Abstract
Background
Extremes of temperature have been associated with short-term increases in daily mortality. We identified subpopulations with increased susceptibility to dying during temperature extremes, based on personal demographics, small-area characteristics and preexisting medical conditions.
Methods
We examined Medicare participants in 135 U.S. cities and identified preexisting conditions based on hospitalization records prior to their deaths, from 1985–2006. Personal characteristics were obtained from the Medicare records, and area characteristics were assigned based on zip-code of residence. We conducted a case-only analysis of over 11 million deaths, and evaluated modification of the risk of dying associated with extremely hot days and extremely cold days, continuous temperatures, and water-vapor pressure. Modifiers included preexisting conditions, personal characteristics, zip-code-level population characteristics, and land-cover characteristics. For each effect modifier, a city-specific logistic regression model was fitted and then an overall national estimate was calculated using meta-analysis.
Results
People with certain preexisting conditions were more susceptible to extreme heat, with an additional 6% (95% confidence interval= 4% – 8%) increase in the risk of dying on an extremely hot day in subjects with previous admission for atrial fibrillation, an additional 8% (4%–12%) in subjects with Alzheimer disease, and an additional 6% (3%–9%) in subjects with dementia. Zip-code level and personal characteristics were also associated with increased susceptibility to temperature.
Conclusions
We identified several subgroups of the population who are particularly susceptible to temperature extremes, including persons with atrial fibrillation.
Numerous multi-city time-series analyses have demonstrated that cold and hot temperatures, as well as temperature extremes, are associated with increased death rates in the days following these weather conditions.1–9 Less is known about water-vapor pressure and mortality. In two Chinese cities, high water-vapor pressure was associated with increased risks of all-cause and cardiorespiratory deaths during influenza outbreaks,10 and another study in several U.S. counties found an association with influenza mortality.11 These findings are important for understanding the health effects of climate change, which affects temperatures, extreme heat, water vapor and other weather conditions.12
Identifying factors that confer susceptibility to temperature extremes can help in developing public health programs that better target the most vulnerable. Greater susceptibility has been reported among the elderly, those of lower socioeconomic status, minorities, and persons with diabetes.5,6,13–16 Community-level modifiers, such as vegetative covering or green space, have rarely been examined as modifiers, and usually only for entire urban areas rather than at smaller spatial scales. An exception is a recent study in Phoenix, AZ that found non-vegetated areas to be associated with heat-associated death in a Census block group.17
Physiologically, the underlying mechanisms for heat-associated mortality may be related to the stress placed on the respiratory and circulatory systems to increase heat loss through skin-surface blood circulation.1,18 This stress, coupled with an increase in blood viscosity and cholesterol levels with high temperatures,19 may increase cardiorespiratory death risk. Recent studies report that changes in temperature and water-vapor pressure were associated with increased blood pressure, lipid levels, inflammatory markers, plasma cholesterol, and plasma fibrinogen and heart rate variability in the elderly or in persons with preexisting cardiovascular disease.20–25 High humidity has been associated with declines in physical and mental capacity, but high humidity often occurs when temperature is high.26
Less is known about medical conditions that confer susceptibility. Diabetes and chronic obstructive pulmonary disease (COPD) may make people more susceptible to temperature extremes.14 A multi-city study5 found that cardiac-arrest deaths increased on extremely cold days, whereas heat-related mortality was higher for those with coexisting atrial fibrillation.
With an aging population, the prevalence of neurologic conditions is increasing, but this is under-investigated as a characteristic of vulnerability to weather. Several studies have identified persons with mental illness, dementia, substance misuse and cognitive impairment as being particularly susceptible to heat.4,7,15,27–31
In this study we applied a case-only approach to examine susceptibility to weather parameters and temperature extremes in 135 U.S. cities. We hypothesized that subjects with specific preexisting conditions, or who were previously admitted to the hospital for specific primary or concomitant medical conditions, are more susceptible to the effect of weather on mortality. The case-only approach does not assume that the stated cause of death was also a preexisting condition, and it tests whether the risk differs for people with versus without the condition, regardless of the proximate cause of death. Based on the literature reviewed above, we focused on COPD, pneumonia, diabetes, congestive heart failure, and atrial fibrillation, as well as neurologic disorders such as Alzheimer disease, Parkinson disease, disorders of the peripheral nervous system, and dementia.
As additional factors that could confer susceptibility, we examined several personal and zip code characteristics that have previously been demonstrated to modify the relationship between heat and health.32 Sex, race, education and poverty may be associated with weather vulnerability because they are associated with differences in health status, air conditioning use, and social isolation and support.33–35 Green space and population density may physically alter the weather within microclimates and lower the temperature, particularly in the summer.17
Methods
Study design and population
As previously described,36 we chose 135 U.S. cities from the 201 counties with the highest number of cardiovascular hospital admissions in 2004–2006. Cities were then defined according to the county or counties corresponding to their metropolitan statistical area.
Health data
The U.S. Medicare program covers hospitalization for most U.S. citizens aged 65 years and older. Using Medicare claims records from the Medicare Provider Analysis and Review File for the years 1985 to 2006, we examined all patients admitted for any conditions; we then selected those who subsequently died and whose date of death was validated. We excluded subjects who died in the hospital after having been in the (climate-controlled) hospital for at least one day. Information on sex and race was available for each subject.
Persons for whom the following conditions were noted as the admission’s primary discharge diagnosis, or as any of nine coexisting conditions, were classified as having the condition: chronic obstructive pulmonary disease (International Classification of Disease 9th revision [ICD-9] 490–496, except 493), pneumonia (480 – 487), diabetes (250), congestive heart failure (CHF; 428), atrial fibrillation (A fib; 427.3), myocardial infarction (MI; 410), stroke (430–438), Alzheimer disease (331.0), Parkinson’s disease (332), dementia (290), disorders of the peripheral nervous system (350–359), and hereditary and degenerative diseases of the central nervous system (330–337).
Modifiers
We defined three types of modifiers: specific cause of admissions (preexisting medical conditions), personal characteristics, and area-level modifiers. U.S. Census zip code tabulation areas for the year 2000 Census were used to define boundaries of the 5-digit zip codes (http://www.census.gov/geo/www/tiger/tigermap.html#ZIP).
In a case-only analysis, the outcome variables are the presence or absence of each specific modifier. Therefore we created an indicator variable for presence or absence of the specific causes of admission specified above, sex, and non-white race.
We obtained area (zip-code) — level modifiers from the U.S. Census for the years 1980, 1990 and 2000 standardized to 2000 boundaries.37 From the 1992 and 2001 National Land Cover Dataset, we calculated percent of area with green space and covered by a water body.32 The census and land-use data were merged with the Medicare patient records by zip code of residence.
We defined, for each of the area-level modifiers, the 25th and 75th percentiles across all the cities for each available year of Census and National Land Cover Dataset data. We then assigned the 1980, 1990 and 2000 Census variable values to the deaths occurring in the years 1985–1989, 1990–1999, and 2000–2006, respectively. For each of the following modifiers, we then computed indicator variables equal to 1 when below the 25th percentile of the modifier: percent of area with green space, area covered by water, and population over 25 years of age who completed college. Indicator variables were equal to 1 when above the 75th percentile of the modifier for: proportions of the population below the poverty level, of non-white race, and population aged 25+ years who had not completed high school, and population density.
Environmental data
We obtained daily weather data from the National Oceanic and Atmospheric Administration (NOAA) for a single weather station for each city, as previously described36. “Extremely cold days” were those with a daily maximum temperature at or below the 1st percentile of the distribution in that city.5,38 Similarly, we defined extremely hot days as those with a daily minimum temperature at or above the 99th percentile. We chose the daily maximum temperature to examine extreme cold and the daily minimum to examine extreme heat because these indicate situations in which little relief exists during the day (for cold) or at night (for heat). Because study cities represented a wide range of climates, in some cases the percentile-based cutoff points to define extreme temperatures were at very mild temperatures. Therefore, cities were excluded from the extreme cold analysis if their cutoff for extreme cold was ≥ 10°C and were excluded from the extreme heat analysis for extreme heat was ≤ 20°C, leaving a total of 111 and 123 cities, respectively.
We also examined the following continuous weather parameters: mean temperature, apparent temperature and water-vapor pressure in order to evaluate associations between mortality and more moderate weather exposures.39 For these continuous exposure variables, we tested the association on the same day and for the average of the date of death and the two previous days.
Apparent temperature (AT, ‘°C) is the perceived air temperature given the humidity; it was calculated with the following formula40:
where Ta is air temperature and Td is dew point temperature. Water-vapor pressure (wvp) is the water content of a mixture of water vapor and other constituents of air; water vapor pressure (hPa) is computed as41,42:
Water vapor pressure is lowest during winter and highest during summer.
Statistical analysis
The case-only approach can investigate how characteristics that do not vary (or vary slowly) over time (e.g., sex or socioeconomic status) modify the effect of a time-varying exposure (e.g., weather parameters) on the outcome of interest (e.g., death) by restricting the sample to cases (e.g., decedents).5,14,43
This approach provides important advantages over traditional analyses, including reduction of potential confounding by variables typically associated with mortality (e.g., smoking history, blood pressure), simplification of modeling, and reduction of result sensitivity to model misspecification bias.14,43
In the case-only design, a negative association does not necessarily indicate a decreased risk of dying on extreme temperature days for the group of persons or causes examined. Rather, it indicates that the risk of dying associated with extreme temperatures is less pronounced for that group of persons or causes than for the others; a positive association shows that people with certain personal, medical, or area characteristics are more highly represented among the group of people who die on the extreme-temperature days. That is, the case-only analysis estimates what would be the coefficient of an interaction term in a Poisson regression, and provides the incremental effect in that group.
We fitted city-specific logistic regression models in which the dependent variable was the presence or absence of the hypothesized modifier that was a personal characteristic, an area-level characteristic or a cause of admission. The predictors were either extreme hot or cold temperature (indicator variables) or continuous forms of air temperature, water-vapor pressure or apparent temperature.
We conducted separate analyses for cold months (November-March) and warm months (May-September) to exclude extremely hot days from the reference group for extremely cold days and vice versa, thus avoiding an over- or underestimation of the interaction in situations where the modifier of interest is relevant for both hot and cold days.
All models included sine and cosine terms with a 365.24 day period to capture possible interactions between season and the characteristic being investigated (e.g., if people with diabetes have a stronger seasonal pattern in mortality than other persons).14
The main effects of temperature on mortality are well established. Because the case-only analysis estimates the modification of effect but not the main effect of temperature, the reported results are estimates of the additional effect in the subgroup with the characteristic being examined. Therefore, this approach serves our goal of determining whether personal and area-level characteristics are markers of vulnerability to temperature-related mortality.
In a second stage of analysis, we combined the city-specific results using a random-effects meta-analysis.44 Cities in which the proportion of cases was less than 1% were excluded from the analysis. We used the I2 statistic to assess the proportion of total variation in effect estimates attributable to between-city heterogeneity.45
For binomial outcomes, results are presented as the relative odds of dying on an extreme-temperature day for persons who had the condition (e.g. Alzheimer), were in a certain demographic group (e.g. female), or lived in a zip code with the characteristic of interest (e.g. less green space) compared with persons who, to continue the examples, did not have Alzheimer disease, were male, or lived in an area with more green space. For the continuous predictors, the relative odds of dying are presented (for interquartile ranges [IQRs] across all cities) for an increase in warm-month temperature (7.7 °C), an increase in warm-month water-vapor pressure (8.1 hPa), a decrease in cold-month temperature (8.6 °C), and a decrease in cold-month water vapor pressure (5.2 hPa). For small coefficients, these are essentially equivalent to the additional percent change in mortality in the subgroup compared with the general population. That is, an OR of 1.04 indicates a 4% additional increase in mortality associated with hot days in the susceptible group.
Results
Analyses included 7,204,031 total deaths across 135 U.S. cities. Table 1 shows descriptive information about the persons who died and the areas (zip codes) in which they lived. eTable 1 presents the same data for each city, while eTable 2 shows the city-level exposure variables and the thresholds used to define extreme temperatures in each study city and in the excluded cities. Minneapolis, MN had the lowest temperature threshold for extreme cold (−16.7°C), while Phoenix, AZ, had the highest temperature threshold for extreme heat (31.7°C).
Table 1.
Descriptive statistics for persons who died (n=7,204,031) across the 135 US cities
| Individual characteristics | No. | % of deaths |
|---|---|---|
| previous admission for: | ||
| Atrial fibrillation | 1,206,427 | 17 |
| Congestive heart failure | 2,790,616 | 39 |
| MI | 686,927 | 10 |
| Stroke | 1,340,368 | 19 |
| Diabetes | 1,410,099 | 20 |
| COPD | 2,025,149 | 28 |
| Pneumonia | 1,779,908 | 25 |
| Alzheimer disease | 370,158 | 5 |
| Parkinson disease | 201,333 | 3 |
| Dementia | 466,675 | 6 |
| Individual-level modifiers | ||
| Non-white race | 1,006,641 | 14 |
| Female | 4,117,023 | 57 |
| Area-level Modifiers by code | No. | % of deaths |
|---|---|---|
| proportion green space <25th percentile | 2,552,223 | 35 |
| proportion water <25th percentile | 1,744,768 | 24 |
| Proportion in poverty level >75th percentile | 2,100,936 | 29 |
| Population density >75th percentile | 2,816,427 | 39 |
| Proportion without high school diploma >75th percentile | 1,964,069 | 27 |
| Proportion with college degree <25th percentile | 1,454,113 | 20 |
| Proportion non-white >75th percentile | 2,133,811 | 30 |
| Proportion black race >75th percentile | 2,287,239 | 32 |
| Zipcode-level descriptives | 25th Percentile | Median | 75th Percentile |
|---|---|---|---|
| Proportion black race | 0.008 | 0.030 | 0.107 |
| Proportion with college degree | 0.134 | 0.226 | 0.373 |
| Proportion Hispanic | 0.014 | 0.040 | 0.132 |
| Proportion without high school diploma | 0.090 | 0.151 | 0.240 |
| Proportion non-white race | 0.052 | 0.141 | 0.337 |
| Population density (persons/km2) | 133 | 616 | 1578 |
| Proportion poverty level | 0.042 | 0.075 | 0.143 |
| Proportion green space | 0.262 | 0.619 | 0.900 |
| Proportion water | 0.000 | 0.004 | 0.012 |
Chicago had the highest number of deaths during the study period (n=414,741), while Salinas, CA and Spokane, WA had the smallest number of deaths (fewer than 17,000 deaths each). Among the decedents 39% had a previous admission for congestive heart failure, and 20% had a previous admission for diabetes. The percent of subjects with neurological disorders and dementia varied between 3% and 7%.
Study cities had a wide range of climates, with warm-month mean temperatures ranging from 14.4°C in Portland, ME to 29.7°C in Phoenix, AZ, and cold-month temperatures varying between −0.5°C in Minneapolis, MN and 24.3°C in Honolulu, Hawaii. The correlation between mean temperature and water-vapor pressure during warm months varied among the cities, with the weakest correlation (0.13) in El Paso, TX and the strongest correlation (0.89) in Hamilton, OH, Utica, NY, Lansing, MI and Cincinnati, OH. In cold months, the lowest correlation of 0.06 was found in Ventura, CA and the highest correlations were observed in several cities in Florida.
Table 2 shows correlations among the zip-code-level-characteristics. Notably, we found a correlation of −0.80 between the proportion of green space and population density, and between the proportion of population who had graduated from college and who had not graduated from high school. In general, we found substantial effect modification by medical conditions and personal- and area-level characteristics of the association between total mortality and weather parameters (extreme hot and cold temperature; warm- and cold-month continuous temperature; and water-vapor pressure) (Figures 1–6), with the strongest associations observed for neurological disease. The results for extreme-hot and extreme-cold temperature defined instead at the 5th and 95th percentiles of temperature and for apparent temperature are presented in eFigures 1–3; they were similar to results presented in the text.
Table 2.
Correlations among the zip code-level characteristics.
| Proportion green space |
Proportion water |
Proportion with college degree |
Proportion poverty level |
Proportion black race |
Proportion Hispanic |
Proportion non-white race |
Proportion without high school diploma |
Population density (persons/km2) |
|
|---|---|---|---|---|---|---|---|---|---|
| Proportion green space | 1 | ||||||||
| Proportion water | 0.28 | 1 | |||||||
| Proportion with college degree | 0.09 | 0.04 | 1 | ||||||
| Proportion poverty level | −0.41 | −0.11 | −0.56 | 1 | |||||
| Proportion black race | −0.43 | −0.11 | −0.25 | 0.51 | 1 | ||||
| Proportion Hispanic | −0.31 | −0.03 | −0.35 | 0.46 | 0.37 | 1 | |||
| Proportion non-white race | −0.47 | −0.15 | −0.36 | 0.61 | 0.80 | 0.53 | 1 | ||
| Proportion without high school diploma | −0.27 | −0.09 | −0.83 | 0.74 | 0.39 | 0.50 | 0.54 | 1 | |
| Population density (persons/km2) | −0.83 | −0.25 | −0.03 | 0.39 | 0.42 | 0.33 | 0.46 | 0.23 | 1 |
Figure 1.
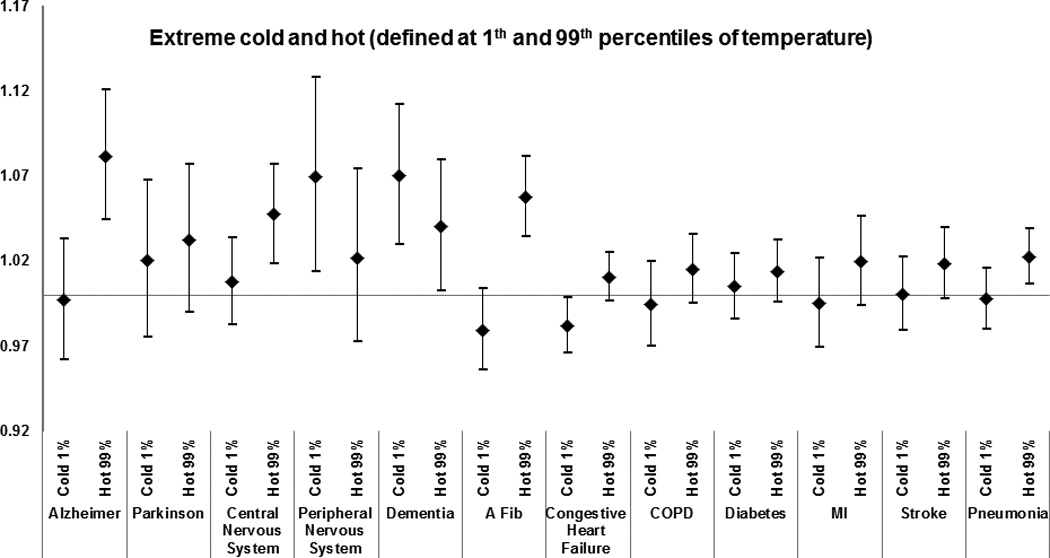
Effect modification by medical condition of the effect of extreme-hot and extreme-cold temperature (defined at 1st and 99th percentiles of temperature) on total mortality. Results from the meta-analysis of 123 and 111 U.S. cities, respectively, during the period 1985–2006, among Medicare enrollees. Estimates represent the relative odds of dying on an extreme-temperature day for persons who had the medical condition compared with persons who did not have the condition. See Methods section for definition of each medical condition.
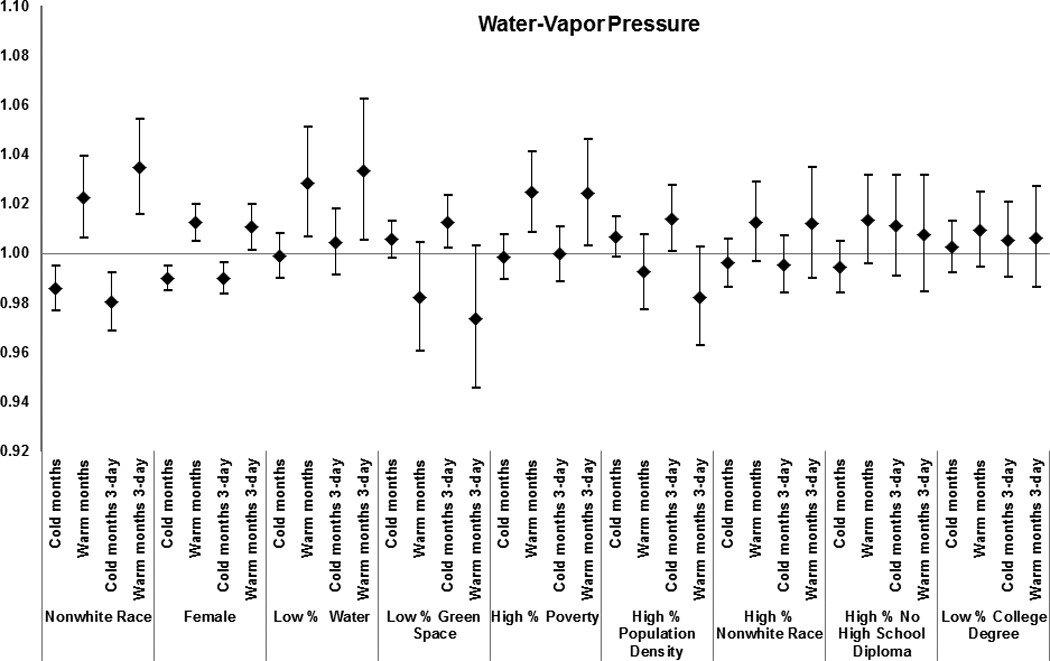
Figure 6.
Effect modification by subject and area-level characteristics of the effect of warm-months’ and cold-months’ water-vapor pressure for the day of death and for the 3-day average on total mortality. Results from the meta-analysis of 123 and 111 U.S. cities, respectively, during the period 1985–2006, among Medicare enrollees. Estimates represent the relative odds of dying for persons who had the medical condition compared with persons who did not have the condition for an increase in warm-months’ water-vapor pressure of 8.1 hPa, and for a decrease in cold-months’ water-vapor pressure of 5.2 hPa.
The following results report the odds ratios for the interactions, i.e. the additional effect of the weather parameters in people with the examined modifier. Having had a previous admission that included Alzheimer disease as a cause was the strongest modifier of the extreme-heat effect (odds ratio of dying = 1.08 [95% confidence interval (CI) = 1.04–1.12]); of warm-month temperature for the day of death (1.03 [1.00–1.05] per 7.7 °C increase in temperature), and for the 3-day average (1.03 [1.00–1.07] per 7.7 °C increase in temperature) and of warm-month water-vapor pressure for the day of death (1.04 [1.02–1.06] per 8.1 hectopascals (hPa) increase in water-vapor pressure) and for the 3-day average (1.06 [1.03–1.08] per 8.1 hPa increase in water-vapor pressure).
In addition to those having Alzheimer’s disease, other persons at greater risk of dying on extremely hot days included those with a previous admission for dementia (1.04 [1.02–1.09]), hereditary and degenerative diseases of the central nervous system (1.05 [1.02–1.08]) and atrial fibrillation (1.06 [1.03–1.08]).
Other causes of admission that modified the associations between mortality and extreme heat were: congestive heart failure (1.01 [1.00–1.02]), diabetes (1.01 [1.00–1.03]), COPD (1.02 [1.00–1.04]), pneumonia 1.02 [1.01–1.04]), and stroke (1.02 [1.00–1.04]). A similar pattern of effect modification was observed for water-vapor pressure and temperature (Figures 3 and 5), with the results for the three-day average having higher odds ratios than for the same day.
Figure 3.
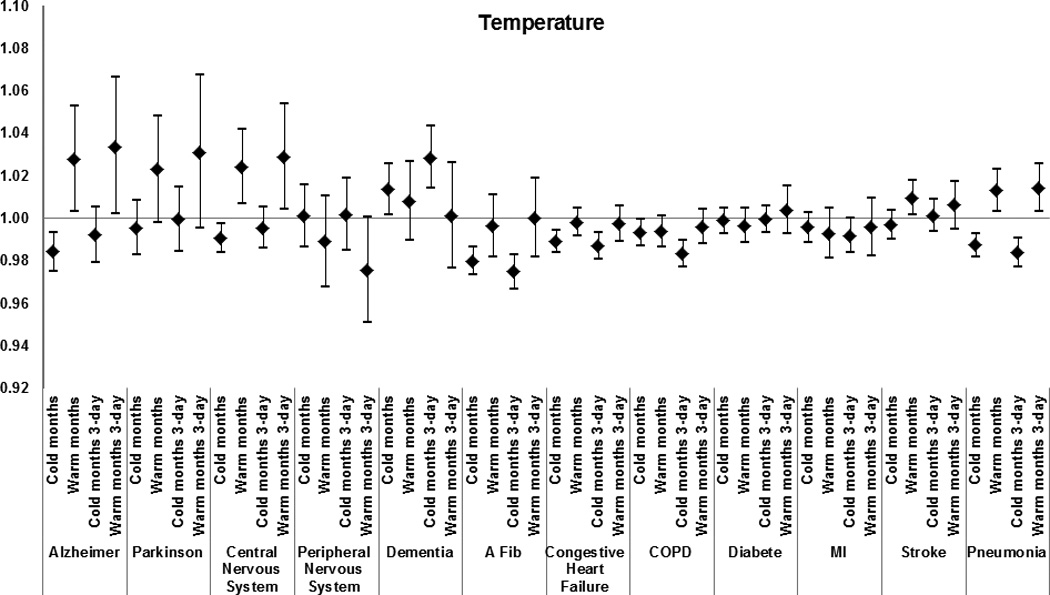
Effect modification by medical condition of the effect of warm-months’ and cold-months’ temperature for the day of death and for the 3-day average on total mortality. Results from the meta-analysis of 123 and 111 U.S. cities, respectively, during the period 1985–2006, among Medicare enrollees. Estimates represent the relative odds of dying for persons who had the medical condition compared with persons who did not have the condition for an increase in warm-months’ temperature of 7.7 °C, and for a decrease in cold-months’ temperature of 8.6 °C.
Figure 5.
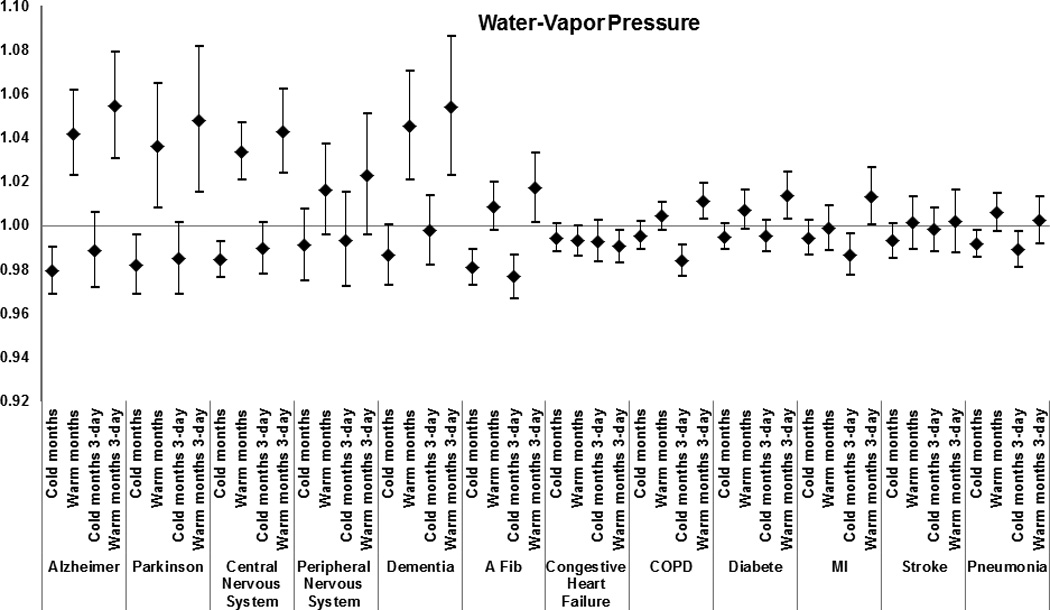
Effect modification by medical condition of the effect of warm-months’ and cold-months’ water-vapor pressure for the day of death and for the 3-day average on total mortality. Results from the meta-analysis of 123 and 111 U.S. cities, respectively, during the period 1985–2006, among Medicare enrollees. Estimates represent the relative odds of dying for persons who had the medical condition compared with persons who did not have the condition for an increase in warm-months’ water-vapor pressure of 8.1 hPa, and for a decrease in cold-months’ water-vapor pressure of 5.2 hPa.
Zip code-level characteristics were modifiers of the association between mortality and extremely hot weather, warm-month water-vapor pressure, and warm-month temperature. Specifically, for a 7.7 °C increase in warm-month temperature, we found enhanced risk for subjects living in codes with higher poverty (1.02 [1.01 – 1.04]); greater population density (1.02 [1.01 – 1.03]); and more residents without high school degrees (1.04 [1.02–1.05]).
Similarly, during extremely hot days mortality was higher in zip codes with higher poverty (1.03 [1.00–1.05]); higher population density (1.02 [0.99–1.05]); and more residents of non-white race (1.03 [0.99–1.06]) and without high school degree (1.04 [1.01–1.06]).
The results for zip codes with less water and green space varied by exposure; with less water (1.03 [1.01–1.05]) modifying the effect of water-vapor pressure on mortality, and less green space (1.03 [1.01–1.05]) modifying the effect of temperature in warm months on mortality. Women and subjects with race defined as non-white had a higher risk of mortality for all exposures examined.
For effects of extreme cold, subjects with a previous admission for dementia (1.07 [1.03–1.11]) and disorder of the peripheral nervous system (1.07 [1.01–1.13]) had a higher risk of mortality. However, the risks of dying with other medical conditions were lower on extremely cold days. Similar results were observed for temperature and water-vapor pressure.
Among the zip code-level characteristics associated with increased susceptibility to cold-month temperature were low proportions of green space (1.01 [0.99–1.01]) and of residents with a college degree (1.01 [1.00–1.02]). A similar pattern of effect modification during cold months was observed for water-vapor pressure (Figure 6).
We found moderate heterogeneity (I2 < 0.5) among the city-specific effect estimates during cold and warm weather, and during extreme cold and hot days.
Discussion
In this large multi-city study, we identified several subpopulations particularly susceptible to temperature extremes, continuous temperature and water-vapor pressure. Subjects with a neurological disorder, dementia and atrial fibrillation were especially vulnerable to each of these exposures. The increase in deaths on extremely high-temperature days was higher also for congestive heart failure, MI, stroke, diabetes, COPD, and pneumonia, and similar increases were observed for the continuous weather parameters. Among personal characteristics, being non-white and female was associated with elevated risk of mortality due to increases in warm-month temperatures and in water-vapor pressure, and on extremely hot days.
Notably, local zip-code-level socioeconomic characteristics modified susceptibility to all the warm-month exposures. During warm months, in zip codes with a low percent of green space, associations between mortality and temperature were higher, but low percent water in a zip code did not modify the effect. By contrast, during the warm months, associations between water-vapor pressure and mortality were higher in zip codes with less water but not in those with less green space Both green space and presence of water may systematically change the experience of temperature and water-vapor pressure exposure for people living in those areas. For example, people living in areas with low percent of green space may experience hotter temperatures than those in verdant areas, and indoor humidity may correlate more strongly with outdoor water-vapor pressure in areas with more surface water. Further analysis using finer-spatial scale data would be necessary to evaluate whether these contrasting patterns would persist.
Our results suggest that some specific medical conditions as well as area-level characteristics increase susceptibility to temperature extremes and to continuous weather parameters. Several studies on the effects of temperature on mortality have also found a greater susceptibility of the elderly to both cold6,46 and hot temperatures.1–4 A reduced thermoregulatory capacity in the elderly, combined with a diminished ability to detect changes in their body temperature, may partly explain their increased susceptibility,47 although physiologic differences in other responses to extreme temperatures may also play a role.48
Three studies have found decreased temperature associated with increases in inflammatory markers,20 low-density lipoprotein, and low levels of high-density lipoprotein21 and blood pressure22 in elderly men, suggesting that these risk factors may be among the underlying mechanisms which may lead to cold-related, but not heat-related, cardiovascular deaths. Similarly, Ren and co-authors23 showed that higher ambient temperature was associated with decreases in heart-rate-variability measures during the warm but not the cold season, concluding that temperature could precipitate cardiovascular events via autonomic-nervous-system dysfunction.
It has also been reported that exposure to cold temperatures increases levels of plasma cholesterol and plasma fibrinogen,19 which could lead to thrombosis through hemoconcentration.
Subjects with neurological disorders and dementia are more vulnerable to extreme heat due to physiological, behavioral and social factors,49 but also because psychiatric medications can increase vulnerability to heat-related morbidity by altering the body’s ability to thermoregulate50,51 due to pharmacologic effects on the parasympathetic pathway.52
Our study therefore confirms and supports previous findings of increased risk of exposure to warm weather in persons with mental illness, dementia, and neurological disorders.4,15,16,27–29,31,53,54
When examining whether chronic conditions recorded on admission records prior to death increase the susceptibility to extreme temperatures, we confirmed previous findings5,14 that people with diabetes are particularly vulnerable to heat. This could be explained by impaired thermoregulation through impaired autonomic control and endothelial function.
An important finding of our study is that subjects with atrial fibrillation are more susceptible to temperature extremes than to other weather parameters. Atrial fibrillation is a main risk factor for stroke, which has been found to be particularly vulnerable to the effects of extreme heat.5,53 An increase in blood viscosity and cholesterol levels with high temperatures19 may interact with atrial fibrillation to facilitate the formation of blood clots that cause stroke.
We found that subjects of non-white race living in areas with lower education, higher poverty, and lower percent of green space and water were more susceptible to extreme heat, temperature, and water-vapor pressure during warm months. Greater susceptibility to dying on extremely hot days has been previously reported for non-whites and those of lower socioeconomic status, and could be related to poorer health status, limited access to health care, and poorer housing conditions.5,13–15,33
Regarding water-vapor pressure, Yang and co-authors10 found a 0.35% increase [0.07 – 0.62] in total mortality with each 1% increase in influenza virus activity during periods of high water-vapor pressure. In Germany, a decrease in air temperature and in water-vapor pressure doubled the risk of ST-segment depression, which could explain the association between weather changes and cardiovascular mortality.25 Our results confirm these previous findings that changes in water-vapor pressure are associated with increased risk of mortality in susceptible populations.
One limitation of our study is that we lack data on medication use and therefore cannot examine whether specific drugs modify the effect of weather on mortality. Another limitation relates to the case-only study design, which cannot estimate the main effects of temperature but only effect modification by certain characteristics. Moreover, those characteristics could not be evaluated as continuous variables or as categorical variables with three or more levels.
Our population may not be representative of the populations of decedents with these conditions, given that it consisted of people who were hospitalized for any reason prior to dying. Medicare billing records probably do not identify pre-existing conditions in decedents as comprehensively as, for example, the clinical information (not just hospitalizations) from the UK General Practice Research Database used by Page and colleagues.31 However, we believe we captured most of the cases of these medical conditions (among persons hospitalized for any reason), given that, for example, Alzheimer disease will generally be noted on an admission for pneumonia, given that the cognitive impairment will affect management of the case in the hospital.
Previous studies have suggested that populations tend to adapt to the local climate.55,56 This adaptation may occur by physiologic acclimatization, behavioral patterns, or other adaptive mechanisms, such as having heating or air conditioning at home.57 The effects of an increase in global temperature may be partly mitigated by these adaptive mechanisms, and, as our results suggest, increasing green space might ameliorate the heat effects.
In conclusion, this study’s identification of subpopulations particularly vulnerable to temperature extremes is of public health relevance, especially if such subpopulations are growing proportions of the population —as is the case for persons with diabetes and for the elderly in many countries.
Supplementary Material
Figure 2.
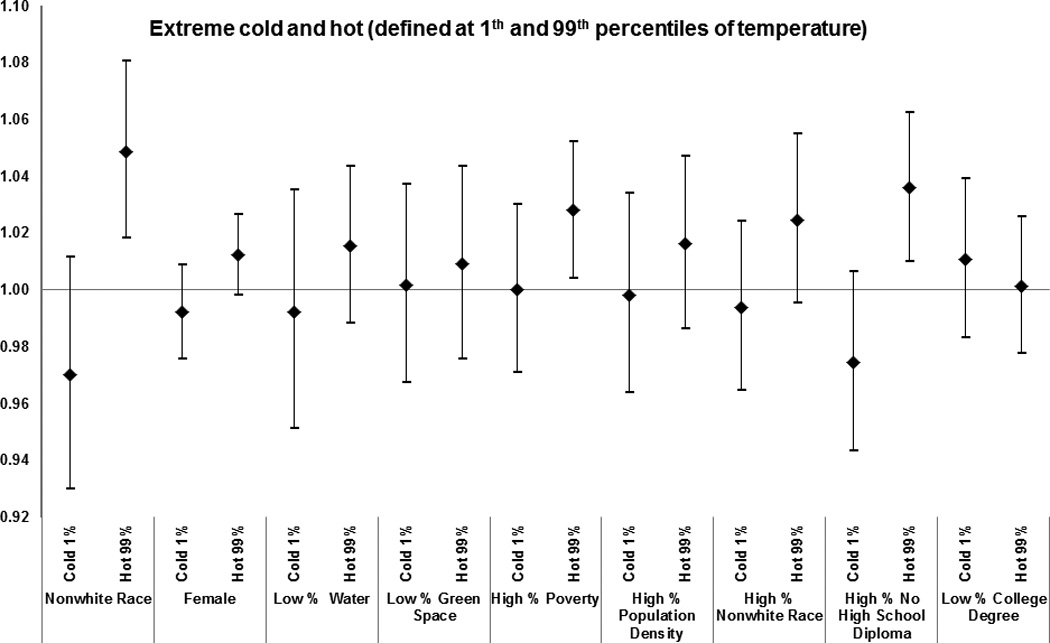
Effect modification by subject and area-level characteristics of the effect of extreme-hot and extreme-cold temperature (defined at 1st and 99th percentiles of temperature) on total mortality. Results from the meta-analysis of 123 and 111 U.S. cities, respectively, during the period 1985–2006, among Medicare enrollees.
Figure 4.
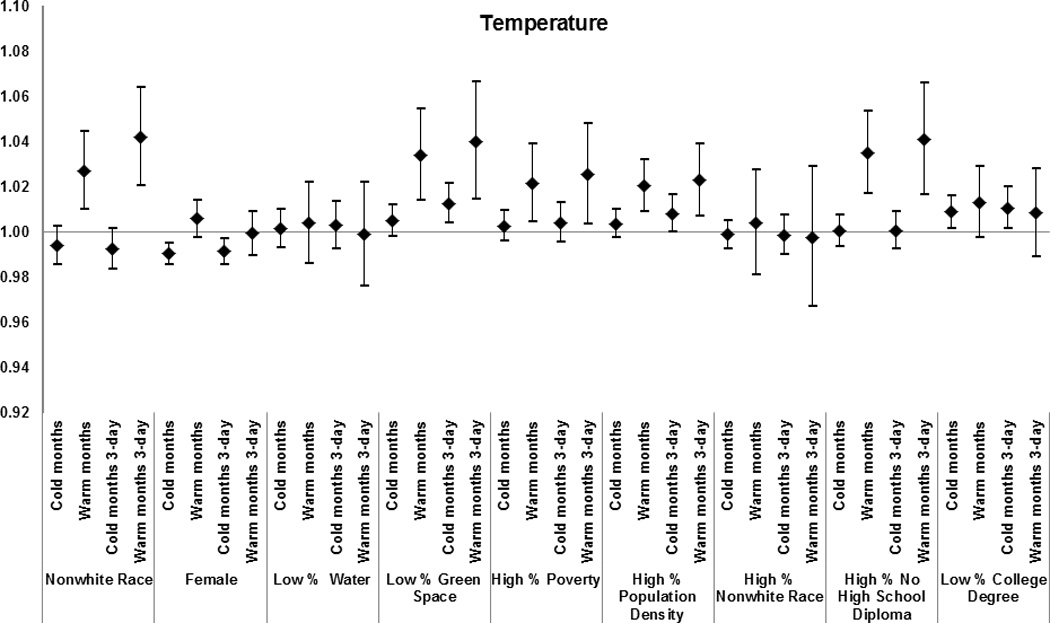
Effect modification by subject and area-level characteristics of the effect of warm-months’ and cold-months’ temperature for the day of death and for the 3-day average on total mortality. Results from the meta-analysis of 123 and 111 U.S. cities, respectively, during the period 1985–2006, among Medicare enrollees. Estimates represent the relative odds of dying for persons who had the medical condition compared with persons who did not have the condition for an increase in warm-months’ temperature of 7.7 °C, and for a decrease in cold-months’ temperature of 8.6 °C.
Acknowledgments
This study was funded in part by: NIEHS R21ES020695-01, NIEHS R21ES020156, NIA T32AG027708
This study was made possible in part by USEPA grant RD 83479801, R83275201, RD-83241601, and EPA R83275201 awarded by the U.S. Environmental Protection Agency. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Conflicts of Interest and Source of Funding
The authors have no Conflicts of Interest
Reference
- 1.Basu R. High ambient temperature and mortality: a review of epidemiologic studies from 2001 to 2008. Environ Health. 2009;8(1):40. doi: 10.1186/1476-069X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gosling S, Lowe J, McGregor G, Pelling M, Malamud B. Associations between elevated atmospheric temperature and human mortality: a critical review of the literature. Climatic Change. 2009;92(3):299–341. [Google Scholar]
- 3.Analitis A, Katsouyanni K, Biggeri A, et al. Effects of cold weather on mortality: results from 15 European cities within the PHEWE project. Am J Epidemiol. 2008;168(12):1397–1408. doi: 10.1093/aje/kwn266. [DOI] [PubMed] [Google Scholar]
- 4.Williams S, Nitschke M, Weinstein P, Pisaniello DL, Parton KA, Bi P. The impact of summer temperatures and heatwaves on mortality and morbidity in Perth, Australia 1994–2008. Environ Int. 2012;40:33–38. doi: 10.1016/j.envint.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Medina-Ramon M, Zanobetti A, Cavanagh DP, Schwartz J. Extreme temperatures and mortality: assessing effect modification by personal characteristics and specific cause of death in a multi-city case-only analysis. Environ Health Perspect. 2006;114(9):1331–1336. doi: 10.1289/ehp.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Neill MS, Zanobetti A, Schwartz J. Modifiers of the temperature and mortality association in seven US cities. Am J Epidemiol. 2003;157(12):1074–1082. doi: 10.1093/aje/kwg096. [DOI] [PubMed] [Google Scholar]
- 7.Basagana X, Sartini C, Barrera-Gomez J, et al. Heat waves and cause-specific mortality at all ages. Epidemiology. 2011;22(6):765–772. doi: 10.1097/EDE.0b013e31823031c5. [DOI] [PubMed] [Google Scholar]
- 8.Turner LR, Barnett AG, Connell D, Tong S. Ambient temperature and cardiorespiratory morbidity: a systematic review and meta-analysis. Epidemiology. 2012;23(4):594–606. doi: 10.1097/EDE.0b013e3182572795. [DOI] [PubMed] [Google Scholar]
- 9.Ostro B, Rauch S, Green R, Malig B, Basu R. The effects of temperature and use of air conditioning on hospitalizations. Am J Epidemiol. 2010;172(9):1053–1061. doi: 10.1093/aje/kwq231. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Chen PY, He JF, et al. Effect modification of environmental factors on influenza-associated mortality: a time-series study in two Chinese cities. BMC infectious diseases. 2011;11:342. doi: 10.1186/1471-2334-11-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barreca AI, Shimshack JP. Absolute humidity, temperature, and influenza mortality: 30 years of county-level evidence from the United States. American journal of epidemiology. 2012;176(Suppl):S114–S122. doi: 10.1093/aje/kws259. [DOI] [PubMed] [Google Scholar]
- 12.Solomon S, Qin D, Manning M, et al., editors. IPCC. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press; 2007. p. 996. [Google Scholar]
- 13.Diaz J, Jordan A, Garcia R, et al. Heat waves in Madrid 1986–1997: effects on the health of the elderly. Int Arch Occup Environ Health. 2002;75(3):163–170. doi: 10.1007/s00420-001-0290-4. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz J. Who is sensitive to extremes of temperature?: A case-only analysis. Epidemiology. 2005;16(1):67–72. doi: 10.1097/01.ede.0000147114.25957.71. [DOI] [PubMed] [Google Scholar]
- 15.Stafoggia M, Forastiere F, Agostini D, et al. Vulnerability to heat-related mortality: a multicity, population-based, case-crossover analysis. Epidemiology. 2006;17(3):315–323. doi: 10.1097/01.ede.0000208477.36665.34. [DOI] [PubMed] [Google Scholar]
- 16.Schifano P, Cappai G, De Sario M, et al. Susceptibility to heat wave-related mortality: a follow-up study of a cohort of elderly in Rome. Environmental health: a global access science source. 2009;8:50. doi: 10.1186/1476-069X-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harlan SL, Declet-Barreto JH, Stefanov WL, Petitti DB. Neighborhood Effects on Heat Deaths: Social and Environmental Predictors of Vulnerability in Maricopa County, Arizona. Environmental health perspectives. 2012 doi: 10.1289/ehp.1104625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 19.Keatinge WR, Coleshaw SR, Easton JC, Cotter F, Mattock MB, Chelliah R. Increased platelet and red cell counts, blood viscosity, and plasma cholesterol levels during heat stress, and mortality from coronary and cerebral thrombosis. Am J Med. 1986;81(5):795–800. doi: 10.1016/0002-9343(86)90348-7. [DOI] [PubMed] [Google Scholar]
- 20.Halonen JI, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. Associations between outdoor temperature and markers of inflammation: a cohort study. Environmental health: a global access science source. 2010;9:42. doi: 10.1186/1476-069X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halonen JI, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. Outdoor temperature is associated with serum HDL and LDL. Environ Res. 2011;111(2):281–287. doi: 10.1016/j.envres.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halonen JI, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. Relationship between outdoor temperature and blood pressure. Occup Environ Med. 2011;68(4):296–301. doi: 10.1136/oem.2010.056507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren C, O’Neill MS, Park SK, Sparrow D, Vokonas P, Schwartz J. Ambient temperature, air pollution, and heart rate variability in an aging population. Am J Epidemiol. 2011;173(9):1013–1021. doi: 10.1093/aje/kwq477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keatinge WR, Coleshaw SR, Cotter F, Mattock M, Murphy M, Chelliah R. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: factors in mortality from coronary and cerebral thrombosis in winter. British Medical Journal Clinical Research Ed. 1984;289(6456):1405–1408. doi: 10.1136/bmj.289.6456.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider A, Schuh A, Maetzel F-K, Rückerl R, Breitner S, Peters A. Weather-induced ischemia and arrhythmia in patients undergoing cardiac rehabilitation: another difference between men and women. International journal of biometeorology. 2008;52(6):535–547. doi: 10.1007/s00484-008-0144-9. [DOI] [PubMed] [Google Scholar]
- 26.Sharma VM, Pichan G, Panwar MR. Differential effects of hot-humid and hot-dry environments on mental functions. International archives of occupational and environmental health. 1983;52(4):315–327. doi: 10.1007/BF02226897. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser R, Rubin CH, Henderson AK, et al. Heat-related death and mental illness during the 1999 Cincinnati heat wave. The American journal of forensic medicine and pathology. 2001;22(3):303–307. doi: 10.1097/00000433-200109000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Hansen A, Bi P, Nitschke M, Ryan P, Pisaniello D, Tucker G. The effect of heat waves on mental health in a temperate Australian city. Environ Health Perspect. 2008;116(10):1369–1375. doi: 10.1289/ehp.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bark N. Deaths of psychiatric patients during heat waves. Psychiatric services (Washington, D.C.) 1998;49(8):1088–1090. doi: 10.1176/ps.49.8.1088. [DOI] [PubMed] [Google Scholar]
- 30.Conti S, Meli P, Minelli G, et al. Epidemiologic study of mortality during the Summer 2003 heat wave in Italy. Environ Res. 2005;98(3):390–399. doi: 10.1016/j.envres.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Page LA, Hajat S, Kovats RS, Howard LM. Temperature-related deaths in people with psychosis, dementia and substance misuse. The British journal of psychiatry: the journal of mental science. 2012;200(6):485–490. doi: 10.1192/bjp.bp.111.100404. [DOI] [PubMed] [Google Scholar]
- 32.Reid CE, O’Neill MS, Gronlund CJ, et al. Mapping community determinants of heat vulnerability. Environ Health Perspect. 2009;117(11):1730–1736. doi: 10.1289/ehp.0900683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Neill MS. Air conditioning and heat-related health effects. Applied Environmental Science and Public Health. 2003;1(1):9–12. [Google Scholar]
- 34.O’Neill MS, Zanobetti A, Schwartz J. Disparities by Race in Heat-Related Mortality in Four US Cities: The Role of Air Conditioning Prevalence. J Urban Health. 2005;82(2):191–197. doi: 10.1093/jurban/jti043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajat S, Kovats RS, Lachowycz K. Heat-related and cold-related deaths in England and Wales: who is at risk? Occup Environ Med. 2007;64(2):93–100. doi: 10.1136/oem.2006.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zanobetti A, O’Neill MS, Gronlund CJ, Schwartz JD. Summer temperature variability and long-term survival among elderly people with chronic disease. Proceedings of the National Academy of Sciences. 2012;109(17):6608–6613. doi: 10.1073/pnas.1113070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anonymous. Demographic data, US Census and GIS products - GeoLytics. [Accessed April 19, 2013];Planners Package: Census 2000 Long Form (SF3) and 1990 Form in 2000 Boundaries. 2006 Available at: http://www.geolytics.com/. [Google Scholar]
- 38.Zhang K, Rood RB, Michailidis G, et al. Comparing exposure metrics for classifying “dangerous heat” in heat wave and health warning systems. Environment international. 2012;46:23–29. doi: 10.1016/j.envint.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanobetti A, Schwartz J. Temperature and Mortality in Nine US Cities. Epidemiology. 2008;19(4):563–570. doi: 10.1097/EDE.0b013e31816d652d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalkstein LS, Valimont KM. An evaluation of summer discomfort in the United States using a relative climatological index. Bulletin American Meteorological Society. 1986;67(7):842–848. [Google Scholar]
- 41.Eaton M, Kells SA. Use of vapor pressure deficit to predict humidity and temperature effects on the mortality of mold mites, Tyrophagus putrescentiae. Experimental & applied acarology. 2009;47(3):201–213. doi: 10.1007/s10493-008-9206-2. [DOI] [PubMed] [Google Scholar]
- 42.Buck AL. New Equations for Computing Vapor Pressure and Enhancement Factor. Journal of Applied Meteorology. 1981;20(12):1527–1532. [Google Scholar]
- 43.Armstrong BG. Fixed factors that modify the effects of time-varying factors: applying the case-only approach. Epidemiology. 2003;14(4):467–472. doi: 10.1097/01.ede.0000071408.39011.99. [DOI] [PubMed] [Google Scholar]
- 44.Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Stat Med. 1995;14(4):395–411. doi: 10.1002/sim.4780140406. [DOI] [PubMed] [Google Scholar]
- 45.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 46.Curriero FC, Heiner KS, Samet JM, Zeger SL, Strug L, Patz JA. Temperature and mortality in 11cities of the eastern United States. Am J Epidemiol. 2002;155(1):80–87. doi: 10.1093/aje/155.1.80. [DOI] [PubMed] [Google Scholar]
- 47.Mercer JB. Cold--an underrated risk factor for health. Environ Res. 2003;92(1):8–13. doi: 10.1016/s0013-9351(02)00009-9. [DOI] [PubMed] [Google Scholar]
- 48.Smolander J. Effect of cold exposure on older humans. International journal of sports medicine. 2002;23(2):86–92. doi: 10.1055/s-2002-20137. [DOI] [PubMed] [Google Scholar]
- 49.Faunt JD, Wilkinson TJ, Aplin P, Henschke P, Webb M, Penhall RK. The effete in the heat: heat-related hospital presentations during a ten day heat wave. Australian and New Zealand journal of medicine. 1995;25(2):117–121. doi: 10.1111/j.1445-5994.1995.tb02822.x. [DOI] [PubMed] [Google Scholar]
- 50.Batscha CL. Heat stroke. Keeping your clients cool in the summer. Journal of psychosocial nursing and mental health services. 1997;35(7):12–17. doi: 10.3928/0279-3695-19970701-19. [DOI] [PubMed] [Google Scholar]
- 51.Flynn A, McGreevy C, Mulkerrin EC. Why do older patients die in a heatwave? QJM: monthly journal of the Association of Physicians. 2005;98(3):227–229. doi: 10.1093/qjmed/hci025. [DOI] [PubMed] [Google Scholar]
- 52.Martin-Latry K, Goumy MP, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalisation. European psychiatry: the journal of the Association of European Psychiatrists. 2007;22(6):335–338. doi: 10.1016/j.eurpsy.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Bouchama A, Dehbi M, Mohamed G, Matthies F, Shoukri M, Menne B. Prognostic factors in heat wave related deaths: a meta-analysis. Arch Intern Med. 2007;167(20):2170–2176. doi: 10.1001/archinte.167.20.ira70009. [DOI] [PubMed] [Google Scholar]
- 54.Babich P, Wang PY, Allen G, Sioutas C, Koutrakis P. Development and evaluation of a continuous ambient PM2.5 mass monitor. Aerosol Science and Technology. 2000;32:309–324. [Google Scholar]
- 55.Braga AL, Zanobetti A, Schwartz J. The time course of weather-related deaths. Epidemiology. 2001;12(6):662–667. doi: 10.1097/00001648-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 56.Medina-Ramon M, Schwartz J. Temperature, Temperature Extremes, and Mortality: A Study of Acclimatization and Effect Modification in 50 United States Cities. Occup Environ Med. 2007;64(12):827–833. doi: 10.1136/oem.2007.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keatinge WR, Donaldson GC, Bucher K, et al. Winter mortality in relation to climate. Int J Circumpolar Health. 2000;59(3–4):154–159. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


