Abstract
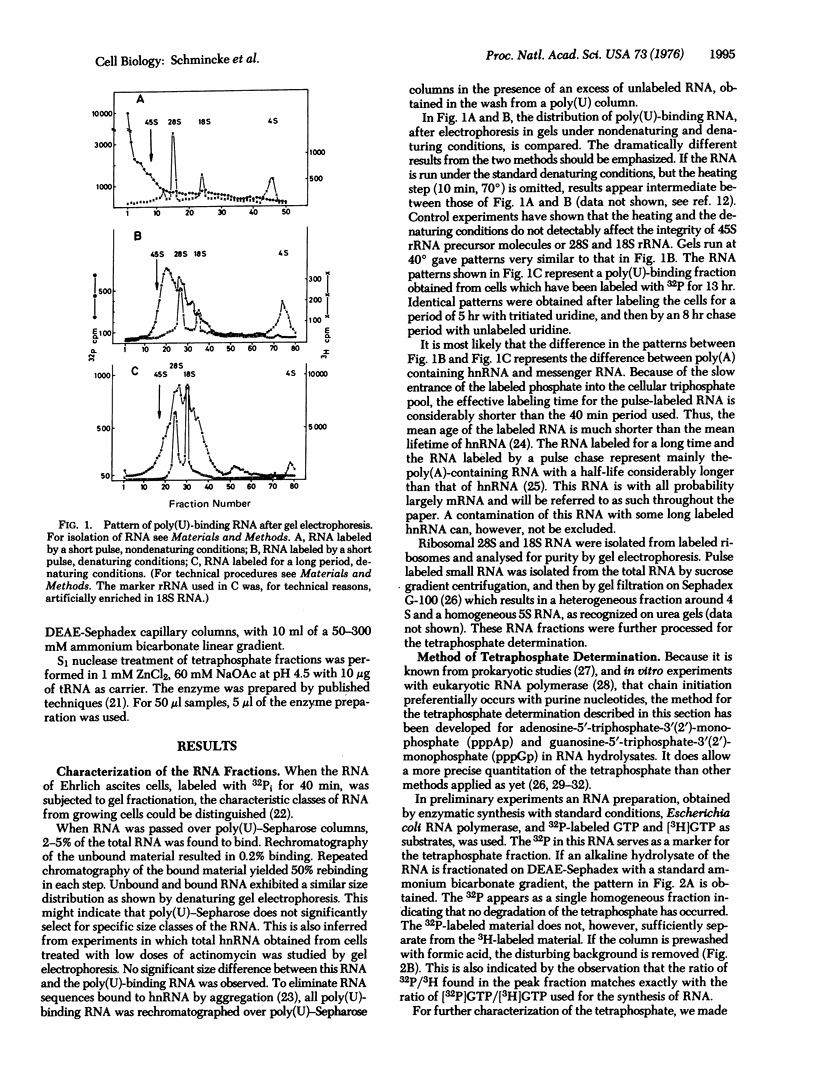
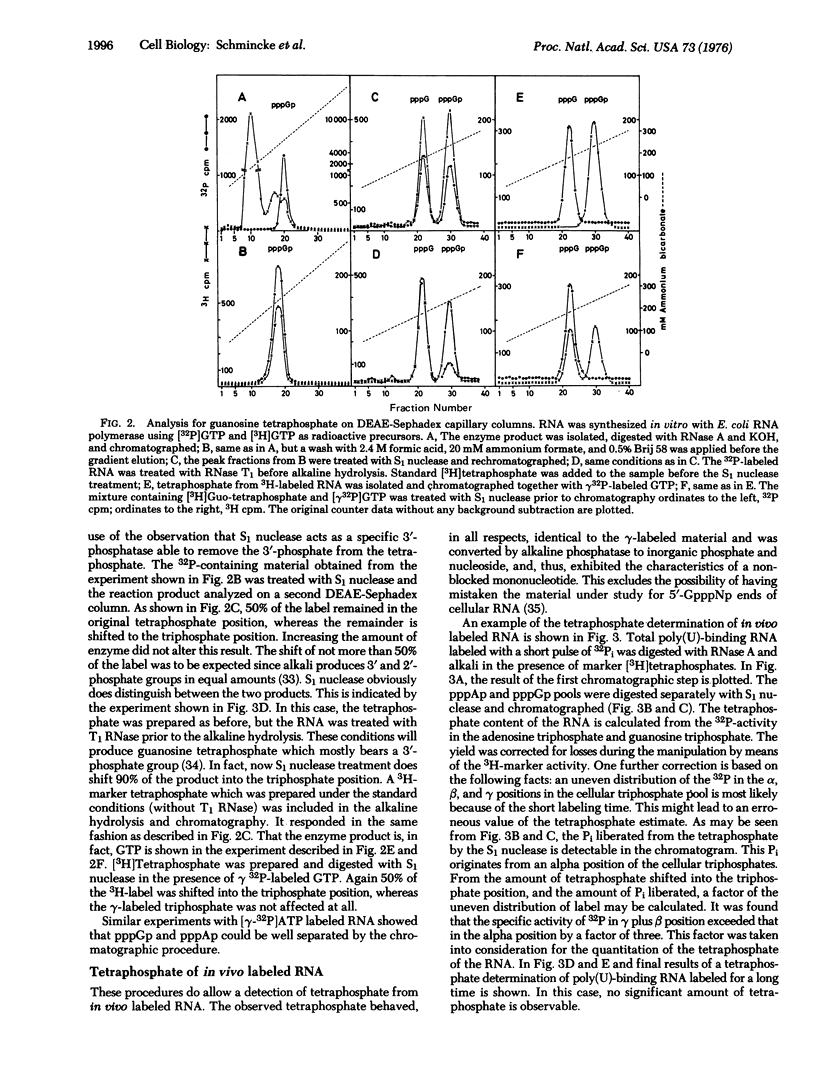
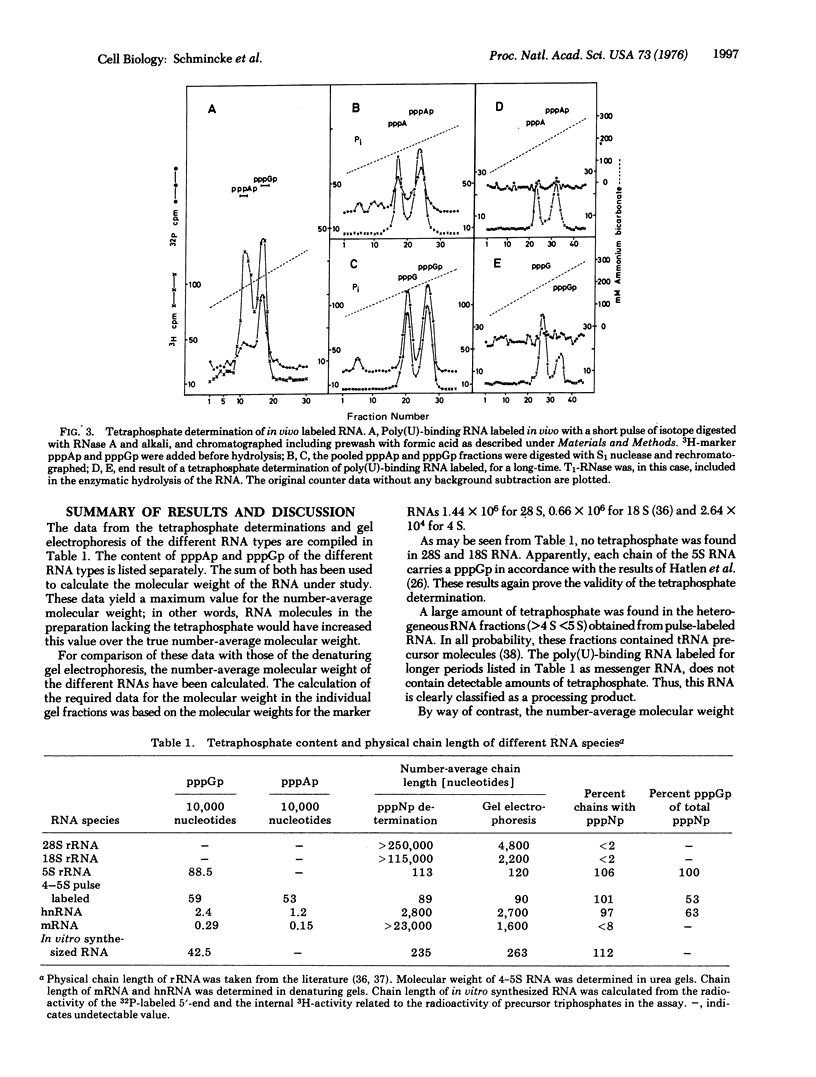
A method for the quantitation of 5"-tetraphosphate ends in 32P-labeled RNA has been developed. The tetraphosphate content of different RNA fractions obtained from Ehrlich ascites cells labeled with 32P for different lengths of time has been determined. Ribosomal RNA and poly(U)-binding RNA, labeled for long periods, (mRNA) lack 5'-terminal tetraphosphate. 5S RNA, pulse labeled 4-5S RNA, and poly(U)-binding hnRNA (heterogeneous nuclear RNA) do contain tetraphosphate. From the amount of the tetraphosphate, molecular weight data can be calculated for these RNA fractions which agree with independent determinations by denaturing gel electrophoresis. The results demonstrate that the majority of the poly(A) containing hnRNA molecules are small (less than 28S) and contain the tetraphosphate of the primary transcript. Therefore, they do not originate from the 3'-end of large molecules by processing events.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Buetti E., Scherrer K., Weil R. Transcription of the polyoma virus genome: synthesis and cleavage of giant late polyoma-specific RNA. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2231–2235. doi: 10.1073/pnas.68.9.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. M., Cory S. Modified nucleosides and bizarre 5'-termini in mouse myeloma mRNA. Nature. 1975 May 1;255(5503):28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- Bramwell M. E. The behaviour of heterogeneous nuclear ribonucleic acid in partially and completely denaturing conditions. Biochem J. 1974 Aug;141(2):477–484. doi: 10.1042/bj1410477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F., Barrell B. G. The sequence of 5 s ribosomal ribonucleic acid. J Mol Biol. 1968 Jun 28;34(3):379–412. doi: 10.1016/0022-2836(68)90168-x. [DOI] [PubMed] [Google Scholar]
- Daneholt B., Hosick H. The transcription unit in Balbiani ring 2 of Chironomus tentans. Cold Spring Harb Symp Quant Biol. 1974;38:629–635. doi: 10.1101/sqb.1974.038.01.067. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Ribonucleic acids from animal cells. Bacteriol Rev. 1968 Sep;32(3):262–290. doi: 10.1128/br.32.3.262-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Organization, transcription, and regulation in the animal genome. Q Rev Biol. 1973 Dec;48(4):565–613. doi: 10.1086/407817. [DOI] [PubMed] [Google Scholar]
- Derman E., Darnell J. E. Relationship of chain transcription to poly(A) addition and processing of hnRNA in HeLa cells. Cell. 1974 Nov;3(3):255–264. doi: 10.1016/0092-8674(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Haselkorn R. Messenger RNA. Annu Rev Biochem. 1969;38:647–676. doi: 10.1146/annurev.bi.38.070169.003243. [DOI] [PubMed] [Google Scholar]
- Georgieff M., Bachenheimer S., Darnell J. E. An examination of the nuclear RNA of adenovirus-transformed cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):475–482. doi: 10.1101/sqb.1974.039.01.059. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P., Ryskov A. P., Coutelle C., Mantieva V. L., Avakyan E. R. On the structure of transcriptional unit in mammalian cells. Biochim Biophys Acta. 1972 Jan 31;259(2):259–283. doi: 10.1016/0005-2787(72)90066-4. [DOI] [PubMed] [Google Scholar]
- Gissinger F., Kedinger C., Chambon P. Animal DNA-dependent RNA polymerases. 10. General enzymatic properties of purified calf thymus RNA polymerases AI and B. Biochimie. 1974;56(3):319–333. doi: 10.1016/s0300-9084(74)80139-2. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Hatlen L. E., Amaldi F., Attardi G. Oligonucleotide pattern after pancreatic ribonuclease digestion and the 3' and 5' termini of 5S ribonucleic acid from HeLa cells. Biochemistry. 1969 Dec;8(12):4989–5005. doi: 10.1021/bi00840a048. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry. 1973 Jun 5;12(12):2330–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- Houssais J. F., Attardi G. High molecular weight nonribosomal-type nuclear RNA and cytoplasmic messenger RNA in HeLa cells. Proc Natl Acad Sci U S A. 1966 Aug;56(2):616–623. doi: 10.1073/pnas.56.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T., Diggelmann H., Scherrer K. Demonstration of globin messenger sequences in giant nuclear precursors of messenger RNA of avian erythroblasts. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1122–1126. doi: 10.1073/pnas.70.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D. Potentiation of hemoglobin messenger ribonucleic acid. A step in protein synthesis initiation involving interaction of messenger with 18 S ribosomal ribonucleic acid. J Biol Chem. 1975 Aug 10;250(15):6085–6092. [PubMed] [Google Scholar]
- Keshgegian A. A., Garibian G. S., Furth J. J. Transcription of chromatin. Initial and terminal nucleotides of ribonucleic acid synthesized by calf thymus and Escherichia coli ribonucleic acid polymerases. Biochemistry. 1973 Oct 23;12(22):4337–4342. doi: 10.1021/bi00746a006. [DOI] [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: sequence components of heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Feb;4(2):77–93. doi: 10.1016/0092-8674(75)90113-0. [DOI] [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: the relationship between heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Jan;4(1):11–20. doi: 10.1016/0092-8674(75)90128-2. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T. Isolation of mRNA from KB-cells by affinity chromatography on polyuridylic acid covalently linked to Sepharose. Eur J Biochem. 1972 Dec 4;31(2):246–254. doi: 10.1111/j.1432-1033.1972.tb02527.x. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo V. S., De Kloet S. R. Disaggregation of "giant" heterogeneous nuclear RNA of mouse Ehrlich ascites cells by thermal denaturation in the presence of formaldehyde. Biochim Biophys Acta. 1971 Sep 30;247(1):74–79. doi: 10.1016/0005-2787(71)90809-4. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Schimke R. T. Ovalbumin messenger RNA: evidence that the initial product of transcription is the same size as polysomal ovalbumin messenger. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4327–4331. doi: 10.1073/pnas.71.11.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds L., Penman S. Pre-4S RNA made in isolated HeLa cell nuclei terminates with U. Cell. 1974 Oct;3(2):185–188. doi: 10.1016/0092-8674(74)90124-x. [DOI] [PubMed] [Google Scholar]
- Penman S., Vesco C., Penman M. Localization and kinetics of formation of nuclear heterodisperse RNA, cytoplasmic heterodisperse RNA and polyribosome-associated messenger RNA in HeLa cells. J Mol Biol. 1968 May 28;34(1):49–60. doi: 10.1016/0022-2836(68)90234-9. [DOI] [PubMed] [Google Scholar]
- Price R. P., Ransom L., Penman S. Identification of a small subfraction of hnRNA with the characteristics of a precursor to mRNA. Cell. 1974 Aug;2(4):253–258. doi: 10.1016/0092-8674(74)90019-1. [DOI] [PubMed] [Google Scholar]
- Reijnders L., Sloof P., Sival J., Borst P. Gel electrophoresis of RNA under denaturing conditions. Biochim Biophys Acta. 1973 Oct 26;324(3):320–333. doi: 10.1016/0005-2787(73)90278-5. [DOI] [PubMed] [Google Scholar]
- Roblin R. The 5'-terminus of bacteriophage R17 RNA: pppGp. J Mol Biol. 1968 Jan 14;31(1):51–61. doi: 10.1016/0022-2836(68)90053-3. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Loening U. E. 5'-Ends of ribosomal and ribosomal precursor RNAs form Xenopus laevis. Eur J Biochem. 1974 Mar 15;43(1):59–67. doi: 10.1111/j.1432-1033.1974.tb03384.x. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Vaughan M. H., Warner J. R., Darnell J. E., Jr The turnover of nuclear DNA-like RNA in HeLa cells. J Cell Biol. 1968 Oct;39(1):112–118. doi: 10.1083/jcb.39.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R., Birnboim H. C., Girard M., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. I. Identification by zone sedimentation of a heterogeneous fraction separate from ribosomal precursor RNA. J Mol Biol. 1966 Aug;19(2):349–361. doi: 10.1016/s0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]


