Abstract
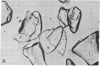
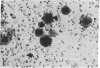
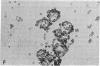
Isoproterenol, corticotropin (ACTH), and triodothyronine immobilized on glass and Sepharose beads by diazotization procedures have been shown to interact with cultured tumor cells of "target tissue" origin. Cells used were rat glioma cells (C6), rat adrenal tumor cells (Y-1), and rat pituitary tumor cells (GH3). The rat glioma cells bound principally to immobilized isoproterenol, whereas the rat adrenal tumor cells bound to immobilized corticotropin, and rat pituitary tumor cells bound to immobilized triiodothyronine. Binding was inhibited by preincubation of the cells in soluble drug or hormone. With C6 cells there was a positive correlation between adenylate cyclase [ATP pyrophosphate-lyase (cyclizing, EC 4.6.1.1] stimulation and the degree of binding to the immobilized isoproterenol. Norepinephrine, bound through the ethanolamine side chain via an amide linkage, did not bind cells, demonstrating specific structural requirements for drug-cell interactions. HeLa cells were shown to bind tightly to diphtheria toxin coupled to Sepharose beads via an amide bond. This binding was inhibited by prior incubation of the Sepharose toxin with purified antitoxin. Toxin bound to Sepharose via an azo bond did not bind cells. These data suggest that the cell affinities are due to cell surface receptors interacting with the immobilized drugs and hormones, and that the observed affinities possibly reflect the relative receptor complement of these cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Creagan R. P., Chen S., Ruddle F. H. Genetic analysis of the cell surface: association of human chromosome 5 with sensitivity to diphtheria toxin in mouse-human somatic cell hybrids. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2237–2241. doi: 10.1073/pnas.72.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of insulin with the cell membrane: the primary action of insulin. Proc Natl Acad Sci U S A. 1969 Jun;63(2):450–457. doi: 10.1073/pnas.63.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Edelman G. M., Rutishauser U., Millette C. F. Cell fractionation and arrangement on fibers, beads, and surfaces. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2153–2157. doi: 10.1073/pnas.68.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine R. A., Yoggeswaran G., Hakorori S. Glycosphingolipids covalently linked to agarose gel or glass beads. Use of the compounds for purification of antibodies directed against globoside and hematoside. J Biol Chem. 1974 Jul 25;249(14):4460–4466. [PubMed] [Google Scholar]
- Melmon K. L., Weinstein Y., Shearer G. M., Bourne H. R., Bauminger S. Separation of specific antibody-forming mouse cells by their adherence to insolubilized endogenous hormones. J Clin Invest. 1974 Jan;53(1):22–30. doi: 10.1172/JCI107542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsnes S., Refsnes K., Pihl A. Mechanism of action of the toxic lectins abrin and ricin. Nature. 1974 Jun 14;249(458):627–631. doi: 10.1038/249627a0. [DOI] [PubMed] [Google Scholar]
- Schimmer B. P., Ueda K., Sato G. H. Site of action of adrenocorticotropic hormone (ACTH) in adrenal cell cultures. Biochem Biophys Res Commun. 1968 Sep 6;32(5):806–810. doi: 10.1016/0006-291x(68)90312-4. [DOI] [PubMed] [Google Scholar]
- Soderman D. D., Germershauden J., Katzen H. M. Affinity binding of intact fat cells and their ghosts to immobilized insulin. Proc Natl Acad Sci U S A. 1973 Mar;70(3):792–796. doi: 10.1073/pnas.70.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter J. C., Dixon J. E., Maroko P. R., Kaplan N. O. Biologically active catecholamines covalentyly bound to glass beads. Proc Natl Acad Sci U S A. 1972 May;69(5):1141–1145. doi: 10.1073/pnas.69.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter J. C., Ross J., Jr, Dixon J. E., Mayer S. E., Kaplan N. O. Immobilized catecholamine and cocaine effects on contractility of cardiac muscle. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1214–1217. doi: 10.1073/pnas.70.4.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlander M. S., Venter J. C., Goodman M., Kaplan N. O., Saks B. Biological activity of catecholamines covalently linked to synthetic polymers: proof of immobilized drug theory. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1009–1013. doi: 10.1073/pnas.73.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderhaar B., Mueller G. C. Binding of estrogen receptor to estradiol immobilized on insoluble resins. Biochim Biophys Acta. 1969 Apr 29;176(3):626–631. doi: 10.1016/0005-2760(69)90229-x. [DOI] [PubMed] [Google Scholar]