Abstract
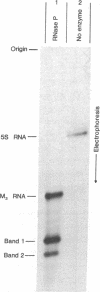
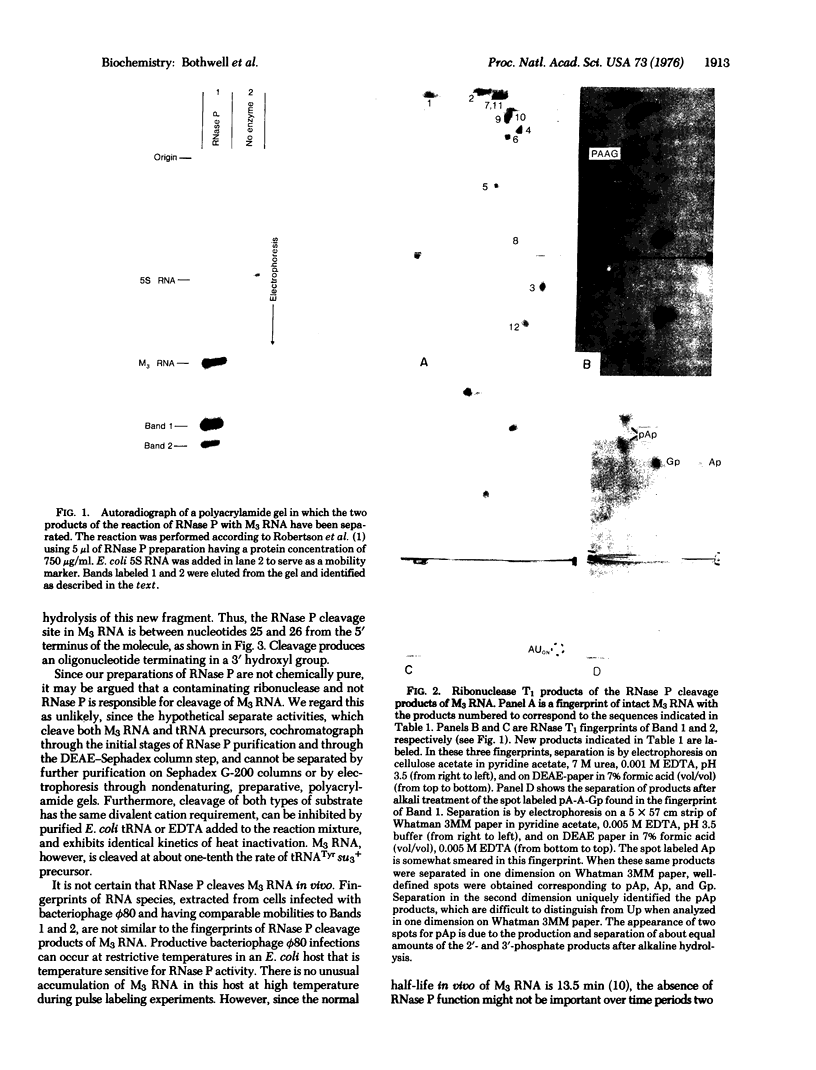
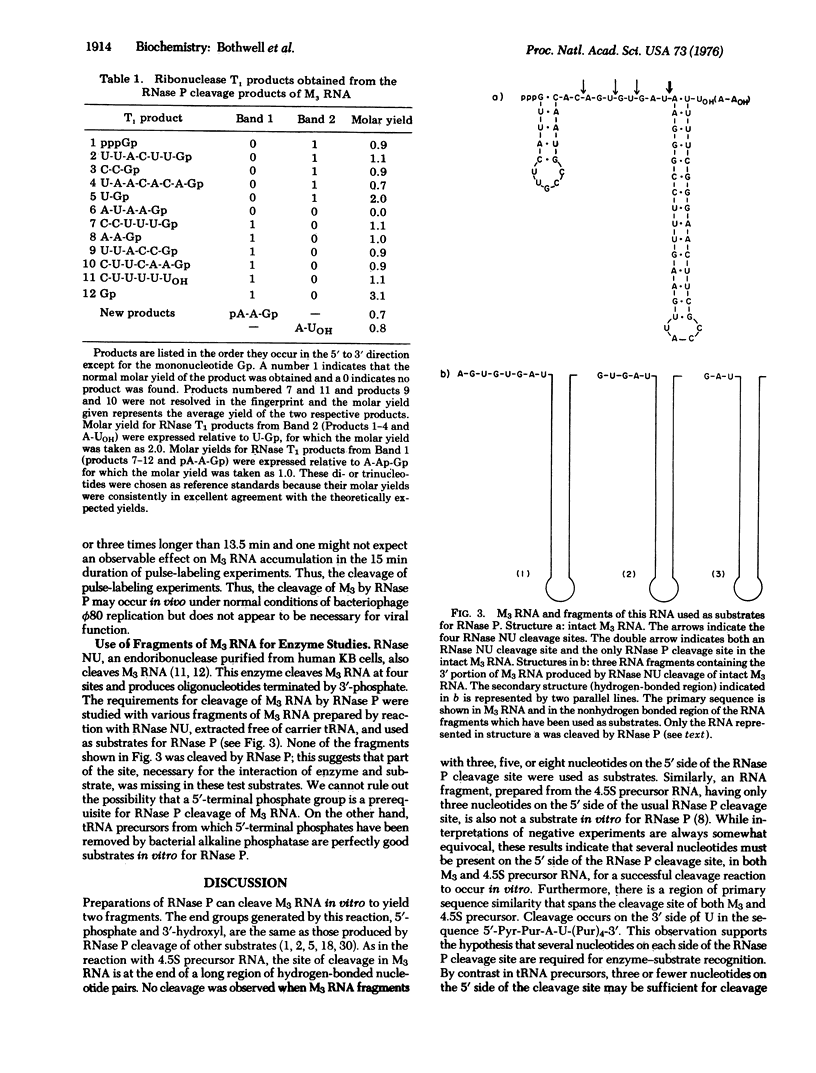
RNase P can cleave in vitro a bacteriophage phi80-induced RNA which is 62 nucleotides long [M3 RNA, G. Pieczenik et al. (1972) Arch. Biochem. Biophys. 152, 152-165] to yield two specific fragments 25 and 37 nucleotides long. As is the case for another substrate of RNase P; the precursor to Escherichia coli 4.5S RNA, the cleavage site in M3 RNA is at the end of a long double-stranded region immediately adjacent to a single-stranded segment. Similar nucleotide sequences span the cleavage site in both substrates. These and other features of the reaction of RNase P with M3 and 4.5S precursor RNA are different from some aspects of the reaction of this enzyme with tRNA precursor molecules. A qualitative scheme is presented that is directed towards the understanding of the differences in RNase P cleavage site specificity for these substrates.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson J. N., Gefter M. L., Barnett L., Landy A., Russell R. L., Smith J. D. Mutant tyrosine transfer ribonucleic acids. J Mol Biol. 1970 Jan 14;47(1):15–28. doi: 10.1016/0022-2836(70)90398-0. [DOI] [PubMed] [Google Scholar]
- Altman S., Bothwell A. L., Stark B. C. Processing of E. coli tRNA Tyr precursor RNA in vitro. Brookhaven Symp Biol. 1975 Jul;(26):12–25. [PubMed] [Google Scholar]
- Altman S., Smith J. D. Tyrosine tRNA precursor molecule polynucleotide sequence. Nat New Biol. 1971 Sep 8;233(36):35–39. doi: 10.1038/newbio233035a0. [DOI] [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Altman S. Characterization of ribonuclease NU cleavage sites in a bacteriophage phi80-induced ribonucleic acid. J Biol Chem. 1975 Feb 25;250(4):1460–1463. [PubMed] [Google Scholar]
- Bothwell A. L., Altman S. Partial purification and properties of an endoribonuclease isolated from human KB cells. J Biol Chem. 1975 Feb 25;250(4):1451–1459. [PubMed] [Google Scholar]
- Chang S. E., Smith J. D. Structural studies on a tyrosine tRNA precursor. Nat New Biol. 1973 Dec 12;246(154):165–168. doi: 10.1038/newbio246165a0. [DOI] [PubMed] [Google Scholar]
- Chang S., Carbon J. The nucleotide sequence of a precursor to the glycine- and threonine-specific transfer ribonucleic acids of Escherichia coli. J Biol Chem. 1975 Jul 25;250(14):5542–5555. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin B. E. Studies and sequences of Escherichia coli 4.5 S RNA. J Biol Chem. 1975 Jul 25;250(14):5426–5437. [PubMed] [Google Scholar]
- Guthrie C., Seidman J. G., Altman S., Barrell B. G., Smith J. D., McClain W. H. Identification of tRNA precursor molecules made by phage T4. Nat New Biol. 1973 Nov 7;246(149):6–11. doi: 10.1038/newbio246006a0. [DOI] [PubMed] [Google Scholar]
- Guthrie C. The nucleotide sequence of the dimeric precursor to glutamine and leucine transfer RNAs coded by bacteriophage T4. J Mol Biol. 1975 Jul 15;95(4):529–547. doi: 10.1016/0022-2836(75)90315-0. [DOI] [PubMed] [Google Scholar]
- Ikemura T., Shimura Y., Sakano H., Ozeki H. Precursor molecules of Escherichia coli transfer RNAs accumulated in a temperature-sensitive mutant. J Mol Biol. 1975 Jul 25;96(1):69–86. doi: 10.1016/0022-2836(75)90182-5. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Sussman J. L., Suddath F. L., Quigley G. J., McPherson A., Wang A. H., Seeman N. C., RICH A. The general structure of transfer RNA molecules. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4970–4974. doi: 10.1073/pnas.71.12.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. A., Rosenberg M., Steitz J. A. Nucleotide sequences of the 5' and 3' termini of bacteriophage T7 early messenger RNAs synthesized in vivo: evidence for sequence specificity in RNA processing. J Mol Biol. 1974 Nov 15;89(4):767–776. doi: 10.1016/0022-2836(74)90051-5. [DOI] [PubMed] [Google Scholar]
- McClain W. H., Seidman J. G. Genetic perturbations that reveal tertiary conformation of tRNA precursor molecules. Nature. 1975 Sep 11;257(5522):106–110. doi: 10.1038/257106a0. [DOI] [PubMed] [Google Scholar]
- Paddock G., Abelson J. Sequence of T4, T2 and T6 bacteriophage species I RNA and specific cleavage by an E. coli endonuclease. Nat New Biol. 1973 Nov 7;246(149):2–6. doi: 10.1038/newbio246002a0. [DOI] [PubMed] [Google Scholar]
- Pieczenik G., Barrell B. G., Gefter M. L. Bacteriophage phi 80-induced low molecular weight RNA. Arch Biochem Biophys. 1972 Sep;152(1):152–165. doi: 10.1016/0003-9861(72)90203-2. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Altman S., Smith J. D. Purification and properties of a specific Escherichia coli ribonuclease which cleaves a tyrosine transfer ribonucleic acid presursor. J Biol Chem. 1972 Aug 25;247(16):5243–5251. [PubMed] [Google Scholar]
- Robertson H. D., Dunn J. J. Ribonucleic acid processing activity of Escherichia coli ribonuclease III. J Biol Chem. 1975 Apr 25;250(8):3050–3056. [PubMed] [Google Scholar]
- Robertson H. D., Webster R. E., Zinder N. D. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968 Jan 10;243(1):82–91. [PubMed] [Google Scholar]
- Robertus J. D., Ladner J. E., Finch J. T., Rhodes D., Brown R. S., Clark B. F., Klug A. Structure of yeast phenylalanine tRNA at 3 A resolution. Nature. 1974 Aug 16;250(467):546–551. doi: 10.1038/250546a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Yamada S., Ikemura T., Shimura Y., Ozeki H. Temperature sensitive mutants of Escherichia coli for tRNA synthesis. Nucleic Acids Res. 1974 Mar;1(3):355–371. doi: 10.1093/nar/1.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl P., Primakoff P. Mutants of Escherichia coli thermosensitive for the synthesis of transfer RNA. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2091–2095. doi: 10.1073/pnas.70.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D., Barnett L., Brenner S., Russell R. L. More mutant tyrosine transfer ribonucleic acids. J Mol Biol. 1970 Nov 28;54(1):1–14. doi: 10.1016/0022-2836(70)90442-0. [DOI] [PubMed] [Google Scholar]
- Vögeli G., Grosjean H., Söll D. A method for the isolation of specific tRNA precursors. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4790–4794. doi: 10.1073/pnas.72.12.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]