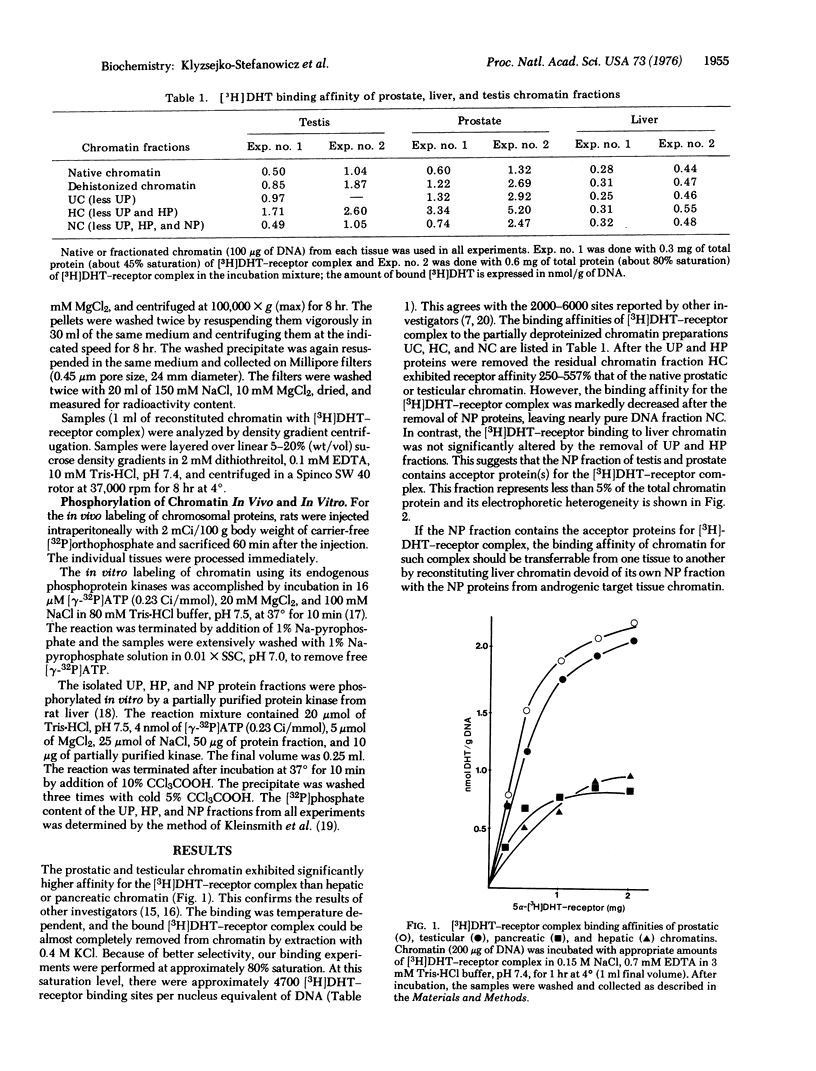
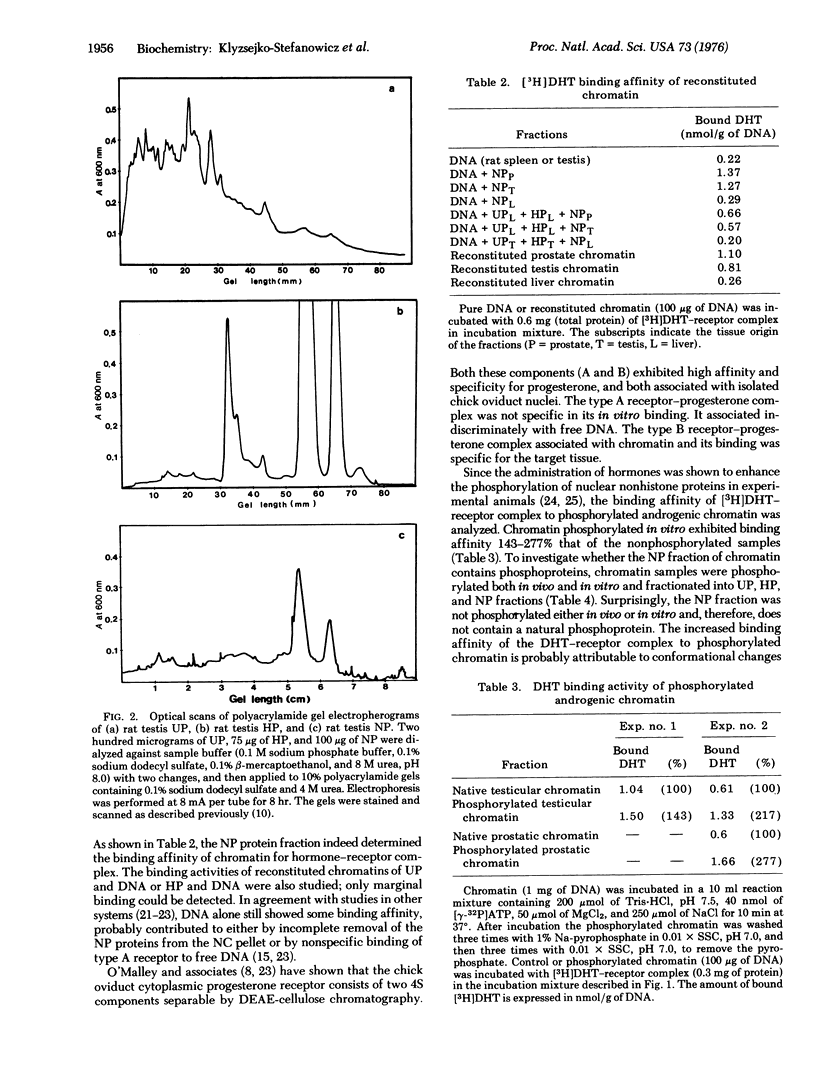
Abstract
Fractionation of chromatin into urea-soluble chromosomal nonhistone proteins (UP), histones (HP), and DNA-associated nonhistone proteins (NP) revealed that the NP fraction from testicular and prostatic chromatin contains organ-specific acceptors for complexes of 5alpha-dihydrotestosterone (17beta-hydroxy-5alpha-androstan-3-one) and its receptor. This acceptor capacity of androgenic tissue chromatin could be transferred to chromatins from non-target tissues with the NP fraction of DNA-associated proteins. Phosphorylation of chromatin enhanced its hormone-receptor binding capacity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed K., Ishida H. Effect of testosterone on nuclear phosphoproteins of rat ventral prostate. Mol Pharmacol. 1971 May;7(3):323–327. [PubMed] [Google Scholar]
- Ahmed K., Wilson M. J. Chromatin-associated protein phosphokinases of rat ventral prostate. Characteristics and effects of androgenic status. J Biol Chem. 1975 Mar 25;250(6):2370–2375. [PubMed] [Google Scholar]
- Bekhor I., Kung G. M., Bonner J. Sequence-specific interaction of DNA and chromosomal protein. J Mol Biol. 1969 Jan;39(2):351–364. doi: 10.1016/0022-2836(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Buller R. E., Schrader W. T., O'Malley B. W. Progesterone-binding components of chick oviduct. IX. The kinetics of nuclear binding. J Biol Chem. 1975 Feb 10;250(3):809–818. [PubMed] [Google Scholar]
- Chiu J. F., Craddock C., Getz S., Hnilica L. S. Nonhistone chromatin protein phosphorylation during azo-dye carcinogenesis. FEBS Lett. 1973 Jul 1;33(2):247–250. doi: 10.1016/0014-5793(73)80204-2. [DOI] [PubMed] [Google Scholar]
- Chiu J. F., Hunt M., Hnilica L. S. Tissue-specific DNA-protein complexes during azo dye hepatocarcinogenesis. Cancer Res. 1975 Apr;35(4):913–919. [PubMed] [Google Scholar]
- Fang S., Liao S. Androgen receptors. Steroid- and tissue-specific retention of a 17 beta-hydroxy-5 alpha-androstan-3-one-protein complex by the cell nuclei of ventral prostate. J Biol Chem. 1971 Jan 10;246(1):16–24. [PubMed] [Google Scholar]
- Hamilton T. H. Control by estrogen of genetic transcription and translation. Binding to chromatin and stimulation of nucleolar RNA synthesis are primary events in the early estrogen action. Science. 1968 Aug 16;161(3842):649–661. doi: 10.1126/science.161.3842.649. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., DeSombre E. R. Mechanism of action of the female sex hormones. Annu Rev Biochem. 1972;41:203–230. doi: 10.1146/annurev.bi.41.070172.001223. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., Suzuki T., Kawashima T., Stumpf W. E., Jungblut P. W., DeSombre E. R. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama M., Dastugue B., Kruh J. Action of phosphoproteins and protein kinase from rat liver chromatin on RNA synthesis. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1345–1350. doi: 10.1016/s0006-291x(71)80233-4. [DOI] [PubMed] [Google Scholar]
- King R. J., Gordon J., Cowan D. M., Inman D. R. The intranuclear localization of [6,7-3H]-oestradiol-17-beta in dimethylbenzanthracene-induced rat mammary adenocarcinoma and other tissues. J Endocrinol. 1966 Oct;36(2):139–150. doi: 10.1677/joe.0.0360139. [DOI] [PubMed] [Google Scholar]
- King R. J., Gordon J. Involvement of DNA in the acceptor mechanism for uterine oestradiol receptor. Nat New Biol. 1972 Dec 6;240(101):185–187. doi: 10.1038/newbio240185a0. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphoprotein metabolism in isolated lymphocyte nuclei. Proc Natl Acad Sci U S A. 1966 May;55(5):1182–1189. doi: 10.1073/pnas.55.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S., Fang S. Receptor-proteims for androgens and the mode of action of androgens on gene transcription in ventral prostate. Vitam Horm. 1969;27:17–90. doi: 10.1016/s0083-6729(08)61124-3. [DOI] [PubMed] [Google Scholar]
- Mainwaring W. I., Irving R. The use of deoxyribonucleic acid-cellulose chromatography and isoelectric focusing for the characterization and partial purification of steroid-receptor complexes. Biochem J. 1973 May;134(1):113–127. doi: 10.1042/bj1340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainwaring W. I., Peterken B. M. A reconstituted cell-free system for the specific transfer of steroid--receptor complexes into nuclear chromatin isolated from the rat ventral prostate gland. Biochem J. 1971 Nov;125(1):285–295. doi: 10.1042/bj1250285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., Means A. R. Female steroid hormones and target cell nuclei. Science. 1974 Feb 15;183(4125):610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Schrader W. T. Progesterone receptor components: identification of subunits binding to the target-cell genome. J Steroid Biochem. 1972 Apr;3(3):617–629. doi: 10.1016/0022-4731(72)90107-0. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Spelsberg T. C., Scharder W. T., Chytil F., Steggles A. W. Mechanisms of interaction of a hormone--receptor complex with the genome of a eukaryotic target cell. Nature. 1972 Jan 21;235(5334):141–144. doi: 10.1038/235141a0. [DOI] [PubMed] [Google Scholar]
- Shyamala G., Gorski J. Estrogen receptors in the rat uterus. Studies on the interaction of cytosol and nuclear binding sites. J Biol Chem. 1969 Mar 10;244(5):1097–1103. [PubMed] [Google Scholar]
- Spelsberg T. C., Mitchell W. M., Chytil F., Wilson E. M., O'Malley B. W. Chromatin of the developing chick oviduct: changes in the acidic proteins. Biochim Biophys Acta. 1973 Jul 27;312(4):765–778. doi: 10.1016/0005-2787(73)90080-4. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Steggles A. W., Chytil F., O'Malley B. W. Progesterone-binding components of chick oviduct. V. Exchange of progesterone-binding capacity from target to nontarget tissue chromatins. J Biol Chem. 1972 Mar 10;247(5):1368–1374. [PubMed] [Google Scholar]
- Spelsberg T. C., Steggles A. W., O'Malley B. W. Progesterone-binding components of chick oviduct. 3. Chromatin acceptor sites. J Biol Chem. 1971 Jul 10;246(13):4188–4197. [PubMed] [Google Scholar]
- Steggles A. W., Spelsberg T. C., Glasser S. R., O'Malley B. W. Soluble complexes between steroid hormones and target-tissue receptors bind specifically to target-tissue chromatin. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1479–1482. doi: 10.1073/pnas.68.7.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft D. The interaction of uterine estrogen receptors with DNA. J Steroid Biochem. 1972 Apr;3(3):515–522. doi: 10.1016/0022-4731(72)90098-2. [DOI] [PubMed] [Google Scholar]
- Tymoczko J. L., Liao S. Retention of an androgen-protein complex by nuclear chromatin aggregates: heat-labile factors. Biochim Biophys Acta. 1971 Dec 21;252(3):607–611. doi: 10.1016/0304-4165(71)90168-1. [DOI] [PubMed] [Google Scholar]
- Wang T. Y., Nyberg L. M. Androgen receptors in the nonhistone protein fractions of prostatic chromatin. Int Rev Cytol. 1974;39:1–33. doi: 10.1016/s0074-7696(08)60937-7. [DOI] [PubMed] [Google Scholar]


