Abstract
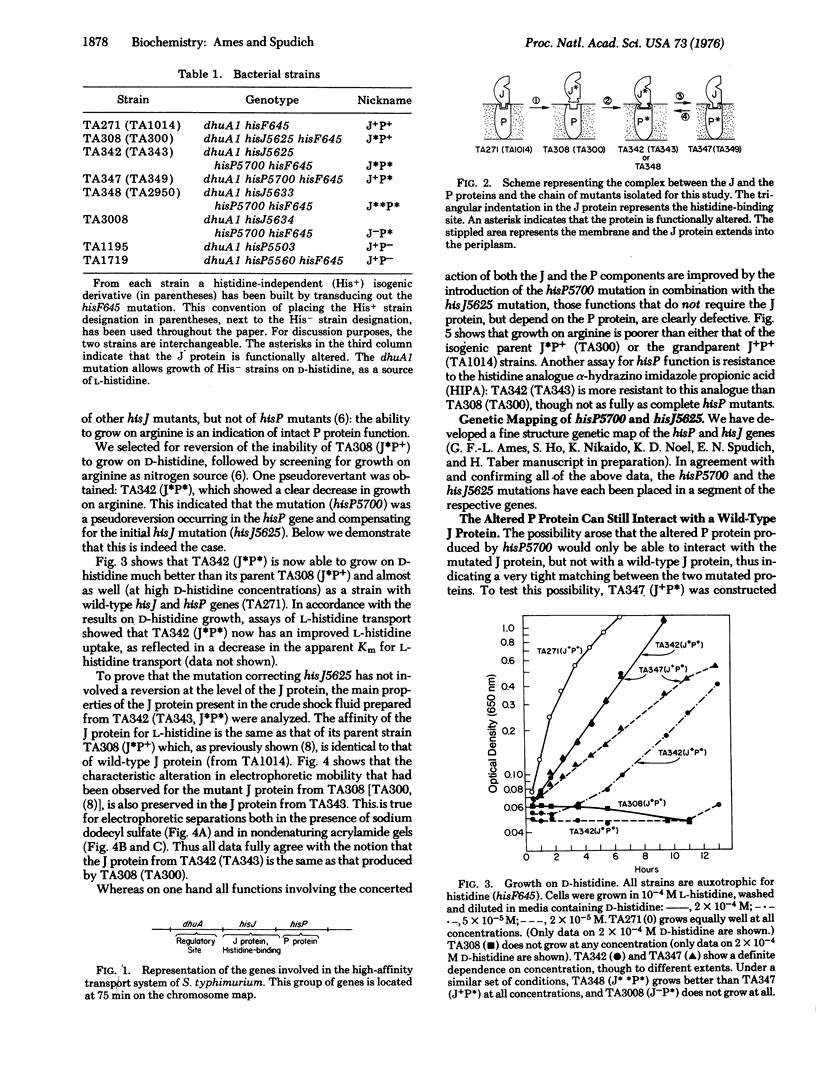
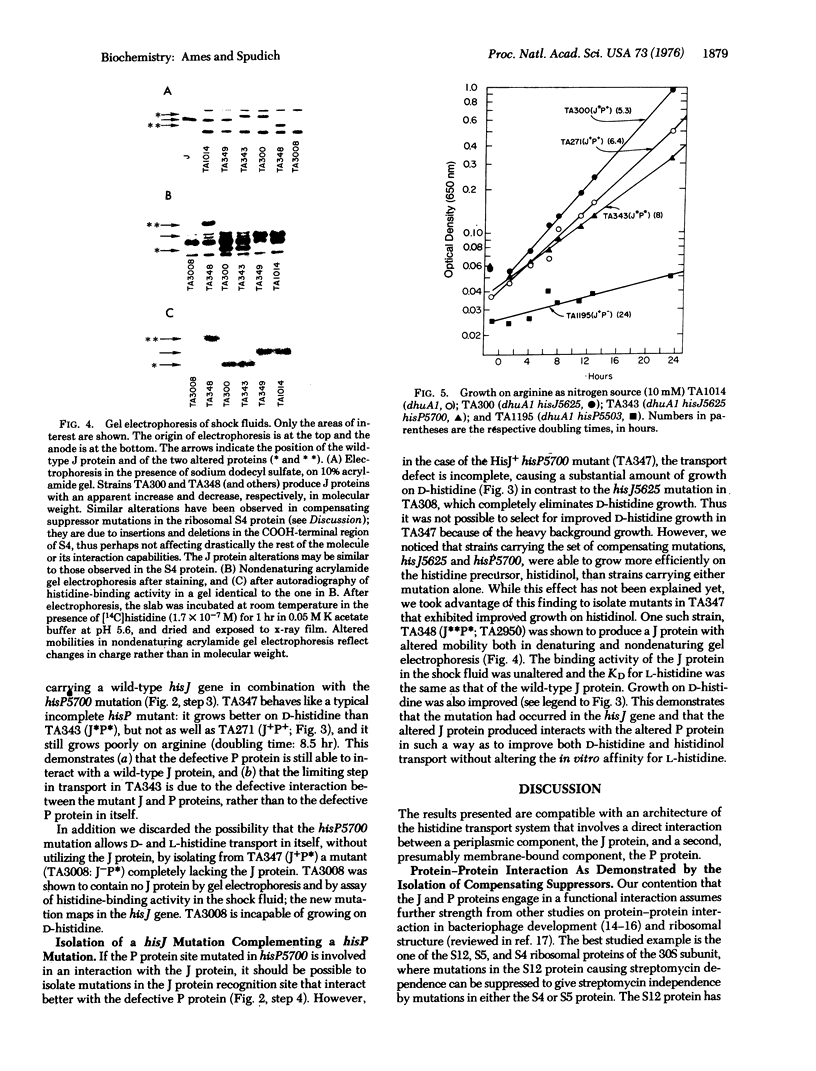
A component of the high-affinity histidine transport system in Salmonella typhimurium, the periplasmic histidine-binding protein J, interacts with another transport component, the P protein. A mutant J protein, with a defective interaction site but intact histidine-binding site, can function in histidine transport if an appropriate compensating mutation is introduced in the P protein. The interaction between the J and P proteins is an obligatory step in transport. The significance of this interaction and of the involvement of the P protein in multiple transport functions is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Lever J. E. The histidine-binding protein J is a component of histidine transport. Identification of its structural gene, hisJ. J Biol Chem. 1972 Jul 10;247(13):4309–4316. [PubMed] [Google Scholar]
- Ames G. F., Lever J. Components of histidine transport: histidine-binding proteins and hisP protein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1096–1103. doi: 10.1073/pnas.66.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Jarvik J., Botstein D. Conditional-lethal mutations that suppress genetic defects in morphogenesis by altering structural proteins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2738–2742. doi: 10.1073/pnas.72.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreishman G. P., Robertson D. E., Ho C. PMR studies of the substrate induced conformational change of glutamine binding protein from E. coli. Biochem Biophys Res Commun. 1973 Jul 2;53(1):18–23. doi: 10.1016/0006-291x(73)91394-6. [DOI] [PubMed] [Google Scholar]
- Kustu S. G., Ames G. F. The hisP protein, a known histidine transport component in Salmonella typhimurium, is also an arginine transport component. J Bacteriol. 1973 Oct;116(1):107–113. doi: 10.1128/jb.116.1.107-113.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S. G., Ames G. F. The histidine-binding protein J, a histidine transport component, has two different functional sites. J Biol Chem. 1974 Nov 10;249(21):6976–6983. [PubMed] [Google Scholar]
- Ordal G. W., Adler J. Properties of mutants in galactose taxis and transport. J Bacteriol. 1974 Feb;117(2):517–526. doi: 10.1128/jb.117.2.517-526.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R., Rotman B. Evidence for binding protein-independent substrate translocation by the methylgalactoside transport system of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Feb;72(2):423–427. doi: 10.1073/pnas.72.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel S., Ferro-Luzzi Ames G., Noel K. D. Hydrophobic chromatography in the purification of the histidine-binding protein J from Salmonella typhimurium. Arch Biochem Biophys. 1973 Nov;159(1):174–179. doi: 10.1016/0003-9861(73)90442-6. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Sommer A., Traut R. R. Identification by diagonal gel electrophoresis of nine neighboring protein pairs in the Escherichia coli 30 S ribosome crosslinked with methyl-4-mercaptobutyrimidate. J Mol Biol. 1975 Oct 5;97(4):471–481. doi: 10.1016/s0022-2836(75)80054-4. [DOI] [PubMed] [Google Scholar]
- Strange P. G., Koshland D. E., Jr Receptor interactions in a signalling system: competition between ribose receptor and galactose receptor in the chemotaxis response. Proc Natl Acad Sci U S A. 1976 Mar;73(3):762–766. doi: 10.1073/pnas.73.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Coppo A., Manzi A., Martire G., Pulitzer J. F. Design of a system of conditional lethal mutations (tab/k/com) affecting protein-protein interactions in bacteriophage T4-infected Escherichia coli. J Mol Biol. 1975 Aug 25;96(4):563–578. doi: 10.1016/0022-2836(75)90139-4. [DOI] [PubMed] [Google Scholar]
- Wilson O. H., Holden J. T. Stimulation of arginine transport in osmotically shocked Escherichia coli W cells by purified arginine-binding protein fractions. J Biol Chem. 1969 May 25;244(10):2743–2749. [PubMed] [Google Scholar]






