corresponding axial velocity range of ±0.9 mm/s and ±0.3 mm/s when one and three A-lines are skipped, respectively (Eq. (1)).
CD is also affected by the mechanical stability of the scanner used in B-scan. Hence, the galvanometric scanner (Thorlabs Inc.) is characterized to find the most stable operation region by calculating the CD between the A-lines for various time points [23]. For the system used, the most stable time period is found to be between 0.25–1.2 seconds after moving to a next step, and the camera is triggered only in this time frame of each step to capture A-scans. Each B scan had 1.5 mm span over the sample consisting of 5000 A-lines by acquiring 25 A-lines at each 200 discrete steps. In the elevational direction, there were 200 B scans along ~1.2 mm. Hence the data cube of each 3D image (C scan) was composed of 1024 by 5000 by 200 (z-x-y) voxels, which took ~6.7 min to acquire. During this time, the volunteer sit on a chair with his elbow supported on the imaging table and his finger is gently fixed in a holder arranged to fit. The stage is tilted 20° with respect to table to have detectable axial component (relative to top view) of the RBC velocity. Images are obtained by Doppler processing of complex signals among A-lines. Phase variance mask is used to separate Doppler flow signals from noisy phase background. Post-processing time took ~15 minutes using commercial software MATLAB®.
Furthermore, UHS-OMAG technique [14] is engineered with a separate scanning protocol to visualize the volumetric microvasculature in the same area. For UHS-OMAG, each frame (B scan) consists of 300 A-lines over 1.5 mm span with imaging rate of 240 fps. In the elevational direction, there were total number of 1920 B scans along ~1.2 mm with 8 repetition in each location. Hence the data cube of each 3D image was composed of 1024 by 300 by 240 (z-x-y) voxels, which took ~8 s to acquire. Moreover, ~7 µm lateral and axial resolutions are good enough to differentiate the arteriole-end capillary from venule-end capillary. ED based algorithm [15] is implemented in MATLAB® to visualize microvasculature, which took ~3 minutes to post-process.
3. Results and discussion
Human finger cuticle with 1.5×1.2 mm2 area, depicted in Fig. 1(a), is imaged to obtain RBC velocity mapping. The majority of the microvasculature in the skin resides in the papillary dermis 1–2 mm below the surface of the skin where capillaries range in diameter from 10 to 35 µm [26]. The cuticle, which is also called the eponychium, is a barrier layer between the skin and the nail plate fusing these structures together. Its supply and waste disposal is carried out by RBCs travelling within loop-shaped capillaries. Imaging this area is favorable because of thin epidermis layer compared to other areas in nail-fold region.
Figure 1.
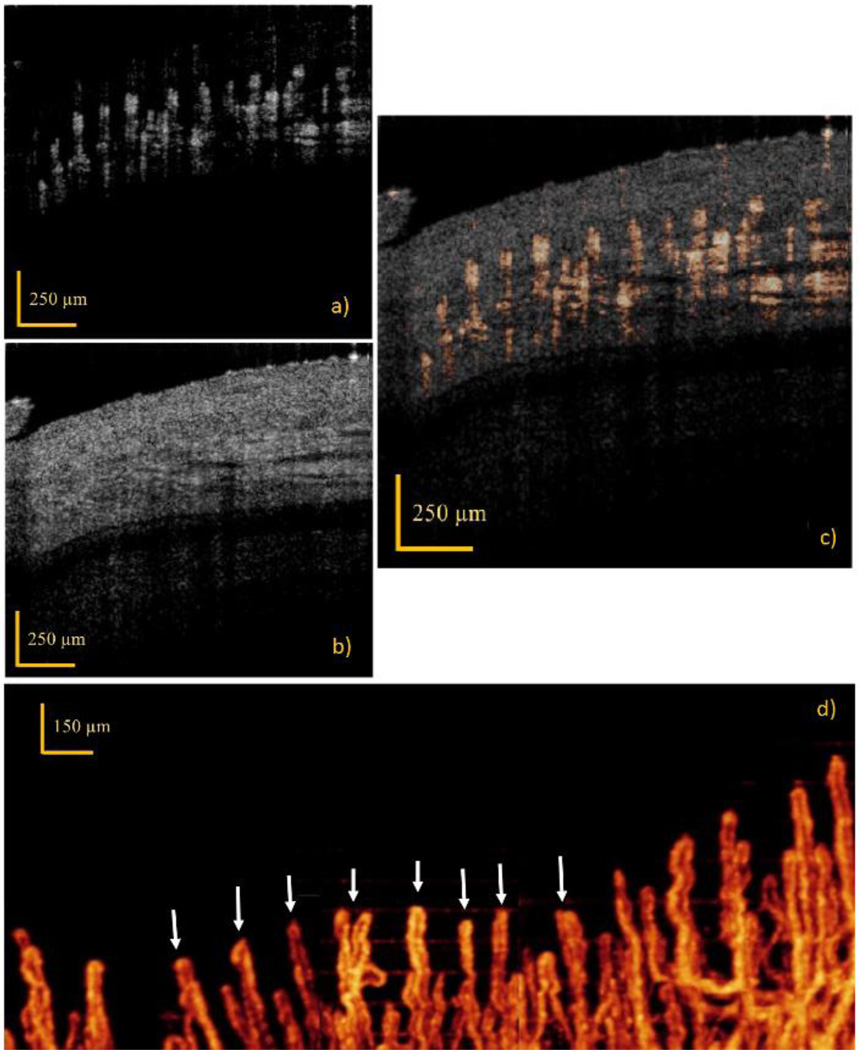
(a) Drawing of the human finger to be imaged. The red rows show the directions of the scanning. (b–c) Maximum projection image of the bidirectional RBC axial velocity mapping in the human finger cuticle capillaries, where (b) shows a range of ±0.3 mm/s, and (c) shows a range of ±0.9 mm/s. The red and green lines corresponds to the arteriole-end and venule-end capillaries, respectively. The white dashed line in (b) shows the portion of the image deteriorated by the bulk motion. (d–e) Cross section views (B-scan) of (b) and (c), respectively. B-scan location is pointed out with the yellow dashed lines.
Before imaging, the volunteer stayed inside the room at a stable temperature of 23 °C for 15 min to have a normal blood flow in capillaries. The fourth finger of right hand is preferred for the imaging due to the high transparency of the skin [9]. Mineral oil is applied on the nail-fold area to enhance the penetration depth and decrease the surface reflection.
The bidirectional en face maximum projection images in Fig. 1(b–c) show the arteriole-end and venule-end capillaries as red and green lines, respectively. RBC axial velocity information is coded with a color bar in a range of ±0.3 mm/s in Fig 1(b). This range enables to map RBC axial velocities accurately in most locations, yet some of the larger velocities stayed out of range. In order to catch few locations with a larger velocity, a range of ±0.9 mm/s is demonstrated in Fig. 1(c). Figure 1(d–e) show the cross section views (B-scan) of Fig. 1(b) and Fig. 1(c), respectively, where B-scan location is pointed out with the yellow dashed lines. From the 2D B-scan images that form the en face projection image in Fig 1(c), the average axial velocities are calculated in MATLAB® as ~0.23 mm/s for arteriole-end and ~0.21 mm/s for venule-end capillaries. By using the tilt angle, 20°, between axial and actual velocity directions, the average absolute velocities are found as ~0.67 mm/s and ~0.61 mm/s for arteriole-end and venule-end capillaries, respectively. These results match with the expected values considering the typical RBC average velocity measured with healthy subjects using NVC is around 0.8 mm/s [22].
Dense scanning protocol enables imaging various RBCs at multiple locations of the capillaries to form continuous lines, however the discontinuities still appear due to lack of RBC passing through the capillaries while focused light is scanning on these areas. Few out-of-range flow is also observed as phase-wrapped signals, seen as yellow color (some of them pointed out with white arrows in Fig 1(b)). In addition, involuntary movements of the finger, occurred during the 6.7 min imaging, deteriorated small portion of the image around the white dashed line in Fig. 1(b).
The volumetric UHS-OMAG imaging results of capillary network in the vicinity of the previously imaged area (Fig 1(b–c)) is given in Fig. 2. The maximum intensity projection view of capillary loop region at 150 – 400µm depth is demonstrated in Fig 2(d), which is arranged to stay in the depth of focus of the lens. Three images captured at adjacent regions are combined to extend the visualized area. The corresponding capillaries shown in the bidirectional flow image (Fig. 1(b–c)) is pointed out in Fig. 2(d) with white arrows. By combining Fig. 2(d) with Fig. 1(b), the size, the shape and the orientation of the arteriole-end and venule-end capillaries can be extracted along with RBC velocity information.
Figure 2.
In vivo imaging results obtained by UHS-OMAG. (a–c) Cross-sectional images along the B-frame, where (a) shows the flow image, (b) show the structural image, and (c) show the flow image stitched on top of the structural image. (d) UHS-OMAG maximum intensity projection view of microcirculation network at 150 – 400µm depth, where 3D vascular signals are projected into a single image. White arrows point out the corresponding capillary loops where RBC velocity is mapped in Fig 1(b). Comparing this figure with Fig. 1(b–c), the arteriole-end capillary can be differentiated from venule-end capillary.
Progression of various connective tissue diseases, diabetes, and RP are reflected on morphological and functional changes in microvasculature. The ability of visualizing structural alterations of capillaries with RBC velocity mapping within a human finger cuticle might be a critical aid in treatment or diagnosis of these diseases. The presented imaging modality enables imaging large number of capillaries in one 3D scan which is crucial to decrease subjectivity and to locate the same set of capillaries of patients at each visit. The velocity range is easily adjustable by slightly changing the frame rate for various clinical studies. Moreover, independent of patient’s skin characteristics, the cutaneous microcirculation in human finger nail-fold area with different depths can also be investigated which is not possible with alternative methods.
Relatively long imaging time of DOMAG, 6.7 min, did not have significant impact on the final image, yet it might be uncomfortable for some patients. The current method will be further tailored in future studies by decreasing the amount of A-scans acquired in each step in order to increase the system imaging speed, and/or by using less number of B-scans to decrease the required imaging time. Furthermore, other imaging protocols may be utilized to achieve shorter imaging time. For instance, DOCT processing can be performed among A-scans of repeated B-scans instead of between adjacent A-scans. In this way, the time interval, T, between processed A-scans can be dramatically increased so that it is sensitive to the capillary blood flows, and on the other hand the strategy would enable faster imaging speed of the system. In addition, DOCT may be implemented into a full field OCT system [27] where adjacent 2D cross sections are processed in 3D volume, which can significantly reduce the overall imaging time.
4. Conclusion
Having a sensitive and reproducible technique of quantifying microcirculatory flow in human finger cuticle enables to measure microvascular disease progression and/or responsiveness to treatment. In this paper, by combining DOMAG and UHS-OMAG techniques, a reliable and comprehensive imaging of the microvasculature in the human finger cuticle is presented. 1.5×1.2 mm2 area of human finger cuticle is imaged using wide velocity range DOMAG to provide RBC velocity mapping in capillary loops with ranges of ±0.9 mm/s and ±0.3 mm/s. Moreover, UHS-OMAG technique is used to visualize capillary morphology with a resolution high enough to differentiate the size and shape of arteriole-end and venule-end capillaries. This approach is promising to facilitate clinical trials of treatment aimed at improving the peripheral circulation, which have so far relied on outcome measures that are either inaccurate or tedious.
Acknowledgments
The work was supported in part by National Institutes of Health grants (R01HL093140, and R01EB009682).
References
- 1.Murray AK, Feng K, Moore TL, Allen PD, Taylor CJ, Herrick AL. Microcirculation. 2011;18(6):440–447. doi: 10.1111/j.1549-8719.2011.00104.x. [DOI] [PubMed] [Google Scholar]
- 2.Tooke JE. Diabetes. 1995;44(7):721–726. doi: 10.2337/diab.44.7.721. [DOI] [PubMed] [Google Scholar]
- 3.Wollersheim H, Reyenga J, Thien TH. Scandinavian journal of clinical & laboratory investigation. 1988;48(1):91–95. [PubMed] [Google Scholar]
- 4.Ingegnoli F, Boracchi P, Gualtierotti R, Lubatti C, Meani L, Zahalkova L, Zeni S, Fantini F. Arthritis and rheumatism. 2008;58(7):2174–2182. doi: 10.1002/art.23555. [DOI] [PubMed] [Google Scholar]
- 5.Sulli A, Secchi ME, Pizzorni C, Cutolo M. Annals of the rheumatic diseases. 2008;67(6):885–887. doi: 10.1136/ard.2007.079756. [DOI] [PubMed] [Google Scholar]
- 6.Clark S, Campbell F, Moore T, Jayson MI, King Ta, Herrick aL. Microvascular research. 1999;57(3):284–291. doi: 10.1006/mvre.1998.2124. [DOI] [PubMed] [Google Scholar]
- 7.Debbabi H, Bonnin P, Ducluzeau PH, Lefthériotis G, Levy BI. American journal of hypertension. 2010;23(5):541–546. doi: 10.1038/ajh.2010.10. [DOI] [PubMed] [Google Scholar]
- 8.Shore AC. British journal of clinical pharmacology. 2000;50(6):501–513. doi: 10.1046/j.1365-2125.2000.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi D, Russo A, Manna E, Binello G, Baldovino S, Sciascia S, Roccatello D. Autoimmunity reviews. 2013;12(8):821–825. doi: 10.1016/j.autrev.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Huang D, Swanson E, Lin C, Schuman J, Stinson W, Chang W, Hee M, Flotte T, Gregory K, et al. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomolins PH, Wang RK. Journal of Physics D: Applied Physics. 2005;38:2519–2535. [Google Scholar]
- 12.Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A. Optics Express. 2007;15(7):4083–4097. doi: 10.1364/oe.15.004083. [DOI] [PubMed] [Google Scholar]
- 13.An L, Qin J, Wang RK. Optics Express. 2010;18:8220–8228. doi: 10.1364/OE.18.008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An L, Shen TT, Wang RK. Journal of Biomedical Optics. 2011;16(10):106013. doi: 10.1117/1.3642638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yousefi S, Zhi Z, Wang RK. IEEE Transactions on Biomedical Engineering. 2011;58(8):2316–2323. doi: 10.1109/TBME.2011.2152839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia Y, Li P, Wang RK. Journal of Biomedical Optics. 2011;16(9):096019. doi: 10.1117/1.3625238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhi Z, Jung Y, Jia Y, An L, Wang RK. Biomedical optics express. 2011;2(5):1059–1068. doi: 10.1364/BOE.2.001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin J, Jiang J, An L, Gareau D, Wang RK. Lasers in surgery and medicine. 2011;43(2):122–129. doi: 10.1002/lsm.20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, Chen Z, Saxer C, Xiang S, de Boer JF, Nelson JS. Optics Letters. 2000;25(2):114. doi: 10.1364/ol.25.000114. [DOI] [PubMed] [Google Scholar]
- 20.Wang RK, An L. Optics express. 2009;17(11):8926–8940. doi: 10.1364/oe.17.008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendargo HC, McNabb RP, Dhalla A-H, Shepherd N, Izatt Ja. Biomedical optics express. 2011;2(8):2175–2188. doi: 10.1364/BOE.2.002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mugii N, Hasegawa M, Matsushita T, Hamaguchi Y, Horie S, Yahata T, Inoue K, Someya F, Fujimoto M, et al. Rheumatology. 2011;50(6):1091–1098. doi: 10.1093/rheumatology/keq430. [DOI] [PubMed] [Google Scholar]
- 23.Shi L, Qin J, Dziennis S, Wang RK. Variable-range Doppler optical microangiography using stabilized step scanning and phase variance binarized mask. Proc. SPIE 8580, Dynamics and Fluctuations in Biomedical Photonics X. 2013:858018. [Google Scholar]
- 24.Ren H, Du C, Yuan Z, Park K, Volkow ND, Pan Y. Molecular psychiatry. 2012;17(10):1017–1025. doi: 10.1038/mp.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meemon P, Rolland JP. Biomedical optics express. 2010;1(3):3116–3121. doi: 10.1364/BOE.1.000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bravermay I. Microcirculation. 1997;4(3):328–340. [Google Scholar]
- 27.Dubois A, Grieve K, Moneron G, Lecaque R, Vabre L, Boccara C. Applied Optics. 2004;43(14):2874–2883. doi: 10.1364/ao.43.002874. [DOI] [PubMed] [Google Scholar]