Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, and usually fatal form of interstitial lung disease (ILD). The precise molecular mechanisms of IPF remain poorly understood. However, analyses of mice receiving bleomycin (BLM) as a model of IPF established the importance of preceding inflammation for the formation of fibrosis. Periostin is a recently characterized matricellular protein involved in modulating cell functions. We recently found that periostin is highly expressed in the lung tissue of patients with IPF, suggesting that it may play a role in the process of pulmonary fibrosis. To explore this possibility, we administered BLM to periostin-deficient mice, and they subsequently showed a reduction of pulmonary fibrosis. We next determined whether this result was caused by a decrease in the preceding recruitment of neutrophils and macrophages in the lungs because of the lower production of chemokines and proinflammatory cytokines. We performed an in vitro analysis of chemokine production in lung fibroblasts, which indicated that periostin-deficient fibroblasts produced few or no chemokines in response to TNF-α compared with control samples, at least partly explaining the lack of inflammatory response and, therefore, fibrosis after BLM administration to periostin-deficient mice. In addition, we confirmed that periostin is highly expressed in the lung tissue of chemotherapeutic-agent–induced ILD as well as of patients with IPF. Taking these results together, we conclude that periostin plays a unique role as an inducer of chemokines to recruit neutrophils and macrophages important in the process of pulmonary fibrosis in BLM-administered model mice. Our results suggest a therapeutic potential for periostin in IPF and drug-induced ILD.
Keywords: bleomycin, chemokine, extracellular matrix protein, idiopathic pulmonary fibrosis, matricellular protein
Clinical Relevance
We demonstrate that periostin, a recently characterized matricellular protein involved in modulating cell functions, plays a unique role as an inducer of chemokines to recruit neutrophils and macrophages important for the process of pulmonary fibrosis in bleomycin-administered model mice. This suggests a therapeutic potential for periostin in idiopathic pulmonary fibrosis and drug-induced interstitial lung disease.
Idiopathic pulmonary fibrosis (IPF), histologically characterized as usual interstitial pneumonia (UIP), is a chronic, progressive, and generally fatal form of interstitial lung disease (ILD) (1, 2). It is triggered by epithelial injury followed by abnormal tissue repair, an abnormal accumulation of fibroblasts and myofibroblasts with an excessive deposition of extracellular matrix (ECM) proteins, and the distortion of lung architecture, ultimately resulting in the replacement of normal functional tissue, which leads to respiratory failure. Although diagnostic approaches have been improved and numerous studies to understand the pathogenesis of IPF have been performed, its precise molecular mechanisms remain poorly understood.
Bleomycin (BLM)-administered mice are widely used as a model of IPF (3). The administration of BLM causes epithelial injury, followed by neutrophil-dominant and lymphocyte-dominant inflammation that leads to fibrosis. In the inflammatory phase, the expression of various chemokines, cytokines (Th1, Th2, Th17, and proinflammatory types), and growth factors is elevated (4–8), and these mediators exert their profibrotic activities through the recruitment, activation, and proliferation of fibroblasts, macrophages, neutrophils, and myofibroblasts. Thus, the importance of preceding inflammation for the formation of fibrosis has been well established in this murine model, although its role remains controversial in patients with IPF (1, 2).
The maintenance of tissue structure is the main function of ECM proteins. However, some ECM proteins, such as osteopontin, secreted protein acidic and rich in cysteine, and thrombospondin, termed “matricellular proteins,” influence cell function by modulating cell–matrix interactions and do not play a direct structural role (9–11). Periostin is a recently characterized matricellular protein belonging to the fasciclin family. Periostin interacts with several integrin molecules (i.e., αvβ1/β3/β5) on cell surfaces, providing signals for tissue development and remodeling. Analyses of periostin-deficient mice showed that periostin is important in the development of bone, tooth, and heart valves by potentiating mesenchymal maturation (12–15). Periostin is involved in the healing processes after myocardial infarction by inducing the proliferation of cardiomyocytes, or after wounds by activating keratinocytes and fibroblasts (16–18; Ontsuka, unpublished data). Furthermore, periostin promotes cancer-cell survival, the epithelial–mesenchymal transition (EMT), invasion, and metastasis via the integrin/phosphatidylinositol 3–kinase/Akt pathway, leading to the development of various tumors (10).
We recently found that periostin is highly expressed in the lung tissues of patients with IPF/UIP in areas of ongoing fibroproliferation (19). The serum periostin concentration is correspondingly up-regulated in these patients, and is inversely correlated with 6-month changes from the detection times in vital capacity and diffusing capacity of the lung for carbon monoxide. These results suggest that the serum concentration of periostin is a relevant biomarker to diagnose IPF and to predict changes of lung function in patients with IPF. Furthermore, these results raise the possibility that periostin contributes to the process of pulmonary fibrosis.
To explore this possibility, we used periostin-deficient mice in a BLM-administered murine model of pulmonary fibrosis. A deficiency of periostin impaired BLM-induced inflammation, resulting in less pulmonary fibrosis. The impaired induction of chemokines and the recruitment of neutrophils and macrophages by TNF-α in periostin-deficient fibroblasts is one mechanism at least partly underlying the reduced inflammation observed in periostin-deficient mice. In addition, the robust expression of periostin in the lung tissue of patients with IPF and with chemotherapeutic-agent–induced ILD strongly suggests the involvement of periostin in the pathogenesis of these clinically relevant lung diseases. These results demonstrate that periostin is important in the process of pulmonary fibrosis by inducing chemokines that recruit neutrophils and macrophages. Furthermore, these insights suggest a therapeutic potential for periostin in IPF and drug-induced ILD.
Materials and Methods
Mice
Seven- to 8-week-old BALB/c or C57BL/6 mice (Japan SLC, Hamamatsu, Japan) and periostin-deficient (Postn−/−) mice or their heterozygous littermates (Postn+/−) were used. Postn−/− mice were generated as previously described (12). The BLM challenge was performed as previously described, with modifications (20, 21). BALB/c mice were instilled intratracheally with 10 mg/kg of BLM (Nihon Kayaku, Tokyo, Japan) dissolved in saline on Day 0. C57BL/6 mice were instilled intraperitoneally with 0.1 g/kg of BLM dissolved in saline three times on Days 0, 7, and 14. Murine lungs were subjected to bronchoalveolar lavage (BAL) or harvested for RT-PCR or ELISA on Day 7, and harvested for histological examination or measurement of hydroxyproline on Day 28. Experiments were undertaken according to the guidelines for the care and use of experimental animals by the Japanese Association for Laboratory Animals Science.
Histology
Lung tissue was subjected to conventional histological staining or to immunostaining using rat anti-periostin monoclonal antibodies (mAbs; clone number SS5D) and rabbit anti-human periostin polyclonal antibody (Ab) (19, 22). The Ashcroft score was estimated (23). Quantification of the area of immunostaining for periostin was performed using photographic analysis in Image J software (http://rsb.info.nih.gov/ij; National Institutes of Health, Bethesda, MD).
Confocal Microscopy
Lung sections were incubated with rabbit anti-periostin polyclonal Ab and anti-actin (smooth muscle; α-SMA) Ab (DAKO, Glostrup, Denmark), followed by incubation with anti-rabbit IgG-Alexa 488 (Invitrogen, Carlsbad, CA), anti-mouse IgG-Alexa 546, and TO-PRO-3 iodide.
Measurement of Hydroxyproline
Hydroxyproline was measured using standard hydroxyproline (Sigma-Aldrich, St. Louis, MO) (21).
Analysis of BAL
BAL fluid (BALF) was collected by lavaging lungs with 500 μl of BAL liquid (0.1% BSA/50 μM EDTA/PBS) three times. BALF cell counts were determined with a hemocytometer. BAL cytospins were prepared, slides were stained with Diff-Quik (Sysmex, Kobe, Japan), and the numbers of eosinophils, neutrophils, and mononuclear cells were counted.
Real-Time PCR
Primer sequences and PCR conditions are available upon request. Real-Time PCR for the screening of chemokine production was performed using an RT2 Profiler PCR Array system (SABiosciences, Frederick, MD).
ELISA
ELISA for IL-1β and TNF-α was performed using Mouse IL-1β ELISA Ready-SET-Go! and Mouse TNF-α ELISA Ready-SET-Go!, respectively (eBioscience, San Diego, CA).
Murine Embryonic Fibroblasts
Postn+/+ and Postn−/− murine embryonic fibroblasts (MEFs) were immortalized by the SV40 large T-antigen. MEFs were seeded and stimulated with TNF-α (PeproTech, Rocky Hill, NJ), or left unstimulated for 3 days. Cells were then disrupted, and total RNA was purified with RNAisoPLUS (Takara, Otsu, Japan).
Specimens of Human Lung Tissue
Five patients with BLM-induced ILD, 5 patients with gefitinib-induced ILD, and 25 patients with UIP were diagnosed at Kurume University Hospital and Dokkyo Medical University, Koshigaya Hospital (Table 1) (19). The details of these patients are described in the online supplement. All protocols were approved by the Ethics Committees of Kurume University or of the Dokkyo Medical University School of Medicine.
TABLE 1.
CHARACTERISTICS OF PATIENTS WITH CHEMOTHERAPEUTIC AGENT–INDUCED ILD
| Histology |
|||||||||
| Patient Number | Age (Years) | Gender | Sample | Carcinoma | ILD | Thoracic Irradiation | Total Dose | Length of Administration | Smoking Status |
| Bleomycin-induced | |||||||||
| 1 | 56 | Male | Autopsy | Squamous (lung) | DAD (organizing/fibrotic) | Unknown | Unknown | Unknown | Unknown |
| 2 | 66 | Male | Autopsy | Squamous (mandible) | DAD (organizing/fibrotic) | 12,000 rad | 190 mg | 28 days | Unknown |
| 3 | 67 | Female | Autopsy | Squamous (genitalia) | DAD (organizing/fibrotic + squamous metaplasia) | 4,000 rad | 300 mg | 70 days | Unknown |
| 4 | 56 | Male | Autopsy | Squamous (hypopharynx) | DAD (organizing/fibrotic + squamous metaplasia) | 3,600 rad | 195 mg | 91 days | Unknown |
| 5 | 69 | Male | Autopsy | Squamous (skin) | DAD (exudative/organizing + acute pulmonary edema) | 6,000 rad | 300 mg | 140 days | Unknown |
| Gefitinib-induced | |||||||||
| 1 | 69 | Female | Biopsy | Adeno | DAD (fibrotic + microscopic honeycombing) | 3.75 g | 15 days | Nonsmoker | |
| 2 | 66 | Female | Biopsy | Adeno | Chronic interstitial fibrosis | 7 g | 28 days | Nonsmoker | |
| 3 | 62 | Female | Biopsy | Adeno | DAD (fibrotic + microscopic honeycombing + squamous metaplasia) | 13 g | 52 days | Nonsmoker | |
| 4 | 62 | Male | Biopsy | Adeno | DAD (fibrotic + microscopic honeycombing) | 9.25 g | 37 days | Nonsmoker | |
| 5 | 73 | Male | Autopsy | Adeno | DAD (fibrotic + microscopic honeycombing + squamous metaplasia) | 1.75 g | 7 days | Smoker | |
Definition of abbreviations: DAD, diffuse alveolar damage; ILD, interstitial lung disease.
Statistical Analyses
The results are presented as means ± SD. Analyses were performed using the two-sided, unpaired Student t test.
Results
Induction of Periostin Expression in BLM-Administered Model Mice
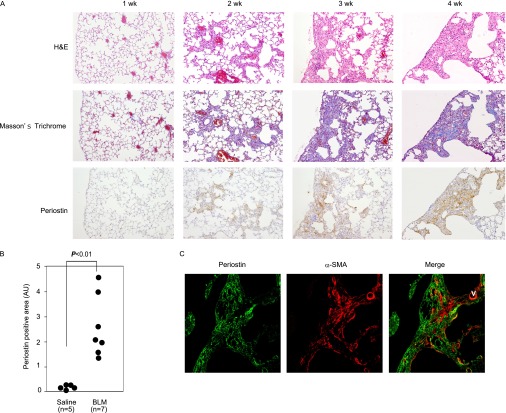
To gain insights into the effects of periostin on pulmonary fibrosis, we used BLM-administered model mice. As shown in Figure 1A, inflammation first occurred in peribronchial regions, followed by fibrosis (∼ 2 weeks), and then the main inflammatory and fibrotic areas moved to subpleural regions (∼ 3 weeks). Fibrosis in subpleural regions was expanded, and had disrupted the normal alveolar structure by 4 weeks. The expression of periostin coincided with the appearance of fibrosis, was scarce at 1 week, and increased thereafter. Periostin was expressed in the peribronchial regions at early time points and in subpleural regions later, as inflammation and fibrosis occurred (Figure 1B). Periostin was frequently observed adjacent to α-SMA–positive myofibroblasts (Figure 1C). These results demonstrate that periostin is a novel component deposited in the lung tissue of BLM-administered mice, and is expressed in areas where ongoing fibroproliferation occurs.
Figure 1.
Expression of periostin in lung tissue of bleomycin (BLM)–challenged mice. (A) Kinetic analysis of periostin expression in lung tissue of BLM-administered BALB/c mice. Staining with hematoxylin-and-eosin (H&E) and Masson trichrome, and immunostaining with anti-periostin antibody (Ab), are shown. (B) Quantification of periostin-positive areas at 4 weeks. (C) Localization of periostin and α–smooth muscle actin (α-SMA) in lungs of pulmonary fibrosis model mice. Lung sections of BALB/c mice at 4 weeks in A were stained with anti-periostin Ab and anti–α-SMA Ab. An immunofluorescence image (green, periostin; red, α-SMA) is shown. AU, arbitrary units; V, vessel; wk, weeks.
Periostin Is Important for the Progression of Pulmonary Fibrosis in BLM-Challenged Mice
To address whether the deposition of periostin in lung tissue contributes to the progression of pulmonary fibrosis or is merely a secondary consequence of pulmonary fibrosis, we assessed fibrosis in BLM-administered, periostin-deficient mice. BLM-challenged wild-type mice began to die 1 week after BLM administration, with greater than 80% mortality at 4 weeks (BALB/c background; Figure 2A). In contrast, almost 90% of BLM-challenged Postn−/− mice survived 4 weeks after the administration of BLM, whereas the survival rate of Postn+/− mice was intermediate between that of wild-type mice and of Postn−/− mice. Histochemical analysis of the lung tissue of surviving Postn−/− mice showed reduced fibrosis and the preservation of normal alveolar structure, compared with Postn+/− mice (Figure 2B). Ashcroft scores were correspondingly lower in Postn−/− mice than in Postn+/− mice (Figure 2C). The main fibrotic areas in Postn−/− mice were located in subpleural regions, as was also the case with Postn+/− and wild-type mice. The amounts of hydroxyproline in the lungs, which reflect collagen content, also profoundly decreased in Postn−/− mice (Figure 2D). Even with model mice showing milder pulmonary fibrosis in the C57BL/6 strain, Postn−/− mice exhibited less fibrosis than wild-type mice (Figures 2B and 2C). These results demonstrate that periostin influences the progression of pulmonary fibrosis in BLM-administered mice.
Figure 2.
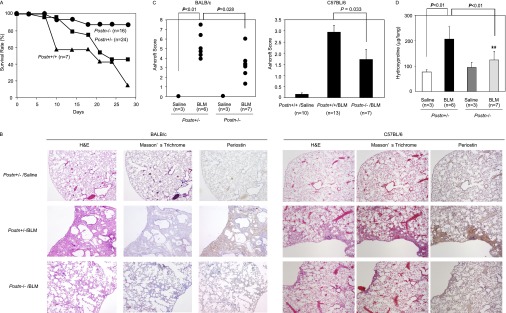
Periostin is important for the progression of pulmonary fibrosis in BLM-challenged mice. BLM was administered intratracheally into wild-type periostin-deficient (Postn−/−) or heterozygous (Postn+/−) mice (BALB/c background) on Day 0, or BLM was administered intraperitoneally into wild-type or Postn−/− mice (C57BL/6 background) three times on Days 0, 7, and 14. Lung tissue was prepared on Day 28. The survival rates (BALB/c; A), lung histology (BALB/c and C57BL/6; B), Ashcroft scores of pulmonary fibrosis (BALB/c and C57BL/6; C), and amounts of hydroxyproline in a lung (BALB/c; D) are shown. Lung histology was analyzed by hematoxylin-and-eosin and Masson trichrome staining, and immunostaining involved anti-periostin Ab.
Periostin Is Important for the Induction of Neutrophil-Dominant and Macrophage-Dominant Inflammation in BLM-Challenged Mice
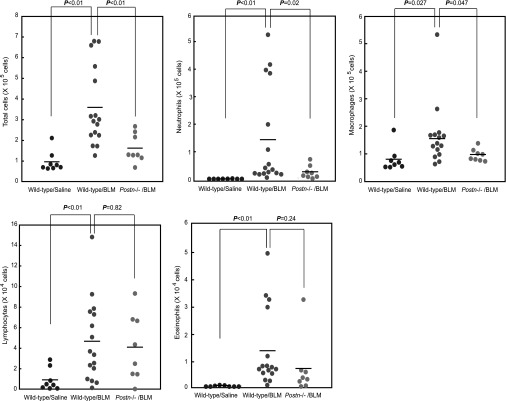
BLM-challenged mice (BALB/c background) began to die 1 week after BLM administration, before the extensive generation of fibrosis and loss of normal alveolar structure were evident, whereas periostin-deficient mice had improved survival rates early in the course of BLM administration (Figure 2A). Because preceding inflammation is important for the formation of BLM-induced pulmonary fibrosis (4–8), we hypothesized that periostin deficiency led to less pulmonary fibrosis by attenuating BLM-induced acute inflammatory responses. To explore this possibility, we quantified leukocytes in BALF and related them to evolving epithelial injury in mice 7 days after BLM administration. After the administration of BLM, total cell counts in BALF were increased in wild-type mice (P < 0.01). However, the infiltration of inflammatory cells in BALF was significantly decreased in periostin-deficient mice (P < 0.01; Figure 3). Neutrophils and macrophages constituted most of the infiltrated cells, and their numbers decreased in periostin-deficient mice (neutrophils, P = 0.02; macrophages, P = 0.047). In contrast, the numbers of lymphocytes were up-regulated by the administration of BLM in both wild-type and periostin-deficient mice. The numbers of eosinophils tended to decrease in periostin-deficient mice compared with wild-type mice, although the down-regulation was not statistically significant. Similar numbers of TUNEL-positive cells were observed in the lung tissue of BLM-challenged wild-type and periostin-deficient mice, suggesting that epithelial injury by the administration of BLM was not influenced by periostin (data not shown). These results show that periostin is critical for the induction of neutrophil-dominant and macrophage-dominant inflammation in BLM-challenged mice, and that impaired inflammation could lead to less pulmonary fibrosis in BLM-administered, periostin-deficient mice.
Figure 3.
Periostin is important for the induction of inflammation in BLM-challenged mice. BLM or saline was administered intratracheally into wild-type or periostin-deficient mice (BALB/c background) on Day 0. Bronchoalveolar lavage fluid (BALF) and the lung tissue were prepared on Day 7. The numbers of total cells, neutrophils, macrophages, lymphocytes, and eosinophils in BALF from saline-administered (n = 8) or BLM-administered wild-type mice (n = 16) or BLM-administered periostin-deficient mice (n = 8) are shown.
Periostin Is Important for the Expression of Chemokines to Recruit Neutrophils and Macrophages in BLM-Challenged Mice
We then addressed whether the impairment of neutrophil-dominant and macrophage-dominant inflammation in BLM-administered, periostin-deficient mice may be attributable to the down-regulation of chemokine production. For that purpose, we first comprehensively screened the expression of chemokines and inflammatory cytokines in lung tissue of BLM-challenged wild-type and periostin-deficient mice, using an RT2 Profiler PCR Array system (SABiosciences). The chemokines or cytokines whose expression folds in BLM-challenged, wild-type mice compared with BLM-challenged, periostin-deficient mice were greater than two included the CC family (Ccl1, Ccl2, Ccl4, Ccl7, Ccl9, and Ccl12), the CXC family (Cxcl1, Cxcl2, Cxcl4, Cxcl10, Cxcl11, and Cxcl13), and the cytokine family (TNF-α Table E1 in the online supplement).
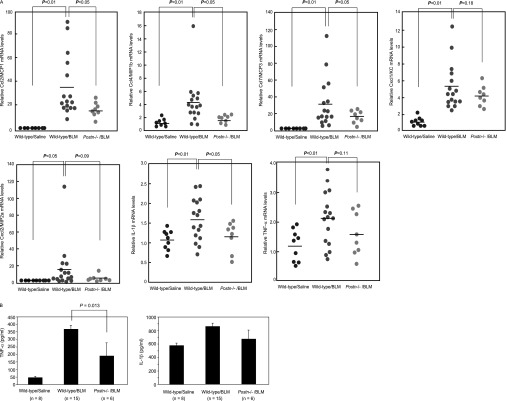
We next focused on several chemokines and cytokines (Ccl2/MCP1, Ccl4/MIP-1B, Ccl7/MCP3, Cxcl1/KC, Cxcl2/MIP-2a, TNF-α, and IL-1β), and analyzed the expression of these factors using real-time PCR. These chemokines and cytokines showed high fold expression. The expression of Ccl2/MCP1, Ccl4/MIP-1B, Ccl7/MCP3, Cxcl1/KC, and Cxcl2/MIP-2a, or their human orthologues, is known to be elevated in lung tissue of BLM-challenged mice or patients with IPF, and this expression plays a significant role in the recruitment of macrophages or neutrophils (4–6, 24, 25). Although the array system did not include IL-1β, IL-1β is known to be tightly correlated with the pathogenesis of pulmonary fibrosis (26). The expressions of these chemokines and cytokines were all up-regulated by the administration of BLM in wild-type mice. However, in periostin-deficient mice, these expressions were markedly down-regulated (Ccl2, P < 0.05; Ccl4, P < 0.01; Ccl7, P < 0.05; and IL-1β, P < 0.05), or showed a tendency to decrease (Cxcl1, Cxcl2, and TNF-α) (Figure 4A). Furthermore, protein concentrations of TNF-α were down-regulated, with a statistical significance in periostin-deficient mice, whereas protein concentrations of IL-1β did not significantly change, probably because the basal protein concentration of IL-1β is relatively high, and the increased amount of IL-1β protein induced by bleomycin is not as high (Figure 4B). In contrast, the expression of other chemokines, such as Ccl5/RANTES, Ccl11/eotaxin, and Ccl17/TARC, was not down-regulated or elevated by the administration of BLM in periostin-deficient mice compared with wild-type mice (data not shown). These results clearly demonstrate that periostin plays an important role in the induction of various chemokines and cytokines to recruit neutrophils and macrophages in BLM-administered mice.
Figure 4.
Periostin is important for the induction of chemokines and proinflammatory cytokines in BLM-challenged mice. RNA (A) or proteins (B) were extracted from lung tissue 7 days after BLM treatment, and prepared as shown in Figure 3. RNA was applied to real-time PCR for Ccl2/MCP1, Ccl4/MIP-1B, Ccl7/MCP3, Cxcl1/KC, Cxcl2/MIP-2a, TNF-α, and IL-1β. The relative mRNA concentrations of each chemokine or cytokine in saline-administered (n = 8) or BLM-administered (n = 16) wild-type mice or BLM-administered, periostin-deficient mice (n = 8) are shown. Protein concentrations of TNF-α and IL-1β from saline-administered (n = 8) or BLM-administered (n = 15) wild-type mice or BLM-administered periostin-deficient mice (n = 6) are shown.
Impairment of Chemokine Production in Periostin-Deficient Fibroblasts
Fibroblasts are known to be one of the main sources of chemokines in pulmonary fibrosis (27). To elucidate the underlying mechanism of how the induction of various chemokines that recruit neutrophils and macrophages is down-regulated in periostin-deficient mice, we investigated chemokine expression by periostin-deficient fibroblasts in response to TNF-α, a potent inducer of chemokine production (28). Upon stimulation with TNF-α, the expression of all these chemokines was significantly up-regulated in wild-type fibroblasts, whereas the expression of Ccl2 (P < 0.01), Ccl7 (P < 0.01), Cxcl1 (P < 0.01), and Cxcl2 (P < 0.01) was markedly down-regulated in periostin-deficient fibroblasts (Figure 5). Notably, TNF-α failed to stimulate the production of Ccl4 and IL-1β by periostin-deficient fibroblasts. These results suggest that an impaired production of chemokines by fibroblasts in response to TNF-α could at least partly explain the underlying mechanism of reduced lung injury and fibrosis observed in BLM-administered, periostin-deficient mice.
Figure 5.
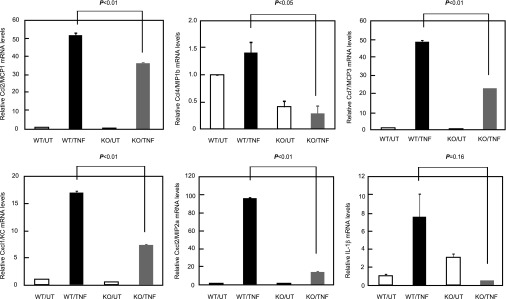
Periostin is important for the induction of chemokines and proinflammatory cytokines in fibroblasts. Wild-type (WT) or periostin-deficient (KO) fibroblasts were stimulated without (UT) or with (TNF) 20 ng/ml of TNF-α for 72 hours. RNA extracted from fibroblasts was applied to real-time PCR for Ccl2/MCP1, Ccl4/MIP-1B, Ccl7/MCP3, Cxcl1/KC, Cxcl2/MIP-2a, and IL-1β.
Expression of Periostin in Lung Tissue of Patients with BLM-Induced or Gefitinib-Induced ILD
Most chemotherapeutic agents, such as BLM and gefitinib, can cause ILD, characterized by acute lung injury with the typical histopathological features of diffuse alveolar damage (DAD) (29). To examine whether the expression of periostin is a histological feature not only of patients with UIP but also of patients with chemotherapeutic-agent–induced ILD, we subjected lung tissue from five patients with BLM-induced ILD and five patients with gefitinib-induced ILD to immunohistochemical analysis (Table 1). Representative histological features and expressions of periostin are shown in Figure 6A.
Figure 6.
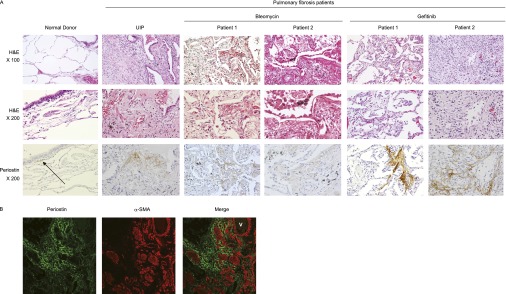
Expression of periostin in lung tissue of patients with BLM-induced or gefitinib-induced ILD. (A) Expression of periostin in control subjects, patients with UIP, BLM-administered patients, and gefitinib-administered patients. Hematoxylin-and-eosin (H&E) staining and immunostaining with anti-periostin Ab in lung tissue obtained from a representative control subject (64-year-old male), a patient with UIP (64-year-old male), BLM-administered patients (56-year-old male and 67-year-old female), and gefitinib-administered patients (66-year-old female and 73-year-old male) are shown. Arrow indicates expressed periostin. (B) Localization of periostin and α-SMA in the lungs of a gefitinib-administered patient (69-year-old female). Lung sections were stained with anti-periostin Ab and anti–α-SMA Ab. An immunofluorescence image (green, periostin; red, α-SMA) is shown. V, vessel.
Periostin was rarely observed in pulmonary epithelial cells, alveolar macrophages, and most bronchiolar basement membranes of normal lung tissue, but was very weakly deposited in some areas around bronchiolar basement membranes. Conversely, the lung tissue of a patient with UIP showed a strong expression of periostin in the interstitia, especially in the fibrotic foci, as previously described (19). The lung tissue of patients with both BLM-induced and gefitinib-induced ILD showed characteristics of the reparatory phase of DAD, with hyaline membranes, regenerating Type II pneumocytes, and interstitial fibrosis. However, fibrosing alveolitis and progressive interstitial fibrosis with microscopic honeycombing were more widely seen in patients with gefitinib-induced ILD, compared with BLM-administered cases. The staining of periostin in BLM-administered patients was evident in thickened alveolar walls and interstitia underneath regenerating epithelial cells. Periostin was frequently expressed adjacent to α-SMA–positive cells in the lung tissue of gefitinib-administered patients as well as in BLM-administered mice (Figure 6B), suggesting that periostin is expressed in ongoing fibrotic areas. These results demonstrate that periostin is involved in the pathogenesis not only of patients with IPF, but also of patients with chemotherapeutic-agent–induced ILD.
Discussion
In this study, using BLM-administered model mice, we demonstrated that periostin, a newly emerged matricellular protein, plays an important role in the process of fibrosis by inducing chemokines to recruit neutrophils and macrophages. Numerous studies using BLM-administered mice were performed to identify key mediators involved in the process of pulmonary fibrosis (3). However, thus far, the involvement of ECM proteins in the process has been poorly understood, except in a few studies involving fibronectin and syndecan-4 (30, 31).
The pathologic roles of periostin in the formation of fibrosis were first suggested based on the characteristics of periostin as a usual ECM protein. Periostin enhances fibrosis by binding to other ECM proteins (collagen I, fibronectin, and tenascin-C) and by inducing collagen fibrillogenesis via activating lysyl oxidase, a catalytic enzyme in the intramolecular and intermolecular cross-linking of collagen (22, 32, 33). This notion is supported by our finding that the colocalization of periostin with tenascin-C was frequently observed in the lung tissues of BLM-administered mice (data not shown). However, periostin is now recognized as important in the development of bone, tooth, and heart valves (12–15), the healing process after myocardial infarction (16, 17), and the development of various tumors (10). In this study, we revealed a novel role of periostin in the process of pulmonary fibrosis as an inducer of chemokines to recruit neutrophils and macrophages.
The important roles of neutrophils and macrophages in the process of pulmonary fibrosis have been well characterized in murine models, although the importance of preceding inflammation for the formation of fibrosis remains controversial in patients with IPF (1, 2). The enforced influx of neutrophils increases the lethality of BLM-treated mice (34), whereas the blockage of neutrophil recruitment protects BLM-induced pulmonary fibrosis (5). Furthermore, neutrophil elastase was reported to be important for TGF-β release from lung tissue (35), and the blockage of macrophage recruitment causes less fibrosis in BLM-induced mice (4, 36, 37). Moreover, the production of matrix metalloproteinase–2, metalloproteinase-9, and connective tissue growth factor from macrophages is assumed to contribute to the generation of pulmonary fibrosis. Based on these reports, we reasoned that the impairment of neutrophil and macrophage recruitment via periostin deficiency would lead to less pulmonary fibrosis. Interestingly, the periostin-dependent infiltration of neutrophils and macrophages was observed at 1 week after the administration of BLM, when the accumulation of periostin was not obvious (Figure 1A), indicating that the basal concentration of periostin is enough for acute responses. Because recurrent tissue injuries are believed to occur during the pathogenesis of IPF (1, 7), accumulated periostin may enhance or sustain the inflammation of IPF.
As yet, no comprehensive view of the target cells of periostin exists. Because fibroblasts are known to be one of the main sources of chemokines in pulmonary fibrosis (27), we examined their capability to produce chemokines in response to TNF-α in the presence or absence of periostin, and we found that fibroblasts are one of the target cells of periostin in the production of chemokines (Figure 5). Furthermore, based on a previous report that periostin modulates the EMT of cancer cells (38) and the present finding that periostin localizes adjacent to α-SMA–positive myofibroblasts in BLM-induced fibrosis (Figure 1C), we suggest that periostin contributes to fibrosis by enhancing the EMT to myofibroblasts. However, we cannot exclude the possibility that periostin acts on immune cells and other parenchymal/stromal cells, thereby contributing to pulmonary fibrosis. Periostin was reported to act on eosinophils, enhancing their recruitment to lesions, or to act on airway epithelial cells, inducing TGF-β activation in the pathogenesis of bronchial asthma (39, 40). Periostin could activate various immune cells and parenchymal/stromal cells, leading to amplified inflammation in the process of pulmonary fibrosis.
In this study, we demonstrated that periostin synergistically induces the production of several chemokines and cytokines (including Ccl2, Ccl4, Ccl7, Cxcl1, Cxcl2, and IL-1β in response to TNF-α and IL-1β Figure 5, and data not shown). In particular, no induction of Ccl4 and IL-1β by TNF-α was observed in the absence of periostin. IL-1β signaling is required, and is sufficient on its own, for BLM-induced pulmonary inflammation and fibrosis (26). TNF-α and IL-1β induce chemokine production through the NF-κB pathway, cooperating with other transcription factors (28). The present findings suggest that the cooperation of TNF-α and periostin is required for the maximum production of chemokines. Periostin was shown to activate extracellular signal–regulated kinases (ERKs) 1 and 2 and phosphatidylinositol 3–kinase (PI3-K) (10). The activation of ERKs and PI3-K is important for TNF-α–activated NF-κB and activator protein–1 (41–43). Periostin may enhance the signal pathway of TNF-α via ERKs and PI3-K. Furthermore, periostin was recently reported to be physically associated with the Notch1 precursor inside cells (44). Further studies are awaited that would clarify how periostin signals cross-talk with TNF-α signals, and whether periostin acts as a secreted protein or an intracellular protein.
BLM-administered mice are used to model drug-induced ILD and IPF (3). This is because DAD (i.e., the typical histopathological features of drug-induced ILD), consisting of the acute/exudative phase (interstitial edema and hyaline membrane formation) and the organizing phase (interstitial fibrosis, Type 2 pneumonia hyperplasia, and squamous metaplasia), is similar to the features of BLM-administered mice (29). We showed that periostin was strongly expressed in the lung tissue of patients receiving BLM or gefitinib in areas of ongoing fibroproliferation, as well as in patients with UIP (Figure 6). Taken together, the importance of periostin in the process of pulmonary fibrosis can be applied to both IPF and drug-induced ILD.
IPF is usually resistant to treatment, including immunosuppressive agents, and carries a poor prognosis, with 5-year mortality rates of approximately 60–80% (7). Although many clinical trials of novel therapies for IPF have been performed, the results have mostly been disappointing. Furthermore, gefitinib-induced ILD is a life-threatening disease, and it was reported that 31 of 70 patients with gefitinib-induced ILD died (45). Our present findings suggest that the interaction between periostin and integrin molecules can be a therapeutic target for IPF and chemotherapy-induced ILD. In fact, the treatment of neutralizing Ab against αv integrin was shown to protect model mice from pulmonary fibrosis (46). Antagonists against αvβ3 integrin have been tested in clinical use for tumors, rheumatoid arthritis, and osteoporosis (47–49). The application of these antagonists can also be expected to prove beneficial for patients with IPF and chemotherapy-induced ILD. Furthermore, targeting periostin, and not αVβ3 integrin, may lead to more specific treatments, although we need to be cautious regarding the adverse effects of inhibiting the physiological roles of periostin, including tissue development, remodeling, and wound healing (12–15, 18).
In conclusion, we show that periostin, a matricellular protein, influences the progression of pulmonary fibrosis by inducing chemokines and proinflammatory cytokines in BLM-administered mice. This suggests a therapeutic potential for targeting periostin in pulmonary fibrosis.
Acknowledgments
The authors thank Dr. Dovie R. Wylie and Mr. Hiroyuki Ideguchi for their critical review of the manuscript and their technical assistance.
Footnotes
This work was supported in part by Grants-in-Aid for Scientific Research (K.I.) and grant KL2 RR025760 (S.K.A.) from the Japanese Society for the Promotion of Science, by A. Shekhar, PI and the Riley Children's Foundation, by the Department of Pediatrics (Neonatal-Perinatal Medicine) at Indiana University, and by the National Institutes of Health (S.J.C.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0115OC on January 12, 2012
References
- 1.Strieter RM. Pathogenesis and natural history of usual interstitial pneumonia: the whole story or the last chapter of a long novel? Chest 2005;128:526S–532S. [DOI] [PubMed] [Google Scholar]
- 2.Gharaee-Kermani M, Gyetko MR, Hu B, Phan SH. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: a potential role for stem cells in the lung parenchyma and implications for therapy. Pharm Res 2007;24:819–841. [DOI] [PubMed] [Google Scholar]
- 3.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2008;294:L152–L160. [DOI] [PubMed] [Google Scholar]
- 4.Baran CP, Opalek JM, McMaken S, Newland CA, O'Brien JM, Hunter MG, Bringardner BD, Monick MM, Brigstock DR, Stromberg PC, et al. Important roles for macrophage colony–stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo RC, Guabiraba R, Garcia CC, Barcelos LS, Roffe E, Souza AL, Amaral FA, Cisalpino D, Cassali GD, Doni A, et al. Role of the chemokine receptor CXCR2 in bleomycin-induced pulmonary inflammation and fibrosis. Am J Respir Cell Mol Biol 2009;40:410–421. [DOI] [PubMed] [Google Scholar]
- 6.Keane MP, Belperio JA, Moore TA, Moore BB, Arenberg DA, Smith RE, Burdick MD, Kunkel SL, Strieter RM. Neutralization of the CXC chemokine, macrophage inflammatory protein–2, attenuates bleomycin-induced pulmonary fibrosis. J Immunol 1999;162:5511–5518. [PubMed] [Google Scholar]
- 7.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2009;2:103–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. Bleomycin and IL-1β–mediated pulmonary fibrosis is IL-17A dependent. J Exp Med 2010;207:535–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norris RA, Moreno-Rodriguez R, Hoffman S, Markwald RR. The many facets of the matricelluar protein periostin during cardiac development, remodeling, and pathophysiology. J Cell Commun Signal 2009;3:275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci 2009;66:2219–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal 2008;2:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, et al. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease–like phenotype. Mol Cell Biol 2005;25:11131–11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, et al. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res 2008;102:752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, Conway SJ, et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res 2007;101:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorts A, Schwanekamp JA, Elrod JW, Sargent MA, Molkentin JD. Genetic manipulation of periostin expression in the heart does not affect myocyte content, cell cycle activity, or cardiac repair. Circ Res 2009;104:e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 2007;13:962–969. [DOI] [PubMed] [Google Scholar]
- 17.Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, et al. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med 2008;205:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, Fukayama M, Kudo A. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS ONE 2011;6:e18410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamoto M, Hoshino T, Kitasato Y, Sakazaki Y, Kawayama T, Fujimoto K, Ohshima K, Shiraishi H, Uchida M, Ohta S, et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J 2011;37:1119–1127. [DOI] [PubMed] [Google Scholar]
- 20.Moore BB, Ballinger MN, White ES, Green ME, Herrygers AB, Wilke CA, Toews GB, Peters-Golden M. Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. J Immunol 2005;174:5644–5649. [DOI] [PubMed] [Google Scholar]
- 21.Hoshino T, Okamoto M, Sakazaki Y, Kato S, Young HA, Aizawa H. Role of proinflammatory cytokines IL-18 and IL-1β in bleomycin-induced lung injury in humans and mice. Am J Respir Cell Mol Biol 2009;41:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 2006;118:98–104. [DOI] [PubMed] [Google Scholar]
- 23.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 1988;41:467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capelli A, Di Stefano A, Gnemmi I, Donner CF. CCR5 expression and CC chemokine levels in idiopathic pulmonary fibrosis. Eur Respir J 2005;25:701–707. [DOI] [PubMed] [Google Scholar]
- 25.Choi ES, Jakubzick C, Carpenter KJ, Kunkel SL, Evanoff H, Martinez FJ, Flaherty KR, Toews GB, Colby TV, Kazerooni EA, et al. Enhanced monocyte chemoattractant protein–3/CC chemokine ligand–7 in usual interstitial pneumonia. Am J Respir Crit Care Med 2004;170:508–515. [DOI] [PubMed] [Google Scholar]
- 26.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VF, Lagente V, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest 2007;117:3786–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agostini C, Gurrieri C. Chemokine/cytokine cocktail in idiopathic pulmonary fibrosis. Proc Am Thorac Soc 2006;3:357–363. [DOI] [PubMed] [Google Scholar]
- 28.Richmond A. NF-κB, chemokine gene transcription and tumour growth. Nat Rev Immunol 2002;2:664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beasley MB. The pathologist's approach to acute lung injury. Arch Pathol Lab Med 2010;134:719–727. [DOI] [PubMed] [Google Scholar]
- 30.Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, et al. An essential role for fibronectin extra Type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med 2008;177:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, Liu N, Jung Y, Homer R, Meltzer EB, et al. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest 2010;120:2049–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, Kudo A. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem 2010;285:2028–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maruhashi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem 2010;285:13294–13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 2002;111:635–646. [DOI] [PubMed] [Google Scholar]
- 35.Chua F, Dunsmore SE, Clingen PH, Mutsaers SE, Shapiro SD, Segal AW, Roes J, Laurent GJ. Mice lacking neutrophil elastase are resistant to bleomycin-induced pulmonary fibrosis. Am J Pathol 2007;170:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M, Takeya M. C–C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol 2004;204:594–604. [DOI] [PubMed] [Google Scholar]
- 37.Tanino Y, Makita H, Miyamoto K, Betsuyaku T, Ohtsuka Y, Nishihira J, Nishimura M. Role of macrophage migration inhibitory factor in bleomycin-induced lung injury and fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 2002;283:L156–L162. [DOI] [PubMed] [Google Scholar]
- 38.Kanno A, Satoh K, Masamune A, Hirota M, Kimura K, Umino J, Hamada S, Satoh A, Egawa S, Motoi F, et al. Periostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells. Int J Cancer 2008;122:2707–2718. [DOI] [PubMed] [Google Scholar]
- 39.Blanchard C, Mingler MK, McBride M, Putnam PE, Collins MH, Chang G, Stringer K, Abonia JP, Molkentin JD, Rothenberg ME. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol 2008;1:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV. Roles of epithelial cell–derived periostin in TGF-β activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA 2010;107:14170–14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karin M, Gallagher E. TNFR signaling: ubiquitin-conjugated TRAFfic signals control stop-and-go for MAPK signaling complexes. Immunol Rev 2009;228:225–240. [DOI] [PubMed] [Google Scholar]
- 42.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-κB activation by tumour necrosis factor requires the Akt serine–threonine kinase. Nature 1999;401:82–85. [DOI] [PubMed] [Google Scholar]
- 43.Reddy SA, Huang JH, Liao WS. Phosphatidylinositol 3–kinase as a mediator of TNF-induced NF-κB activation. J Immunol 2000;164:1355–1363. [DOI] [PubMed] [Google Scholar]
- 44.Tanabe H, Takayama I, Nishiyama T, Shimazaki M, Kii I, Li M, Amizuka N, Katsube K, Kudo A. Periostin associates with Notch1 precursor to maintain Notch1 expression under a stress condition in mouse cells. PLoS ONE 2010;5:e12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, Ariyoshi Y, Fukuoka M. Predictive factors for interstitial lung disease, antitumor response, and survival in non–small-cell lung cancer patients treated with gefitinib. J Clin Oncol 2006;24:2549–2556. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi F, Takahashi K, Okazaki T, Maeda K, Ienaga H, Maeda M, Kon S, Uede T, Fukuchi Y. Role of osteopontin in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 2001;24:264–271. [DOI] [PubMed] [Google Scholar]
- 47.Cacciari B, Spalluto G. Non peptidic alphaVbeta3 antagonists: recent developments. Curr Med Chem 2005;12:51–70. [DOI] [PubMed] [Google Scholar]
- 48.Lainer-Carr D, Brahn E. Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis. Nat Clin Pract Rheumatol 2007;3:434–442. [DOI] [PubMed] [Google Scholar]
- 49.Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br J Cancer 2004;90:561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.