Abstract
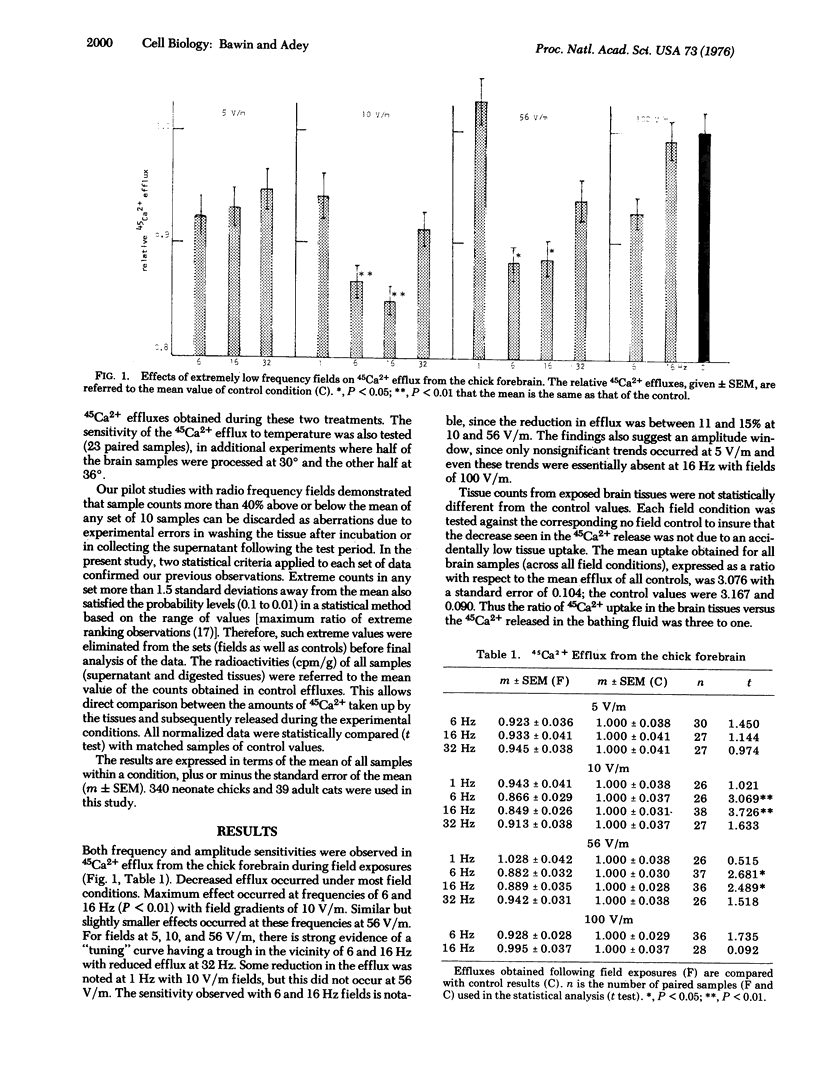
Weak sinusoidal electric fields modify the calcium efflux from freshly isolated chick and cat cerebral tissues bathed in Ringer's solution, at 36 degrees. Following incubation (30 min) with radioactive calcium (45Ca2+), each sample, immersed in fresh solution, was exposed for 20 min to fields at 1, 6, 16, 32, or 75 Hz, with electric gradients of 5, 10, 56, and 100 V/m in air. 45Ca2+ efflux in the solution was then measured in 0.2 ml aliquots and compared with efflux from unexposed control samples. Field exposures resulted in a general trend toward a reduction in the release of the preincubated 45Ca2+. Both frequency and amplitude sensitivities were observed. Maximum decreases occurred at 6 and 16 Hz (12-15%). Thresholds were around 10 and 56 V/m for chick and cat tissues, respectively. Similar but nonsignificant trends occurred during other field exposures. All results were statistically compared with matched samples of controls. Tissue gradients could not be measured, but estimates were of the order of 0.1 muV/cm. The susceptibility of the electrochemical equilibrium in the neuronal membrane to small extracellular perturbations is discussed and a possible role for weak intrinsic cerebral fields in neuronal excitability is suggested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adey W. R. Evidence for cerebral membrane effects of calcium, derived from direct-current gradient, impedance, and intracellular records. Exp Neurol. 1971 Jan;30(1):78–102. doi: 10.1016/0014-4886(71)90224-x. [DOI] [PubMed] [Google Scholar]
- Bawin S. M., Kaczmarek L. K., Adey W. R. Effects of modulated VHF fields on the central nervous system. Ann N Y Acad Sci. 1975 Feb 28;247:74–81. doi: 10.1111/j.1749-6632.1975.tb35984.x. [DOI] [PubMed] [Google Scholar]
- Bennett M. V. Electrolocation in fish. Ann N Y Acad Sci. 1971 Dec 3;188:242–269. doi: 10.1111/j.1749-6632.1971.tb13102.x. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Thiéry J., Tung Y., Kittel C. On the cooperativity of biological membranes. Proc Natl Acad Sci U S A. 1967 Feb;57(2):335–341. doi: 10.1073/pnas.57.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Watanabe S., Lux H. D. Relations between EEG phenomena and potentials of single cortical cells. II. Spontaneous and convulsoid activity. Electroencephalogr Clin Neurophysiol. 1966 Jan;20(1):19–37. doi: 10.1016/0013-4694(66)90137-4. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L. Quantum sensitivity of rods in the toad retina. Science. 1975 Mar 7;187(4179):838–841. doi: 10.1126/science.1114328. [DOI] [PubMed] [Google Scholar]
- Gavalas R. J., Walter D. O., Hamer J., Adey W. R. Effect of low-level, low-frequency electric fields on EEG and behavior in Macaca nemestrina. Brain Res. 1970 Mar 17;18(3):491–501. doi: 10.1016/0006-8993(70)90132-0. [DOI] [PubMed] [Google Scholar]
- Grodsky I. T. Possible physical substrates for the interaction of electromagnetic fields with biologic membranes. Ann N Y Acad Sci. 1975 Feb 28;247:117–124. doi: 10.1111/j.1749-6632.1975.tb35988.x. [DOI] [PubMed] [Google Scholar]
- Hagins W. A. Electrical signs of information flow in photoreceptors. Cold Spring Harb Symp Quant Biol. 1965;30:403–418. doi: 10.1101/sqb.1965.030.01.040. [DOI] [PubMed] [Google Scholar]
- Henn F. A., Hamberger A. Glial cell function: uptake of transmitter substances. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2686–2690. doi: 10.1073/pnas.68.11.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hydén H. A calcium-dependent mechanism for synapse and nerve cell membrane modulation. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2965–2968. doi: 10.1073/pnas.71.8.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L. K., Adey W. R. The efflux of 45CA2+ and (3H)gamma-aminobutyric acid from cate cerebral cortex. Brain Res. 1973 Dec 7;63:331–342. doi: 10.1016/0006-8993(73)90100-5. [DOI] [PubMed] [Google Scholar]
- Keeton W. T. Magnets interfere with pigeon homing. Proc Natl Acad Sci U S A. 1971 Jan;68(1):102–106. doi: 10.1073/pnas.68.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramwell P. W., Shaw J. E. Biological significance of the prostaglandins. Recent Prog Horm Res. 1970;26:139–187. doi: 10.1016/b978-0-12-571126-5.50008-x. [DOI] [PubMed] [Google Scholar]
- Rojas E., Taylor R. E., Atwater I., Bezanilla F. Analysis of the effects of calcium or magnesium on voltage-clamp currents in perfused squid axons bathed in solutions of high potassium. J Gen Physiol. 1969 Oct;54(4):532–552. doi: 10.1085/jgp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson H. W., Dill R. E., Matthews J. L., Martin J. H. An ultrastructural investigation of calcium-dependent granules in the rat neuropil. Brain Res. 1970 Aug 27;22(2):157–162. doi: 10.1016/0006-8993(70)90001-6. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Cooperative binding to linear biopolymers. 1. Fundamental static and dynamic properties. Eur J Biochem. 1970 Feb;12(3):442–453. doi: 10.1111/j.1432-1033.1970.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Simpson L. L. The role of calcium in neurohumoral and neurohormonal extrusion processes. J Pharm Pharmacol. 1968 Dec;20(12):889–910. doi: 10.1111/j.2042-7158.1968.tb09672.x. [DOI] [PubMed] [Google Scholar]
- Wever R. Einfluss schwacher elektro-magnetischer Felder auf die circadiane Periodik des Menschen. Naturwissenschaften. 1968 Jan;55(1):29–32. doi: 10.1007/BF00593403. [DOI] [PubMed] [Google Scholar]
- Wever R. Human circadian rhythms under the influence of weak electric fields and the different aspects of these studies. Int J Biometeorol. 1973 Sep;17(3):227–232. doi: 10.1007/BF01804614. [DOI] [PubMed] [Google Scholar]