Abstract
Binge drinking, a common pattern of alcohol ingestion, is known to potentiate liver injury caused by chronic alcohol abuse. This study was aimed to investigate theeffects of acute binge alcohol onhypoxia-inducible factor-1α (HIF-1α)-mediated liver injury and rolesof alcohol metabolizing enzymes in alcohol-induced hypoxia and hepatotoxicity. Mice and human specimens assigned as binge or non-binge group were analyzed for blood alcohol concentration (BAC), alcohol metabolizing enzymes, HIF-1α-related protein nitration and apoptosis. Binge alcohol promoted acute liver injury in mice with elevated levels of ethanol-inducible cytochrome P450-2E1 (CYP2E1) and hypoxia, both of which were co-localized in centrilobular areas. We observed positive correlations among elevated BAC, CYP2E1, and HIF-1α in mice and humans exposed to binge alcohol. TheCYP2E1 protein levels(r=0.629, p=0.001) and activities (r=0.641, p=0.001) showed significantly positive correlation with BACs in human livers. HIF-1α levels were also positively correlated with BACs (r=0.745, p<0.001) or CYP2E1activities(r=0.792, p<0.001) in humans. Binge alcoholpromotedprotein nitration and apoptosis with significant correlations observed between inducible nitric oxide synthase and BACs, CYP2E1, or HIF-1α in human specimens. Binge alcohol-induced HIF-1α activation and subsequent protein nitration orapoptosisseen in wild-type were significantly alleviatedin the corresponding Cyp2e1-null mice while pretreatment with an HIF-1α inhibitor PX-478 prevented HIF-1α elevation with a trend of decreased levels of 3-nitrotyrosine and apoptosis, supporting the roles of CYP2E1and HIF-1α in binge alcohol-mediatedprotein nitration and hepatotoxicity. Thus binge alcohol promotes acute liver injury in mice and humans at least partly through a CYP2E1-HIF-1α-dependent apoptosis pathway.
Keywords: Binge ethanol, Alcohol metabolizing enzymes, CYP2E1, Hypoxia, Protein Nitration, Apoptosis, Human Liver Injury
Introduction
Alcoholic liver disease (ALD) is a major cause of morbidity and mortality globally, ranging from simple steatosis to severe forms of liver injury such as steatohepatitis, cirrhosis and hepatocellular carcinoma [1-4]. Drinking pattern is known to markedly affect ALD [3].Binge drinking, a prevalent pattern of alcohol ingestion with more than 75% of alcohol consumed [5],is defined by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) as consuming more than 4~5 alcoholic beverages within 2 hours and elevating blood alcohol concentration (BAC) to greater than 0.08% [6].Binge drinking can cause alcoholic hepatitis and liver cirrhosis with increased mortality rates [7,8]. The binge alcohol phenomenon is now prevalentin young populations in many Western countries and particularly alarming [8]. Although binge drinking is more common than chronic alcoholism and causes more social and biomedical problems, less attention has been paid to acute binge alcohol liver injury.
Alcohol ingestion causes central venous hypoxia due to increased hepatic oxygen consumption with a smaller increase in oxygen delivery [1]. Hypoxia plays a role in pathophysiological conditions such as inflammation, oxidative stress and is chemic apoptosis [9]. Hypoxia-inducible factor 1 (HIF-1), the major transcriptional factor activated during hypoxia, is known to regulatehypoxia-associated responses [10]. Under normoxic condition, HIF-1α is rapidly degraded in a ubiquitin-dependent manner, whereas it escapes degradation in hypoxia. Accumulated HIF-1α dimerizes with HIF-1β, translocates to nucleus, and regulates numerous gene transcription programs [11,12]. Hypoxia can lead to apoptosis by permeabilization of mitochondrial membrane, resulting in release of mitochondrial cytochrome c into cytosol [10]. In hypoxia, HIF-1α also regulates inducible nitric oxide synthase (iNOS) expression for production of nitric oxide (NO) [13,14].
Ethanol is metabolized to acetaldehyde by alcohol dehydrogenase (ADH), ethanol-inducible cytochrome P450 2E1 (CYP2E1) and catalase (CAT), despite playing a minor role, while acetaldehyde is further oxidized to acetate by mitochondrial aldehyde dehydrogenase (ALDH2) [4,15]. Elevated CYP2E1 was reported to promote hypoxia and activate HIF-1α in mice with chronic ethanol feeding [12]. Since CYP2E1 is induced by binge as well as chronic ethanol ingestion [16,17], we studied the effects of binge alcohol on HIF-1α-related liver injury. However, comprehensive comparison analysis on the semetabolizing enzymes inalcohol-induced hypoxia and HIF-1α activation has not been fully studied, especially in case of binge alcohol ingestion as the most common pattern of alcohol drinking [5,8,18]. Therefore, the primary aim of this study was to determine the relationships between the major alcohol metabolic enzymes including CYP2E1 and HIF-1α-related liver injury following binge alcohol exposure. Significant progress has been made in developing various animal models for studying the mechanisms of ALD, which cannot be performed step wise in humans[2,19]. Therefore, the secondary aim of this study was to compare the outcomes and study the mechanisms of liver injury in humans and mice exposed to binge alcohol.
Materials and Methods
Animal experiments
All animal experimental procedures were carried out by following the NIH guidelines for small animal experiments and approved by the NIAAA Institutional Animal Care and Use Committee. All mice were housed in temperature- and light-controlled animal facilities, and allowed to have access to water and standard rodent chow ad libitum. All mice were fed with the NIH31 autoclavable rodent diet, which contains 5.5% total fat where animal fat (exclusively derived from menhaden fish oil) is 15% while vegetable oil (mostly from soy) represents 85%. Female wild-type (WT) mice on 129/Svj background (n=3-4/group) were orally administered with 3 doses of ethanol (6 g/kg) or dextrose (control) at 12-h intervals and sacrificed at 1, 2, 4, 9, and 24 h after the lastdoseby method recently described [17]. The whole blood was centrifuged at 3,000 rpm for 5 min, and plasma was separated immediately. Plasma ALT activity was determined using a clinical chemistry analyzer (IDEXX Vet Test, IDEXX Laboratories, Westbrook, ME, USA). Plasma BAC was also measured by using a kit (Sigma Chemical, St. Louis, MO, USA). A small piece from the largest liver lobe of each mouse was collected for liver histology and immunohistochemistry. The remaining liver tissues were frozen in liquid nitrogen and stored at −80 −C until further analysis. To determine the role of CYP2E1 in binge alcohol-induced hypoxic liver injury, age-matched female WT and Cyp2e1-null mice (n=3/group) were administered with 3 ethanol doses and euthanized at 6 h after the last dose [17]. To determine the role of HIF-1α in bingealcohol-mediated liver injury, WT mice (n=3-5/group) were pretreated intraperitoneally with an HIF-1α-specific inhibitor PX-478 (Oncothyreon, Seattle, WA, USA) (5 mg/kg/dose) at 1 h before each ethanol exposure, as described [20], and sacrificed at 6 h after the last ethanol administration.
Case selection of autopsied human specimens
Collection and analysis of autopsied human specimenswere approved by the Institutional Review Board for Human Subjects Research and carried outby a certified staff in the Department of Forensic Medicine of the Seoul National University (Seoul, Korea) following the standard operating procedure for conducting autopsies. Characterization of autopsied human specimens was also approved(exempted) by the NIH Office of Human Subjects Research Protection. The collection of 26 autopsied blood and livers were performed within 24 h post mortem, as follows. During the autopsy procedures, the following information was collected from the police reports and personal medical records: gender, age at death, histological evaluation of liver, history of binge alcohol drinking, BAC, body mass index and smoking history. Based on history of binge alcohol drinking in previous days before autopsies, BAC, and liver histology, we selected 12 specimens as a binge group and 12 cases as a non-binge group. Femoral venous blood samples were collected from autopsy cadavers. Obtained blood was stored in a refrigerator and used for determining alcohol concentration within 24 h of collection. Each blood sample was analyzed using head space-gas chromatography equipped with a glass column and a flamed ionization detector. Two hundred μL of a 0.02% solution of 2-methyl-2-propanol (tert-butyl alcohol, Aldrich Chemical, Milwaukee, WI, USA) was used as an internal standard. The limit of detection for BAC was 0.001%. A small portion of liver from each individual was fixed in 10% buffered formalin for histology or frozen in liquid nitrogen followed by storage at −80 −C for future analyses of immunoblot and enzyme activity measurements.
Hepatic tissue extraction and Western blotting
Total liver homogenates were prepared in ice-cold RIPA buffer. Cytosol, mitochondrial and nuclear fractions were also prepared by differential centrifugations, as described previously [21,22], and all extraction buffers were pre-equilibrated with nitrogen gas to remove the dissolved oxygen. Protein concentrations of liver homogenates were determined using the bicinchoninic acid protein assay reagent (Pierce, Rockford, IL, USA). Equal amounts of liver homogenates were resolved on 12% SDS-PAGE gels and electrically transferred to PVDF membranes. Membranes were initially blocked with 3% (w/v) non-fat milk proteins, subsequently probed overnight with a specific primary antibody to each target protein as indicated below at 4° C, and incubated with a secondary antibody conjugated with horse radish alkaline phosphatase (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at room temperature for 1 h. Image detection was performed using Super Signal West Pico Kit (Pierce) according to the manufacturer’s instructions. GAPDH or β-actin was used as a loading control for whole extracts or cytosol proteins. ATP synthase and lamin A/C were used as a loading control for mitochondria and nuclear proteins, respectively. All other information regarding antibody concentrations are presented in Table 2.
Table 2.
Primary antibodies used in this study
| Antigen | Species | Working | dilution | Buffer* | Source |
|---|---|---|---|---|---|
| ADH | Rabbit | 1:5000 | 3% milk | Cell Signaling | |
| CYP2E1 | Rabbit | 1:10000 | 3% milk | Abcam | |
| Catalase | Rabbit | 1:2000 | 3% milk | Abcam | |
| ALDH2 | Goat | 1:3000 | 3% milk | Santa Cruz Biotechnology | |
| ATP-Synthase | Rabbit | 1:3000 | 3% milk | Santa Cruz Biotechnology | |
| PPARα | Rabbit | 1:1000 | 3% milk | Abcam | |
| HIF-1α | Rabbit | 1:500 | 3% milk | Santa Cruz Biotechnology | |
| HPH-2 | Mouse | 1:1000 | 3% milk | Thermo Scientific | |
| BNIP3 | Rabbit | 1:2000 | 3% milk | Cell Signaling | |
| P53 | Mouse | 1:1000 | 3% milk | Cell Signaling | |
| Bax | Rabbit | 1:2000 | 3% milk | Cell Signaling | |
| iNOS | Rabbit | 1:1000 | 3% milk | Abcam | |
| 3-nitrotyrosine | Mouse | 1:1000 | 3% milk | Abcam | |
| GAPDH | Rabbit | 1:2000 | 3% milk | Cell Signaling | |
| β-actin | Rabbit | 1:2000 | 3% milk | Cell Signaling | |
| Lamin A/C | Rabbit | 1:1000 | 3% milk | Cell Signaling | |
TTBS represents:Tris buffered saline, pH 7.4 with 0.1% Tween 20.
Liver histology, alcohol metabolic enzyme activities, NO analysis, and TUNEL assay
Liver specimens were fixed overnight in 10% buffered formalin and embedded in paraffin. Sections of formalin-fixed livers on paraffin blocks were stained with hematoxylin/eosinand analyzed under microscopy for histology examinations. Activities of mitochondrial low Km ALDH2 (Abcam, Cambridge, MA, USA) and cytosolic ADH (Sigma) were measured by using a kit specific for each enzyme by following the manufacturer’s instructions. Cytosolic proteins and mitochondrial lysates were used for measuring ADH and ALDH2 activities, respectively.CYP2E1 activity was examined by the rate of p-nitrophenoloxidation to p-nitrocatechol with 1 mg cytosol fractionfrom each liver as described previously [23]. As an indicator of NO production, plasma nitrate and nitrite concentrations were measured using the enzymatic nitric oxide assay kit (Oxford Biomedical Research, Oxford, MI, USA). According to the manufacturer’s instructions, this assay kit containing the Griess reagent determines the conversion of nitrate to nitrite by nitrate reductase. The ApopTag peroxidase in situ apoptosis detection kit (Millipore, Billerica, MA, USA) was used to identify apoptotic hepatocytes by the TUNEL method.
Immunohistochemistry (IHC) and detection of liver hypoxia
Immnunohistochemical staining for CYP2E1 and HIF-1α was conducted on formalin-fixed and paraffin-embedded slides with Rabbit Specific HRP/DAB (ABC) detection IHC kit (Abcam, USA) according to the manufacturer’s instructions. For detection of liver hypoxia, Hypoxyprobe-1 Plus kit (Hypoxyprobe, Burlington, MA, USA) was used for IHC staining. The mice were orally administered with 3 consecutive doses of ethanol (6 g/kg/dose) at 12-h intervals and sacrificed at 6 h after the last ethanol dose, as described [17]. Pimonidazole was injected intraperitoneally at a dose of 60 mg/kg body weight at 1 h before euthanasia.
Immunofluorescent and confocal microscopy
Liver tissues were fixed formalin and embedded in paraffin according to standard procedure. Tissue sections were cut in 5μm slices and transferred onto glass slides. Sections were deparaffinized in xylene 3 times for 5 minutes each and rehydrated in following steps of: in 100% alcohol 2 times for 10 minutes, in 95% alcohol 2 times for 10 minutes and rinsed twice for 5 minutes in distilled water. Pretreatment occurred in boiled 0.1 M sodium citrate buffer (pH 7.2) by using microwave. These pretreated slides were cooled for 30 minutes at room temperature and rinsed in distilled water three times for 5 minutes. After rinsing in PBS, the sections were pre-incubated in 10% normal donkey serum in PBS (with 0.2% Triton X-100) for 1 hour at room temperature and then incubated at 4°C overnight with the primary antibody (anti-CYP2E1 from Abcam, Cambridge, United Kingdom). After overnight incubation, slides were washed with PBS three times for 5 minutes and incubated in the dark for 2 hour at room temperature with asecondary antibody (goat anti-rabbit IgG-FITC from Santa Cruz Biotechnology, Dallas, TX). Slides were washed in PBS and incubated at 4°C overnight with another primary antibody (anti-HIF1α from Abcam). After then slides were washed in PBS and incubated in the dark for 2 hour at room temperature with a secondary antibody (goat anti-mouse IgG2b-TR from Santa Cruz Biotechnology). After washing in PBS, slides were mounted in Vectashield Mounting Medium (H-1400; Vector Laboratories, Ontario, Canada). Immunostained slides were observed with confocal microscopy (A1; Nikon, Tokyo, Japan).
Immunoprecipitation and immunoblot analyses
The immunoprecipitation of ADH was performed using Dynabeads Protein G (Novex, Life Technologies, Carlsbad, CA, USA) following the manufacturers recommendations and separated on 12% SDS-PAGE gels for immunoblot analysis using the specific antibody against 3-nitrotyrosine or ADH, as described in the section of Western Blotting.
Quantification and Statistical analysis
Band densities were quantified using a gel digitizing software (UN-SCAN-IT, Orem, UT, USA). The results are expressed as means ± SEM. All data were analyzed by the Student’s t test and aP value of 0.05 or less was considered statistically significant. Pearson correlation analysis was performed using Minitab® 14 software (Minitab Inc., State College, PA, USA) with exclusion of 1 outlier. All other methods are not described here were repeated at least twice and performed as same as described [17,24].
Results
Effects of binge alcohol on liver injury and alcohol metabolic enzymes in mice
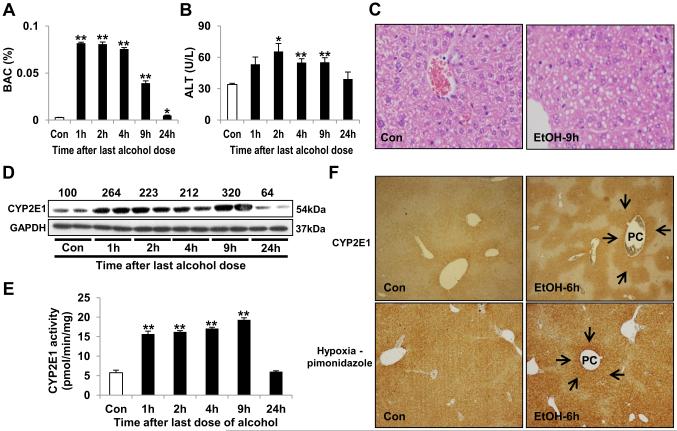
Time-dependent effects of binge alcohol were evaluated in mice treated with 3 consecutive oral doses of ethanol at 12 h intervals. At 1 h after the third ethanol dose (or 25 h following the first ethanol dose), BAC reached a maximum, 0.082% (Fig. 1A). It was higher than 0.08%, a concentration defined as binge drinking [6],indicating that our protocol was sufficient to study effects of binge alcohol. Plasma alanine aminotransferase (ALT) was significantly elevated at 2, 4 and 9 h (Fig. 1B). Histological analysis of liver tissues revealed micro-vesicular lipid droplets accumulationat 24 h(i.e., 48 h from the first ethanol dose) in binge alcohol-exposed mice (Fig. 1C and Supplementary Fig. 1A), along with increased neutrophil infiltration and scattered inflammatory foci as shown in our previous findings [17]. Consistent with our recent results [17], binge alcohol suppressed the hepatic levels of peroxisome proliferator activated receptor-alpha, a transcription factor involved in fatty acid oxidation and inflammation (Supplementary Fig. 1B), likely contributing to fat accumulation and inflammatory liver injury, as indicated by elevated ALT and liver histology.
Fig. 1. Binge alcohol increasedCYP2E1 and hypoxia in mice.
(A,B) The changes of BAC (A) and plasma ALT (B) after binge alcohol exposure.(C) Representative H&E staining of livers at 9 h after the last ethanol dose. (D) Immunoblot analysis of CYP2E1in whole liver lysates.(E) CYP2E1αctivityin cytoplasm.(F) Representative immunohistochemicalstainings(arrows) of CYP2E1 and pimonidazole in livers at 6 h (i.e., 30 h from the first ethanol dose). PC, pericentral regions. Data (n=4/group) expressed as means ± SE(*p< 0.05 and **p< 0.01).
Among major alcohol metabolizing enzymes, ADH was decreased by binge alcohol up to 9 h (Supplementary Fig. 1C), whereas hepatic CYP2E1 was increased up to 9 h while they returned to basal levels at 24 h after the last ethanol dose (Fig. 1D). CAT, being considered as a minor enzymein hepatic alcohol oxidation [15], was reduced by binge alcohol while ALDH2 level was unchanged (Supplementary Fig. 1C). Hepatic activities of ADH and CYP2E1 showed consistent patterns with their protein levels. Binge alcohol led to time-dependent reduction of ADH activity up to 9 h (Supplementary Fig. 1D). Hepatic CYP2E1 activity was increased and reached a maximum at 9 h after thelast alcohol exposure (Fig. 1E).
Binge alcohol increased hypoxia, HIF-1α-related apoptosis and protein nitration in mice
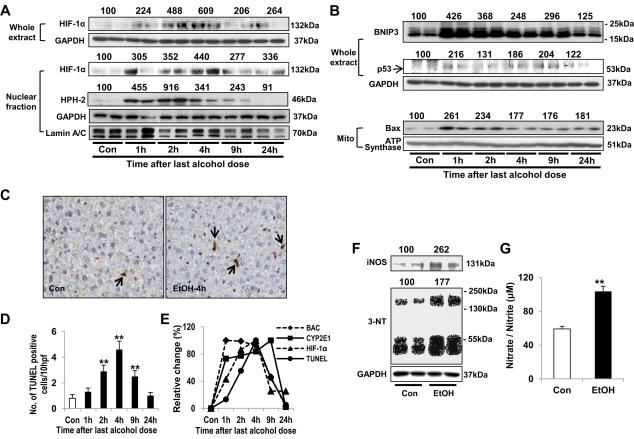
To evaluate whether hypoxia actually occurred in binge alcohol-exposed mice, liver tissues were stained with CYP2E1 and a hypoxia-specific marker pimonidazole, as reported [12]. Immunohistochemical staining for CYP2E1 and pimonidazole were strongly positive in binge alcohol-exposed mice (Fig. 1F). Moreover, CYP2E1 and pimonidazole were co-localized in the centrilobular areas. Binge alcohol elevated HIF-1α expression sin whole liver extracts and nuclear fractions, although the levels of laminused as a loading control for nuclear proteins slightly decreased in alcohol-exposed samples while the GAPDH levels seem unchanged (Fig. 2A), indicating binge alcohol-mediated hypoxia followed by stabilization and translocation of HIF-1α to nucleus. Since hydroxylation of HIF-1α by HIF-specific prolyl hydroxylase-2 (HPH-2) leads to proteasome-mediated degradation of HIF-1α [12], we also measured the HPH-2 level. Interestingly, however, nuclear HPH-2 showed a similar time-dependent expression with that of HIF-1α protein (Fig. 2A), supporting the previous results of HIF-1α-dependent HPH-2 up-regulation [25,26].
Fig. 2. Effects of binge alcohol on hepatic levels of HIF-1α-related apoptosis and protein nitration in mice.
(A,B) Immunoblot analyses of HIF-1α (A) and itsapoptosis-related target proteins (B), as indicated.(C,D) Representative TUNEL staining (C) for apoptotic hepatocytes (arrows) at 4 h (i.e., 28 h from the first ethanol dose) and quantitative analysis (D). (E) The time-dependent changes in BAC, CYP2E1 activity, HIF-1α and apoptotic hepatocytes.(F) Immunoblot analysis of iNOS and 3-NT at 9 husing whole liverlysates. Densitometric analysis for A,B, and F was performed for each target protein in each lane and normalized to either GAPDH (whole lysates), lamin A/C (nuclear fraction), or ATP synthase (mitochondrial fraction). Each number on top of the panels represents the average density of two lanes compared to the WT control (100).(G) Plasma nitrate/nitrite concentrationat 9 h (i.e., 33 h from the first ethanol dose). Data expressed as means ± SE(*p< 0.05 and **p< 0.01).
HIF-1α induces apoptosis by stabilizing p53 and/or increasing the expression of Bcl-2/adenovirus E1B 19 kDa interacting protein-3 (BNIP3) and pro-apoptotic Bax [10,27]. In our study, p53, BNIP3 and mitochondrial Baxwere elevated following binge alcohol (Fig. 2B). Terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) analysis demonstrated significantly increased hepatocyte apoptosis by binge alcohol (Figs. 2C-D and Supplementary Fig. 2). Collectively, the highest BAC was observed between 1~4 h while CYP2E1 activity continuously elevated between 1~9 h following the last ethanol exposure. In contrast, maximal levels of HIF-1α and apoptotic rate were observed at 4 h(i.e., 28 h from the first ethanol dose) (Fig. 2E).
Hypoxia can also lead to NO production with subsequent peroxynitrite formation [28,29]through HIF-1α-mediated iNO Sinduction [13]. Consistently, hepatic iNOS expression was increased at 9 h following the binge alcohol treatment (Fig. 2F), which alsoelevated plasma nitrate/nitritecontents (Fig. 2G). Hepatic nitrated proteins, determined by 3-nitrotyrosine (3-NT) immunoblot, were increasedin binge alcohol-exposed mice(Fig. 2F).
Changes in the alcohol metabolizing enzymes in binge alcohol-exposed humans
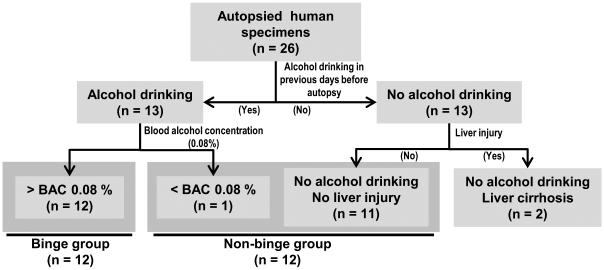
Information for the collected human specimens is summarized in Table 1 and Supplementary Fig. 3. Based on history of binge alcohol drinking in previous days before autopsies and BAC determined post-mortally, 26 individuals were firstly divided into 13 drinkers and 13 non-drinkers (Fig. 3). Twelve from 13 alcohol drinkers showed higher BACs than 0.08%, a concentration defined as binge drinking[6]. Two non-drinkers with liver cirrhosis were excluded from this study to rule out chronic effect. Consequently, total 24 individuals were assigned into 2 groups, a binge (n = 12; 3 females and 9 males) and a non-binge group (n = 12; 7 females and 5 males). Average ages of binge and non-binge group were 46.7 and 41.5, respectively. No significant difference inbody mass index (BMI) was observed between the two groups (data not shown but provided to the reviewers). Although smoking in male individuals was more prevalent in binge group (8/9) than non-binge group (3/5), there was no significant difference in the quantity of smoking between the two groups (data not shown but provided to the reviewers).
Table 1.
Clinical data of human subjects
| Sample No. |
Gender | Age at death |
Drinking binge |
BAC (%) |
Autopsy diagnosis | BMI (kg/m2) |
Smoking (py) |
Group |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 21 | Yes | 0.319 | - | 19.92 | 0 | Binge |
| 2 | Female | 38 | Yes | 0.179 | Fatty liver | 24.52 | 0 | Binge |
| 3 | Female | 49 | Yes | 0.477 | Acute alcohol intoxication | 24.00 | 0 | Binge |
| 4 | Female | 45 | Yes | 0.023 | - | 24.89 | 0 | Non-binge |
| 5 | Female | 46 | No | 0.000 | Acute cardiac death | 24.22 | 0 | Non-binge |
| 6 | Female | 18 | No | 0.000 | Acute cardiac death | 23.23 | 0 | Non-binge |
| 7 | Female | 54 | No | 0.000 | - | 22.47 | 0 | Non-binge |
| 8 | Female | 28 | No | 0.000 | - | 21.93 | 0 | Non-binge |
| 9 | Female | 37 | No | 0.000 | Acute cardiac death | 28.16 | 0 | Non-binge |
| 10 | Female | 45 | No | 0.000 | Acute cardiac death | 21.23 | 0 | Non-binge |
| 11 | Male | 64 | Yes | 0.634 | Acute alcohol intoxication | 23.63 | 40 | Binge |
| 12 | Male | 50 | Yes | 0.225 | Stroke | 22.23 | 30 | Binge |
| 13 | Male | 51 | Yes | 0.472 | Acute alcohol intoxication | 19.37 | 26 | Binge |
| 14 | Male | 45 | Yes | 0.427 | Acute alcohol intoxication, Fatty liver | 24.85 | 20 | Binge |
| 15 | Male | 44 | Yes | 0.187 | Acute cardiac death | 25.71 | 28 | Binge |
| 16 | Male | 44 | Yes | 0.223 | Liver cirrhosis | 21.61 | 20 | Binge |
| 17 | Male | 74 | Yes | 0.270 | Acute cardiac death | 26.26 | 55 | Binge |
| 18 | Male | 42 | Yes | 0.189 | Acute cardiac death | 24.67 | 0 | Binge |
| 19 | Male | 38 | Yes | 0.095 | Acute cardiac death, Fatty liver | 25.26 | 15 | Binge |
| 20 | Male | 32 | No | 0.000 | Acute cardiac death | 28.03 | 12 | Non-binge |
| 21 | Male | 56 | No | 0.000 | Acute cardiac death | 24.13 | 0 | Non-binge |
| 22 | Male | 64 | No | 0.000 | Acute cardiac death | 21.88 | 42 | Non-binge |
| 23 | Male | 47 | No | 0.000 | Acute cardiac death | 26.70 | 24 | Non-binge |
| 24 | Male | 26 | No | 0.000 | Acute cardiac death | 22.72 | 0 | Non-binge |
| 25 | Male | 62 | No | 0.000 | Acute cardiac death, Liver cirrhosis | 20.42 | 0 | - |
| 26 | Female | 31 | No | 0.000 | Liver cirrhosis | 17.18 | 0 | - |
Fig. 3.
Flow chart of study groups of human specimens
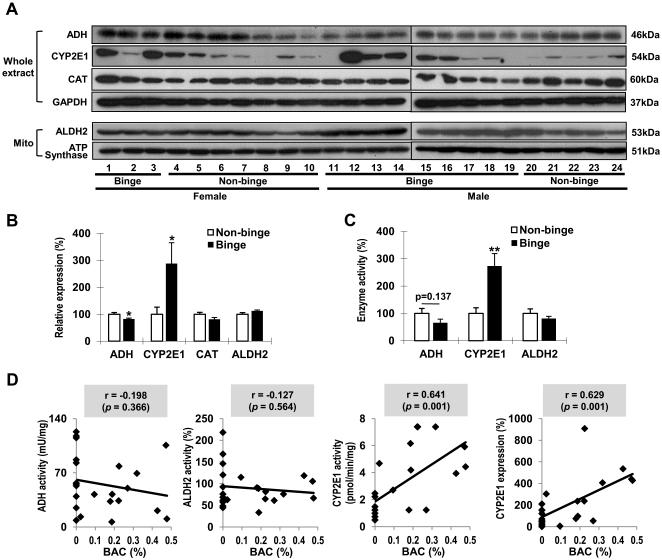
ALDH2 and CAT levels were similar between binge and non-binge groups (Figs. 4A and B). In contrast, significantly reduced ADH (18%) and elevated CYP2E1 (188%) were observed in binge group. Activity measurements showed decreasing trends in both ADH (34.5%) and ALDH2 (18.8%) in binge group, although they were statistically insignificant(Fig. 4C). ADH proteins in binge alcohol-exposed humans and mice were found to be nitrated (data not shown but provided to the reviewers). These results may reflect that nitration of ADH might have contributed to decreased ADH levels in bingealcohol-exposed mice and humans, likely through accelerated ubiquitin-dependent protein degradation of nitrated proteins, as reported [30,31]. Hepatic CYP2E1 activity was significantly(173%) elevated in binge group. We further analyzed statistical correlation between BACs and the major alcohol metabolizing enzymes to determine which proteins were correlated with BACs following binge alcohol ingestion. The activities of ADH and ALDH2 showed no significant correlations with BACs, where as the CYP2E1 activities (r = 0.641, p = 0.001) and protein levels (r = 0.629, p = 0.001) were significantly correlated with BACs (Fig. 4D). There was no marked difference in correlation coefficients between female and male individuals(Supplementary Fig. 4). Furthermore, the hepatic CYP2E1 protein levels and activities did not statistically correlate with the quantities of smoking consumption in humans(data not shown but provided to the reviewers), supporting the primary role of binge alcohol in the CYP2E1 elevation.
Fig. 4. Effects of binge alcohol on alcohol metabolizing enzymes in humans.
(A,B) Immunoblot analyses of the alcohol metabolizing enzymes (A) and quantitative analysis (B).(C) The activities of alcohol metabolizing enzymes. Data expressed as means ± SE(*p< 0.05 and **p< 0.01).(D) Correlative analysison BACs and the alcohol metabolizing enzymes. BACs were plotted against: ADH, ALDH2, CYP2E1 activities, or CYP2E1 expressions with linear regression lines.r, Pearson correlation coefficient.
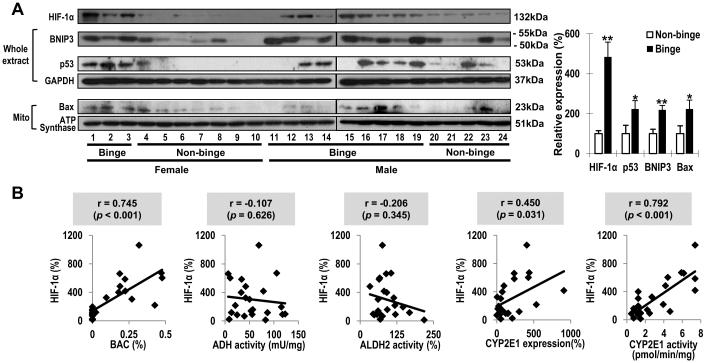
Binge alcohol elevated HIF-1αand its apoptosis-related downstream target proteins in humans
Hepatic HIF-1α was significantly increased (384%) in binge group(Fig. 5A). Subsequently, p53 (124%), BNIP3 (119%) and mitochondrial Bax (124%) were significantly elevatedin binge group. Particularly, HIF-1α levels were positively correlated with BACs (r = 0.745, p< 0.001), CYP2E1 levels (r = 0.450, p = 0.031) and CYP2E1 activities (r = 0.792, p< 0.001), respectively (Fig. 5B). In contrast, there was no significant relationship between HIF-1α and ADH or ALDH2 activity.
Fig. 5. Effects of binge alcohol on HIF-1α and itsapoptosis-related target proteins in humans.
(A) Immunoblot analyses of HIF-1α and downstream target proteins and quantitative analysis. Data expressed as means ± SE(*p< 0.05 and **p< 0.01). (B) Correlative analysis among BACs, the alcohol metabolizing enzymes and HIF-1α. HIF-1αlevels were plotted against: BACs, ADH, ALDH2, CYP2E1 activities or CYP2E1 protein levels.
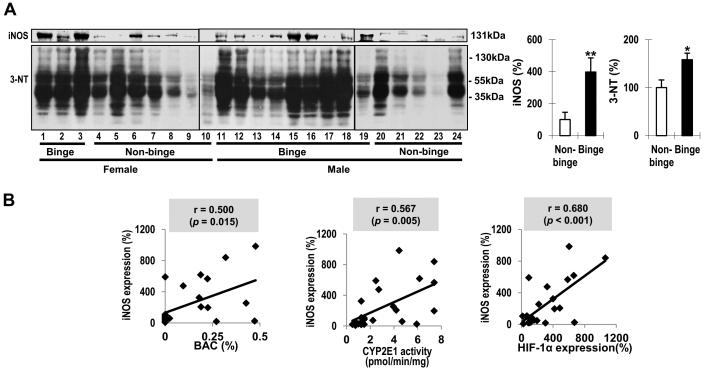
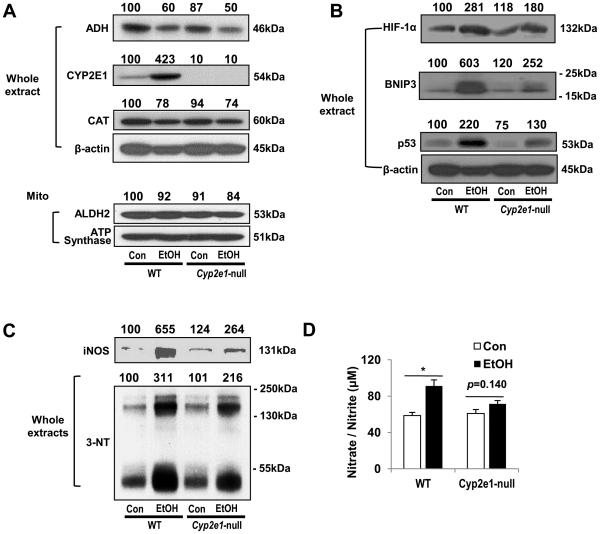
Consistent with the results in mice, hepatic iNOS was significantly increased by 239% in binge alcohol group(Fig. 6A). Moreover, its levels showed positive relationships with BACs (r = 0.500, p = 0.015), CYP2E1 activities (r = 0.567, p = 0.005), and HIF-1α (r = 0.680, p< 0.001), respectively(Fig. 6B), suggesting that HIF-1α was likely involved in binge alcohol-mediated induction of iNOS. The levels of nitrated proteins markedly differed among non-alcoholic individuals, whereas more proteins in many individuals in binge group appeared nitrated. The densitometric analysis revealed that3-NTlevels were 58% elevated (p< 0.05) in binge group than non-binge group (Fig. 6A) and significantly correlated with BACs(r = 0.448, p = 0.032) (Supplementary Fig. 5). The 3-NTlevels exhibiteda tendency of positive correlations with CYP2E1 and HIF-1α, albeit statistically insignificant, possibly due to rapid degradation of some nitrated proteins, as reported [30,31]. The increased levels of iNOS and 3-NT observed in binge alcohol-exposed WT were alleviated in the corresponding Cyp2e1-null mice (Fig. 7C). Consistently, plasma nitrate/nitrite levels, significantly increased (55%) in binge alcohol-exposed WT, were significantly attenuated in the corresponding Cyp2e1-null mice (Fig. 7D).
Fig. 6. Effects of binge alcohol on protein nitration in humans.
(A) Immunoblot analyses of iNOS and 3-NT in whole liver lysates and quantitative analysis. Data expressed as means ± SE(*p< 0.05 and **p< 0.01). (B) Correlative analysis between iNOS and BACs, CYP2E1, or HIF-1α. iNOS levels were plotted against: BACs, CYP2E1 activities or HIF-1α. r, Pearson correlation coefficient.
Fig. 7. Effects of Cyp2e1deletioninbinge alcohol-exposed mice for 6 hours.
(A-D) Immunoblot analyses of the alcohol metabolizing enzymes (A), HIF-1α-related apoptosis target proteins(B), protein nitration (C). Densitometric analysis for A, B, and C was performed for each target protein in each lane and normalized to either GAPDH (whole lysates) or ATP synthase (mitochondrial fraction). Each number on top of the panels represents the average density of two lanes compared to the WT control (100). (D) Plasma nitrate/nitrite concentration was evaluated as shown. Data expressed as means ± SE(*p < 0.05 and **p< 0.01).
Role of CYP2E1or HIF-1α in binge alcohol-induced liver injury in mice
WT and Cyp2e1-null mice were treated with binge alcohol to evaluate the role of hepatic CYP2E1 in promoting HIF-1α-related liver injury, as described [17]. There wasno significant genotype difference inbinge alcohol-mediated reduction of ADH and CAT (Fig. 7A). ALDH2 level was unchanged by binge alcohol in both WT and Cyp2e1-null mice. However, CYP2E1, expressed in only WT mice,was in creased by binge alcohol. The greatly elevated levels of HIF-1α, BNIP3 and p53 in the binge alcohol-exposed WT were attenuated in the corresponding Cyp2e1-null mice (Fig. 7B), providing a possible explanation for our recent finding that binge-alcohol-induced apoptosis was markedly prevented in Cyp2e1-null mice [17]. Immunohistochemistry for hepatic CYP2E1 and HIF-1α revealed that CYP2E1 and HIF-1α levels were markedly increased and co-localized in central vein regions in binge alcohol-exposed WT mice (× 200) (Supplementary Figs. 6C and D, respectively), compared to the control mice (Supplementary Figs. 6A and B, respectively). In contrast, the levels of CYP2E1 in control and ethanol-exposed Cyp2e1-null mice were very low or minimal, as expected (Supplementary Figs. 6E and G, respectively), while the level of HIF-1α seemed minimally elevated in binge alcohol-exposed Cyp2e1-null mice (Supplementary Fig. 6H), compared to the corresponding control (Supplementary Figs. 6F) or alcohol-exposed WT (Supplementary Fig. 6D). Consistently, the levels of fluorescein is othiocyanate (green-fluorescence)-stained CYP2E1and Texas Red-stained HIF-1α(red-fluorescence) were marked increased in binge alcohol-exposed WT mice (Supplementary Figs. 7B and D, respectively), compared to their corresponding controls (Supplementary Figs. 7A and C, respectively). In addition, these two proteins were elevated and co-localized, as evidenced by the increased yellow merged images (Supplementary Fig. 7F), compared to the control (Supplementary Fig. 7E).
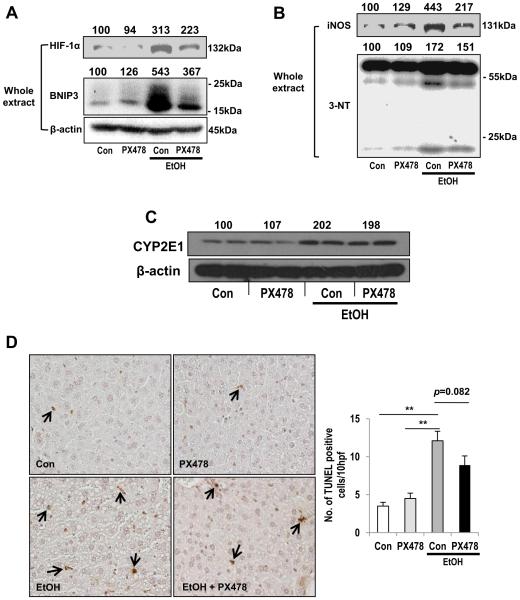
To further investigate the role of HIF-1α in binge alcohol-mediated hepatotoxicity, WT mice were pretreated with an HIF-1α-specific inhibitor PX-478 prior to binge alcohol exposure. PX-478, at the dose used, partially suppressed binge alcohol-mediatedincreases in HIF-1α and BNIP3 (Fig. 8A). Binge alcohol-mediated iNOS induction and subsequent nitrotyrosine formation were also attenuated by PX-478 pretreatment (Fig. 8B), confirming a previous report on the involvement of HIF-1α in iNOS expression [13]. In contrast, PX-478 did not cause any effect on alcohol-mediated elevation of CYP2E1 levels (Fig. 8C), suggesting that the effect of PX-478 was not due to interference with CYP2E1 upregulation. However, PX-478 partially blocked binge alcohol-induced hepatocyte apoptosis and plasma ALT elevation proportionally to the HIF-1α levels, which likely explains why the amelioration did not reach the statistically significant levels (Fig. 8D and Supplementary Fig. 8). Never-the-less, these results suggest an important role of HIF-1α, at least partially, in binge alcohol-induced liver injury.
Fig. 8. Effects of HIF-1α inhibition in binge alcohol-exposed mice.
(A-D) Immunoblot analyses of HIF-1α, BNIP3 (A), iNOS, 3-NT (B), CYP2E1 (C). Densitometric analysis was performed for each target protein and normalized to GAPDH or β-actin. Each number on top of the panels represents the average density of two lanes compared to the WT control (100). Representative TUNEL staining for apoptotic hepatocytes (arrows) (D) in WT mice exposed to binge alcohol for 6 h(i.e., 30 h from the first ethanol dose) in the absence or presence of PX-478 pretreatment. Data expressed as means ± SE(*p < 0.05 and **p< 0.01).
Discussion
As the primary site of alcohol metabolism, liver is a major target of alcohol-induced injury [1-7,15]. Induction of CYP2E1, known to create potentially toxic metabolites that contribute to oxidative tissue damage [15-17], has been associated with hypoxia and HIF-1α activation in response to chronic alcohol feeding in rodents through elevated hepatic oxygen consumption [12]. CYP2E1 is induced by both binge and chronic ethanol ingestion [12,16,17]. In this study, we aimed to investigate the potential mechanism of binge alcohol-mediated liver injury and the relationship between the alcohol metabolizing enzymes including CYP2E1 and HIF-1α-related liver injury inhumans and miceexposed to binge alcohol as a common type of alcohol ingestion [5,8,32].
The binge groups in both humans and mice exhibited BACs greater than 0.08%, the legal limit concentration defined as binge drinking [6]. Mice exposed to binge alcohol showed increases in hepatic steatosis and inflammation. Importantly, among the major alcohol metabolizing enzymes, only CYP2E1 was significantly elevatedin binge group. CYP2E1 is critical in the development of non-alcoholic steatohepatitis and alcoholic hepatitis through increased oxidative/nitrative stress, gut leakiness, endotoxemia, and inflammation-mediated events [17,24,33,34]. In addition, CYP2E1 was shown to play an important role in the production of hydroxyethyl radicals during alcohol consumption, and human alcoholic individuals showed increased levels of autoantibodies against CYP2E1-hydroxyethyl adducts [35,36]. In this study, hepatic CYP2E1 levels and activities in humans and mice were selectively elevated by binge alcohol while the other alcohol metabolizing enzymes did not change or slightly decreased. The increased CYP2E1 amounts and activities positively correlated withthose of BACcompared to other factors such as smoking and BMI, andwerelikely induced through protein stabilization in the presence of alcohol, as reported [37].
Alcohol ingestion can lead to hypoxia around the central vein [12] through increased hepatic oxygen consumption along with a smaller increase in oxygen delivery [1]. All cytochrome P450 enzymes require molecular oxygen for their catalytic activities [15,34]. Especially, CYP2E1 has been known to contribute to ethanol-induced hypoxic damage through increased microsomal oxygen consumption [34,38]. Indeed, we observed co-localization of CYP2E1, HIF-1α and pimonidazole-sensitive hypoxia in the same areas of centrilobule, where oxygen tension is lowest [1,12,39], in binge alcohol-exposed mouse liver tissues. The results of centrilobular hypoxia in binge alcohol-exposed mice are also consistent with the pericentralhypoxia in mice chronically fed an alcohol liquid diet in a CYP2E1-dependent manner, as previously reported [12]. Moreover, significant correlationsamong BAC, CYP2E1 and HIF-1α were observed in humans. Collectively, this datasuggests a criticalrole of hepatic CYP2E1 in binge alcohol-induced hypoxia and subsequent HIF-1α activation.
The underlying mechanisms by which elevated CYP2E1 induces HIF-1α in alcohol-exposed tissues are unknown and need to be studied in the future. The levels of HIF-1α can be regulated by many different pathways during its synthesis and degradation. However, based on our results (Fig. 2A) and the previous reports on the elevated levels of HPH-2 in response to alcohol exposure, this enzyme does not seem to be the main reason for HIF-1α up-regulation, despite its critical role in the hydroxylation of HIF-1α needed for ubiquitin conjugation and subsequent degradation. For instance, alcohol administration was shown to increase the catalytic activities and levels of HPH, as reported by at least three different laboratories [40-42]. Therefore, it is more likely that the elevated levels of HIF-1α in alcohol-exposed mice (Fig. 2) and human specimens (Fig. 5) could result from CYP2E1-related increased oxidative stress, which can oxidatively-modify one of the enzymes in the 26S proteasomal multi-protein complex involved in the ubiquitin-dependent degradation of HIF-1α. For instance, various subunits of the 26S proteasomal complex can be covalently modified: 1) adduct formation with 4-hydroxynonenal [43]; 2) phosphorylation [44]; and 3) oxidative modification of Cys residues [45] in a CYP2E1-dependent manner, since proteasomal activities were not significantly decreased in alcohol-exposed Cyp2e1-null mice [46]. In addition to the suppression by chronic alcohol administration, proteasomal activities can be also suppressed by acute binge alcohol exposure [47]. These types of modifications of the proteasomal subunits likely result in suppressed proteasomal activity, thus leading to increased HIF-1α levels.
Hypoxic cells initiate a cascade of events that induce apoptosis to prevent the accumulation of injured cells from hypoxia-induced mutations, while cells also try to adapt to hypoxic condition to survive [10]. One of the key regulators in these processes is a transcription factor HIF-1α [10]. Elevated HIF-1α can increase stability of a tumor suppressor gene p53, which induces apoptosis by up-regulating Bax under stress conditions [10,27]. HIF-1α can inhibitanti-apoptotic effect of Bcl-2 through induction of BNIP3 [10]. It is also known that HIF-1α can induce various anti-apoptotic proteins, such as survivin, inhibitor of apoptosis protein 2, and vesicular endothelial growth factorto facilitate cell survival and promote angiogenesis [48,49], while cells with high concentrations of HIF-1α can be more resistant to apoptosis under hypoxic conditionsespecially in cancer cells [50]. Our preliminary results showed that the levels of survivin were slightly elevated at 1 h but significantly decreased at later time points after ethanol exposure. Thus, our current observations with normal tissues seem to favor toward increased apoptosis with elevated levels of pro-apoptotic proteins such as p53, BNIP3 and mitochondrial Bax and decreased levels of survivin, all of which are involved in HIF-1α-dependent apoptosis [10,27], in both human and mice exposed to binge alcohol. This result is in agreement with a recent study showing that moderate ethanol exposure leads to hypoxia/HIF1α-induced signaling in liver cells with induction of p53-dependent apoptosis of hepatocytes, contributing to increased hepatic fibrosis during chronic CCl4 exposure [51].
As a post-translational modification, protein nitration is known to modify protein structure, alter their functions, and increase susceptibility to protein degradation [30,31]. NO, required for peroxynitrite formation in the presence of reactive oxygen species, is mostly produced by the action of NOS enzymes, including iNOS [28-31]. In hypoxia, HIF-1α can stimulate iNOS expression [13]. It has been also shown that hepatic Kupffer cell activation following ethanol exposure increased iNOS expression [52]. The NO produced from iNOS might have damaged the mitochondrial respiratory chain, contributing to hypoxia, as described [52]. Our results (Figs. 2 and 6) also showed the elevation of hepatic iNOS and 3-NT possibly associated with HIF-1α activation in binge alcohol-exposed humans and mice. It is also possible that CYP2E1 plays a permissive role in iNOS effects although this needs further investigation. Particularly, significant relationships among BAC, CYP2E1, HIF-1α andiNOS provide evidence about the involvement of HIF-1α in binge alcohol-induced iNOS induction and subsequent events such as protein nitration under hypoxia, at least partially, in binge alcohol-exposed humans and mice. Although not conducted in this study, it is likely that increased protein nitration, that is likely mediated by the increased CYP2E1 and iNOS protein levels, in different sub-cellular fractions might be also involved in inactivation of their functions, contributing to mitochondrial dysfunction, endoplasmic reticulum stress, and apoptosis in alcoholexposure, as recently demonstrated [17,52,53].
In agreement with the results with chronic alcohol feeding [12], our present results of Cyp2e1-null mice following binge alcohol suggest that CYP2E1 most likely plays an important role in promoting binge alcohol-induced apoptosis and protein nitration throughCYP2E1-HIF-1α-dependent mechanism, although further experiment using Cyp2e1 transgenic mice is needed to achieve higher signal to noise effects in clearly establishing the role of CYP2E1. Our results with the HIF-1α inhibitor showed proportionate to inhibition of HIF-1α and its downstream targets along with partial protection against binge alcohol-induced hepatotoxicity, suggesting that HIF-1αplays at least a partial role in CYP2E1-mediated apoptotic injury in response to binge alcohol. In this regard, other factors such as gut leakiness, immune cell activation, oxidative/nitrative protein modifications followed by mitochondrial dysfunction, and epigenetic elements may also contribute to binge alcohol-induced acute liver injury [4,17,24,32,54].
By comparing the data, we found that the elevation of CYP2E1, HIF-1α, p53, BNIP3, iNOS and 3-NT observed in binge alcohol-exposed humansgenerally agreed with those of the corresponding mice.CYP2E1 is regulated by both exogenous substrates such as ethanol and isoniazidas well as endogenous substrates including fatty acids and ketone bodies [33-37,55]. In addition, our study represents the first evidence of a strong and possibly direct relationship between CYP2E1 and HIF-1α activation in human sexposed to acute binge alcohol. We concluded that binge alcohol promotes acute hypoxic liver injury in mice and humans at least partly through activating the CYP2E1-HIF1α-dependent apoptosis pathway. Furthermore, based on the similar results, our rodent model seems to represent a good surrogate tool to predict the effects of binge alcohol on people. Along with a recent report with chronic alcohol exposure [12], our findings clearly indicateCYP2E1αnd HIF-1α as potential targets in development of preventive and/or therapeutic agents against alcohol-induced liver injury.
Supplementary Material
Highlights.
Hepatic CYP2E1 was related to hypoxia and HIF-1α induction following binge alcohol Binge alcohol promoted HIF-1α-related apoptosis and protein nitration
Effects of binge alcohol in humans were replicated in mouse model of binge alcohol-induced liver injury
CYP2E1 and HIF-1α can be valuable targets on alcohol-induced liver injury
Acknowledgement
This research was supported by the Intramural Program of National Institute on Alcohol Abuse and Alcoholism and a National Research Foundation grant funded by the Korean Government (800-20120365 to SHY). We are also grateful to Dr. Klaus Gawrisch for his support.
Abbreviations
- HIF-1α
hypoxia inducible factor-1α
- BAC
blood alcohol concentration
- CYP2E1
cytochrome P450 2E1
- ALD
alcoholic liver disease
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
- iNOS
inducible nitric oxide synthase
- NO
nitric oxide
- ADH
alcohol dehydrogenase
- CAT
catalase
- LDH2
mitochondrial aldehyde dehydrogenase 2
- WT
wild-type
- ALT
alanine aminotransferase
- HPH-2
HIF-specific prolyl hydroxylase-2
- BNIP3
Bcl-2/adenovirus E1B 19 kDa interacting protein-3
- TUNEL
terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick-end labeling
- 3-NT
3-nitrotyrosine
- BMI
body mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflicts of interest
References
- [1].Tsukamoto H, Xi XP. Incomplete compensation of enhanced hepatic oxygen consumption in rats with alcoholic centrilobular liver necrosis. Hepatology. 1989;9:302–306. doi: 10.1002/hep.1840090223. [DOI] [PubMed] [Google Scholar]
- [2].Brandon-Warner E, Schrum LW, Schmidt CM, McKillop IH. Rodent models of alcoholic liver disease: of mice and men. Alcohol. 2012;46:715–725. doi: 10.1016/j.alcohol.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat. Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stewart S, Jones D, Day CP. Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends Mol. Med. 2001;7:408–413. doi: 10.1016/s1471-4914(01)02096-2. [DOI] [PubMed] [Google Scholar]
- [5].CDC . Fact Sheets: Binge Drinking. Vol. 2011. Center for Disease Control and Prevention; Atlanta, GA: 2010. [Google Scholar]
- [6].NIAAA . Moderate & Binge Drinking. NIAAA; Rockville, MD: 2012. [Google Scholar]
- [7].Mathurin P, Lucey MR. Management of alcoholic hepatitis. J. Hepatol. 2012;56:S39–45. doi: 10.1016/S0168-8278(12)60005-1. [DOI] [PubMed] [Google Scholar]
- [8].Mathurin P, Deltenre P. Effect of binge drinking on the liver: an alarming public health issue? Gut. 2009;58:613–617. doi: 10.1136/gut.2007.145573. [DOI] [PubMed] [Google Scholar]
- [9].Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate hypoxic signaling. Curr. Opin. Cell Biol. 2009;21:894–899. doi: 10.1016/j.ceb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J. Clin. Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindauubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- [12].Wang X, Wu D, Yang L, Gan L, Cederbaum AI. Cytochrome P450 2E1 potentiates ethanol induction of hypoxia and HIF-1α in vivo. Free Radic. Biol. Med. 2013;63:175–186. doi: 10.1016/j.freeradbiomed.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].He T, Ai M, Zhao XH, Xing YQ. Inducible nitric oxide synthase mediates hypoxia-induced hypoxia-inducible factor-1 alpha activation and vascular endothelial growth factor expression in oxygen-induced retinopathy. Pathobiology. 2007;74:336–343. doi: 10.1159/000110027. [DOI] [PubMed] [Google Scholar]
- [14].Robinson MA, Baumgardner JE, Otto CM. Oxygen-dependent regulation of nitric oxide production by inducible nitric oxide synthase. Free Radic. Biol. Med. 2011;51:1952–1965. doi: 10.1016/j.freeradbiomed.2011.08.034. [DOI] [PubMed] [Google Scholar]
- [15].Lieber CS. Metabolism of alcohol. Clin. Liver Dis. 2005;9:1–35. doi: 10.1016/j.cld.2004.10.005. [DOI] [PubMed] [Google Scholar]
- [16].Dupont I, Bodenez P, Berthou F, Simon B, Bardou LG, Lucas D. Cytochrome P-450 2E1 activity and oxidative stress in alcoholic patients. Alcohol Alcohol. 2000;35:98–103. doi: 10.1093/alcalc/35.1.98. [DOI] [PubMed] [Google Scholar]
- [17].Abdelmegeed MA, Banerjee A, Jang S, Yoo SH, Yun JW, Gonzalez FJ, Keshavarzian A, Song BJ. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic. Biol. Med. 2013;65:1238–1245. doi: 10.1016/j.freeradbiomed.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Arteel GE, Raleigh JA, Bradford BU, Thurman RG. Acute alcohol produces hypoxia directly in rat liver tissue in vivo: role of Kupffer cells. Am. J. Physiol. 1996;271:G494–500. doi: 10.1152/ajpgi.1996.271.3.G494. [DOI] [PubMed] [Google Scholar]
- [19].de la M Hall P, Lieber CS, DeCarli LM, French SW, Lindros KO, Järveläinen H, Bode C, Parlesak A, Bode JC. Models of alcoholic liver disease in rodents: a critical evaluation. Alcohol. Clin. Exp. Res. 2001;25:254S–261S. doi: 10.1097/00000374-200105051-00041. [DOI] [PubMed] [Google Scholar]
- [20].Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol. Cell. Biol. 2013;33:904–917. doi: 10.1128/MCB.00951-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abdelmegeed MA, Moon KH, Hardwick JP, Gonzalez FJ, Song BJ. Role of peroxisome proliferator-activated receptor-alpha in fasting-mediated oxidative stress. Free Radic. Biol. Med. 2009;47:767–778. doi: 10.1016/j.freeradbiomed.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Song BJ, Suh SK, Moon KH. A simple method to systematically study oxidatively modified proteins in biological samples and its applications. Methods Enzymol. 2010;473:251–264. doi: 10.1016/S0076-6879(10)73013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab. Dispos. 1985;13:548–552. [PubMed] [Google Scholar]
- [24].Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J. Hepatol. 2012;57:860–866. doi: 10.1016/j.jhep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bruning U, Cerone L, Neufeld Z, Fitzpatrick SF, Cheong A, Scholz CC, Simpson DA, Leonard MO, Tambuwala MM, Cummins EP, Taylor C.T. MicroRNA-155. promotes resolution of hypoxia-inducible factor1 alpha activity during prolonged hypoxia. Mol. Cell. Biol. 2011;19:4087–4096. doi: 10.1128/MCB.01276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dalgard CL, Lu H, Mohyeldin A, Verma A. Endogenous 2-oxoacids differentially regulate expression of oxygen sensors. Biochem. J. 2004;380:419–424. doi: 10.1042/BJ20031647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Suzuki H, Tomida A, Tsuruo T. Dephosphorylated hypoxia-inducible factor 1αlpha as a mediator of p53-dependent apoptosis during hypoxia. Oncogene. 2001;20:5779–5788. doi: 10.1038/sj.onc.1204742. [DOI] [PubMed] [Google Scholar]
- [28].Hampl V, Cornfield DN, Cowan NJ, Archer SL. Hypoxia potentiates nitric oxide synthesis and transiently increases cytosolic calcium levels in pulmonary artery endothelial cells. Eur. Respir. J. 1995;8:515–522. [PubMed] [Google Scholar]
- [29].Zhu X, Heunks LM, Versteeg EM, van der Heijden HF, Ennen L, van Kuppevelt TH, Vina J, Dekhuijzen PN. Hypoxia-induced dysfunction of rat diaphragm: role of peroxynitrite. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L16–26. doi: 10.1152/ajplung.00412.2003. [DOI] [PubMed] [Google Scholar]
- [30].Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem. Biophys. Res. Commun. 2003;305:776–783. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- [31].Abello N, Kerstjens HA, Postma DS, Bischoff R. Protein tyrosine nitration: selectivity, physicochemical and biological consequences, denitration, and proteomics methods for the identification of tyrosine-nitrated proteins. J. Proteome Res. 2009;8:3222–3238. doi: 10.1021/pr900039c. [DOI] [PubMed] [Google Scholar]
- [32].Shukla SD, Pruett SB, Szabo G, Arteel GE. Binge ethanol and liver: new molecular developments. Alcohol. Clin. Exp. Res. 2013;37:550–557. doi: 10.1111/acer.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- [34].Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Albano E, Clot P, Morimoto M, Tomasi A, Ingelman-Sundberg M, French SW. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology. 1996;23:155–163. doi: 10.1002/hep.510230121. [DOI] [PubMed] [Google Scholar]
- [36].Clot P, Albano E, Eliasson E, Tabone M, Aricò S, Israel Y, Moncada C, Ingelman-Sundberg M. Cytochrome P4502E1 hydroxyethyl radical adducts as the major antigen in autoantibody formation among alcoholics. Gastroenterology. 1996;111:206–216. doi: 10.1053/gast.1996.v111.pm8698201. [DOI] [PubMed] [Google Scholar]
- [37].Roberts BJ, Shoaf SE, Jeong KS, Song BJ. Induction of CYP2E1 in liver, kidney, brain and intestine during chronic ethanol administration and withdrawal: evidence that CYP2E1 possesses a rapid phase half-life of 6 hours or less. Biochem. Biophys. Res. Commun. 1994;205:1064–1071. doi: 10.1006/bbrc.1994.2774. [DOI] [PubMed] [Google Scholar]
- [38].Ross AD, Varghese G, Oporto B, Carmichael FJ, Israel Y. Effect of propylthiouracil treatment on NADPH-cytochrome P450 reductase levels, oxygen consumption and hydroxyl radical formation in liver microsomes from rats fed ethanol or acetone chronically. Biochem. Pharmacol. 1995;49:979–989. doi: 10.1016/0006-2952(95)00007-m. [DOI] [PubMed] [Google Scholar]
- [39].French SW. The role of hypoxia in the pathogenesis of alcoholic liver disease. Hepatol. Res. 2004;29:69–74. doi: 10.1016/j.hepres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- [40].Pawlicka A, Bankowski E, Chrostek L, Szmitkowski M. An increase of collagen biosynthesis precedes other symptoms of ethanol-induced liver damage in rats. Drug Alcohol Depend. 1988;22:113–116. doi: 10.1016/0376-8716(88)90045-2. [DOI] [PubMed] [Google Scholar]
- [41].Yamada S, Yamada M, Murawaki Y, Hirayama C. Increase in lipoperoxide and prolyl hydroxylase activity in rat liver following chronic ethanol feeding. Biochem. Pharmacol. 1990;40:1015–1019. doi: 10.1016/0006-2952(90)90487-6. [DOI] [PubMed] [Google Scholar]
- [42].Giménez A, Caballería J, Parés A, Alié S, Deulofeu R, Andreu H, Rodés J. Influence of dietary zinc on hepatic collagen and prolyl hydroxylase activity in alcoholic rats. Hepatology. 1992;16:815–819. doi: 10.1002/hep.1840160331. [DOI] [PubMed] [Google Scholar]
- [43].Bardag-Gorce F, Li J, French BA, French SW. The effect of ethanol-induced CYP2E1 on proteasome activity: the role of hydroxynonenal. Exp. Mol. Pathol. 2004;78:109–115. doi: 10.1016/j.yexmp.2004.10.005. [DOI] [PubMed] [Google Scholar]
- [44].Bardag-Gorce F, Venkatesh R, Li J, French BA, French SW. Hyperphosphorylation of rat liver proteasome subunits: the effects of ethanol and okadaic acid are compared. Life Sci. 2004;75:585–597. doi: 10.1016/j.lfs.2003.12.027. [DOI] [PubMed] [Google Scholar]
- [45].Kim BJ, Hood BL, Aragon RA, Hardwick JP, Conrads TP, Veenstra TD, Song BJ. Increased oxidation and degradation of cytosolic proteins in alcohol-exposed mouse liver and hepatoma cells. Proteomics. 2006;6:1250–1260. doi: 10.1002/pmic.200500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bardag-Gorce F, Yuan QX, Li J, French BA, Fang C, Ingelman-Sundberg M, French SW. The effect of ethanol-induced cytochrome p4502E1 on the inhibition of proteasome activity by alcohol. Biochem. Biophys. Res. Commun. 2000;279:23–29. doi: 10.1006/bbrc.2000.3889. [DOI] [PubMed] [Google Scholar]
- [47].D'Souza AJ, Desai SD, Rudner XL, Kelly MN, Ruan S, Shellito JE. Suppression of the macrophage proteasome by ethanol impairs MHC class I antigen processing and presentation. PLoS One. 2013;8:e56890. doi: 10.1371/journal.pone.0056890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wu XY, Fu ZX, Wang XH. Effect of hypoxia-inducible factor 1-a on survivin in colorectal cancer. Mol. Med. Rep. 2010;3:409–415. doi: 10.3892/mmr_00000273. [DOI] [PubMed] [Google Scholar]
- [49].Dong Z, Wang JZ, Yu F, Venkatachalam MA. Apoptosis-resistance of hypoxic cells: multiple factors involved and a role for IAP-2. Am. J. Pathol. 2003;163:663–671. doi: 10.1016/S0002-9440(10)63693-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Akakura N, Kobayashi M, Horiuchi I, Suzuki A, Wang J, Chen J, Niizeki H, Kawamura K, Hosokawa M, Asaka M. Constitutive expression of hypoxia-inducible factor-1αlpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res. 2001;61:6548–6554. [PubMed] [Google Scholar]
- [51].Roychowddhury S, Chiang JC, McMullen MR, Nagy LE. Moderate chronic ethanol feeding exacerbates carbon tetrachloride-induced hepatic fibrosis via hepatocytes-specific hypoxia-inducible factor 1α . Pharma. Res. Per. 2014;2:e00061. doi: 10.1002/prp2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zelickson BR, Benavides GA, Johnson MS, Chacko BK, Venkatraman A, Landar A, Betancourt AM, Bailey SM, Darley-Usmar VM. Nitric oxide and hypoxia exacerbate alcohol-induced mitochondrial dysfunction in hepatocytes. Biochim.Biophys.Acta. 2011;1807:1573–1582. doi: 10.1016/j.bbabio.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Abdelmegeed MA, Jang S, Banerjee A, Hardwick JP, Song BJ. Robust protein nitration contributes to acetaminophen-induced mitochondrial dysfunction and acute liver injury. Free Radic. Biol. Med. 2013;60:211–222. doi: 10.1016/j.freeradbiomed.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Moon KH, Hood BL, Kim BJ, Hardwick JP, Conrads TP, Veenstra TD, Song BJ. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of Rats. Hepatology. 2006;44:1218–1230. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- [55].Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J. Hepatol. 2013;58:395–398. doi: 10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.