Imetelstat, a novel telomerase inhibitor, failed to improve significantly median PFS and OS as maintenance therapy (±bevacizumab) in advanced NSCLC. Telomere length (TL) biomarker results were consistent with the hypothesis that telomerase inhibition is of greater benefit to patients with tumors possessing shorter telomeres; the patients with shorter TL had a trend toward longer median PFS and OS.
Keywords: non-small-cell lung cancer, biomarker, imetelstat
Abstract
Background
Continuation or ‘switch’ maintenance therapy is commonly used in patients with advancd non-small-cell lung cancer (NSCLC). Here, we evaluated the efficacy of the telomerase inhibitor, imetelstat, as switch maintenance therapy in patients with advanced NSCLC.
Patients and methods
The primary end point of this open-label, randomized phase II study was progression-free survival (PFS). Patients with non-progressive, advanced NSCLC after platinum-based doublet (first-line) chemotherapy (with or without bevacizumab), any histology, with Eastern Cooperative Oncology Group performance status 0–1 were eligible. Randomization was 2 : 1 in favor of imetelstat, administered at 9.4 mg/kg on days 1 and 8 of a 21-day cycle, or observation. Telomere length (TL) biomarker exploratory analysis was carried out in tumor tissue by quantitative PCR (qPCR) and telomerase fluorescence in situ hybridization.
Results
Of 116 patients enrolled, 114 were evaluable. Grade 3/4 neutropenia and thrombocytopenia were more frequent with imetelstat. Median PFS was 2.8 and 2.6 months for imetelstat-treated versus control [hazard ratio (HR) = 0.844; 95% CI 0.54–1.31; P = 0.446]. Median survival time favored imetelstat (14.3 versus 11.5 months), although not significantly (HR = 0.68; 95% CI 0.41–1.12; P = 0.129). Exploratory analysis demonstrated a trend toward longer median PFS (HR = 0.43; 95% CI 0.14–1.3; P = 0.124) and overall survival (OS; HR = 0.41; 95% CI 0.11–1.46; P = 0.155) in imetelstat-treated patients with short TL, but no improvement in median PFS and OS in patients with long TL (HR = 0.86; 95% CI 0.39–1.88; and HR = 0.51; 95% CI 0.2–1.28; P = 0.145).
Conclusions
Maintenance imetelstat failed to improve PFS in advanced NSCLC patients responding to first-line therapy. There was a trend toward a improvement in median PFS and OS in patients with short TL. Short TL as a predictive biomarker will require further investigation for the clinical development of imetelstat.
introduction
Telomeres consist of tandem repeats of the TTAGGG DNA sequence, which function to cap the ends of all mammalian chromosomes. Capping prevents chromosomal fusion and also prevents their ends from being misinterpreted as DNA double-strand breaks [1]. When telomeres get critically short, they can no longer cap the telomere ends, triggering a DNA damage signal and resulting in senescence and/or apoptosis [2, 3].
Telomere loss is prevented by the enzyme telomerase, which counters this loss by adding TTAGGG repeats to the chromosome ends [4]. Telomerase inhibition leads to the loss of a cancer cell's ability to maintain telomere length (TL), similarly resulting in cell cycle arrest or apoptosis/senescence.
Imetelstat is a covalently lipidated 13-mer thiophosphoramidate oligonucleotide that acts as a potent specific inhibitor of telomerase. Imetelstat's mechanism of action is not an antisense-based approach. Rather, it binds with high affinity and acts as a competitive inhibitor of human telomerase enzymatic activity [5, 6].
Preclinical studies with imetelstat have shown its ability to inhibit telomerase in tumor cells and to compromise cancer cell viability in vitro. Imetelstat has broad tumor growth inhibition activity in multiple xenograft models, including metastatic non-small-cell lung cancer (NSCLC) and breast cancer [7, 8].
Platinum-based doublet, first-line chemotherapy [9, 10], with or without bevacizumab [11, 12], is the current treatment standard for patients with advanced NSCLC. Additional randomized data with pemetrexed and erlotinib have demonstrated that ‘maintenance therapy’ in NSCLC, either as continuation [13] or switch [14, 15], is effective.
In this study, we evaluated the efficacy of switch maintenance therapy with imetelstat in advanced NSCLC patients and explored the potential use of TL as a surrogate (predictive biomarker) of imetelstat activity.
patients and methods
eligibility criteria
Eligible patients had pathologically confirmed stage IV (per AJCC) or recurrent locally advanced NSCLC (not eligible for curative intent therapy); had experienced no progression after completing first-line platinum-doublet chemotherapy (four cycles); had Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1; were age ≥18 years; had measurable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 [16]; and had adequate hematologic (ANC ≥1500/mm3, hemoglobin ≥9 g/dl, platelet count ≥75 000 μl), renal (<1.5 mg/dl or creatinine clearance >45 ml/min), and hepatic [aspartate aminotransferase (AST) and alanine aminotransferase (ALT) <2.5× the ULN, or <5× the upper limit of normal (ULN) if documented liver metastases, serum bilirubin <2.0 mg/dl and/or alkaline phosphatase <2.5× ULN or ≤5× ULN if liver or bone metastasis documented] function.
Exclusion criteria included patients with progressive disease, symptomatic or untreated central nervous system disease, and clinically significant infections or cardiovascular disease. Owing to bevacizumab use, history of pulmonary hemorrhage, therapeutic anticoagulation, and major surgery within 4 weeks before imetelstat were also exclusionary.
All patients provided written informed consent, and study approval was obtained from the institutional review boards at each of the participating centers. This study was registered with ClinicalTrials.gov (NCT01137968).
study design and treatment plan
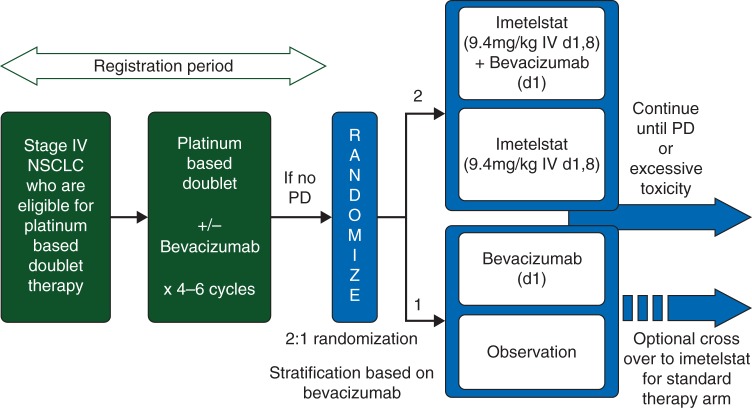
This was an open-label, multicenter, randomized phase II study of imetelstat switch maintenance therapy in patients with advanced NSCLC, non-progressive after four cycles of first-line, platinum-doublet chemotherapy, with or without bevacizumab. Patients receiving bevacizumab continued on the drug after randomization, and patients in the control arm had the option of crossover to imetelstat at progression (Figure 1).
Figure 1.
Study design.
Eligible patients were randomized 2:1 to imetelstat, administered intravenously at 9.4 mg/kg on days 1 and 8 every 21 days in addition to standard of care (bevacizumab or observation) or to standard of care alone. Randomization occurred between 21 and 42 days after the last dose of chemotherapy and was carried out by permuted block design and stratified according to receipt of bevacizumab during first-line chemotherapy.
Therapy continued until disease progression or unacceptable toxicities, and patients receiving both drugs (bevacizumab and imetelstat) could continue on one if the other was stopped. Crossover to imetelstat was allowed upon progressive disease.
clinical assessments
Safety evaluations consisted of a history and physical examination, including vital signs and PS, as well as laboratory measurements. Toxicity assessments were carried out using the National Cancer Institute Common Toxicity Criteria (NCI CTCAE) v4.0 [17].
For the study's primary end point (progression-free survival, PFS), tumor status was assessed every 6 weeks for 36 weeks and every 9 weeks thereafter for the remainder of the study. Responses were assessed with RECIST v1.1, and confirmation of a partial or complete response was required. As there was no blinded placebo control group, the lack of a central (independent) radiographic response assessment represented a potential limitation to our primary outcome's measures.
assessment of biomarkers
Methods for the assessment of biomarkers are described in supplementary Methods, available at Annals of Oncology online.
dose modifications
Criteria for patient dose modifications are described in supplementary Methods, available at Annals of Oncology online.
statistical methods
The primary study end point was PFS. Secondary end points included objective response rate (ORR), overall survival (OS), and safety profile. A prespecified exploratory analysis of PFS by tumor TL was also carried out.
Assuming median (m)PFS of 4.5 and 2.6 months for chemotherapy with and without bevacizumab, respectively, and an exponential survival with a hazard ratio (HR) of 0.5 for the imetelstat effect, the estimated mPFS for imetelstat without and with bevacizumab was 5.2 and 9 months, respectively. With a 2:1 randomization ratio in favor of the imetelstat arm, a total of 96 patients were planned and the estimated number of PFS events required was 67.
Kaplan–Meier estimates, log-rank tests, and Cox proportional hazards models were used for PFS and OS (time-to-event) analyses. ORR results, including exact 95% two-sided CIs, were calculated using standard methods; χ2 and Wilcoxon rank-sum tests were used to test for differences in baseline characteristics.
Efficacy analyses by TL were prespecified for patients grouped into the shortest 1/2, shortest 1/3, and shortest 1/4 of TL.
results
patient characteristics
Originally, the protocol specified enrollment of 96 patients, with the primary analysis to be conducted after 67 PFS events. However, a retrospective review of all CT scan reports identified seven ineligible patients (disease progression by RECIST v1.1 at randomization). For this reason, enrollment was increased to 116 and the primary analysis of PFS was conducted after 77 events. Exclusion of these seven patients from the analyses did not change the results substantially. Similarly, results using only the first 67 events were similar to those reported for the 77 events. A final and mature analysis of survival data was also conducted with 66 OS events.
Thus, between July 2010 and April 2012, 116 patients were randomized and 114 completed a post-screening visit, including administration of protocol-specified therapy (i.e. efficacy population). Fifty-two patients received imetelstat alone, 24 received imatelstat plus bevacizumab, 12 received bevacizumab only, and 26 patients received neither. Baseline patient characteristics were well balanced between arms (Table 1). Squamous histology was present in 18.4%; 31.6% received concomitant bevacizumab.
Table 1.
Patient characteristics
| Imetelstat |
Observation |
Total | |||||
|---|---|---|---|---|---|---|---|
| Bevacizumab | No-bevacizumab | Sub-total | Bevacizumab | No-bevacizumab | Sub-total | ||
| No. (%) | 24 (21.0) | 52 (45.6) | 76 (66.7) | 12 (10.5) | 26 (22.8) | 38 (33.3) | 114 (100.0) |
| Age (years) | |||||||
| Median | 56.9 | 65.3 | 63.1 | 65.1 | 63.5 | ||
| Range | 42–76 | 44–81 | 53–80 | 30–84 | 30–84 | ||
| Gender | |||||||
| Male (%) | 11 (9.6) | 27 (23.6) | 38 (33.3) | 8 (7.0) | 19 (16.70 | 27 (23.6) | 65 (57.0) |
| Female (%) | 13 (11.4) | 25 (21.9) | 38 (33.3) | 4 (3.5) | 7 (6.1) | 11 (9.6) | 49 (43.0) |
| Race | |||||||
| White (%) | 17 (14.9) | 44 (38.6) | 61 (53.5) | 11 (9.6) | 20 (17.5) | 31 (27.2) | 92 (80.7) |
| Black (%) | 2 (1.7) | 3 (2.6) | 5 (4.4) | 0 (0.0) | 4 (3.5) | 4 (3.5) | 9 (7.9) |
| Asian (%) | 5 (4.4) | 5 (4.4) | 10 (8.8) | 1 (0.9) | 2 (1.7) | 3 (2.6) | 13 (11.4) |
| Ethnicity | |||||||
| Hispanic (%) | 0 (0.0) | 2 (1.7) | 2 (1.7) | 1 (0.9) | 1 (0.9) | 2 (1.7) | 4 (3.5) |
| Non-hispanic (%) | 23 (20.1) | 38 (33.3) | 61 (53.5) | 11 (9.6) | 22 (19.3) | 33 (28.9) | 94 (82.5) |
| Other/na (%) | 1 (0.9) | 12 (10.5) | 13 (11.4) | 0 (0.0) | 3 (2.6) | 3 (2.6) | 16 (14.0) |
| ECOG PS | |||||||
| 0 (%) | 8 (7.0) | 19 (16.7) | 27 (23.6) | 4 (3.5) | 10 (8.8) | 14 (12.3) | 41 (36.0) |
| 1 (%) | 16 (14.0) | 32 (28.1) | 48 (42.1) | 7 (6.1) | 16 (14.0) | 23 (20.2) | 71 (62.3) |
| Not available (%) | 0 (0.0) | 1 (0.9) | 1 (0.9) | 1 (0.9) | 0 (0.0) | 1 (0.9) | 2 (1.7) |
| Disease stage | |||||||
| IIIB (%) | 2 (1.7) | 2 (1.7) | 4 (3.5) | 0 (0.0) | 1 (0.9) | 1 (0.9) | 5 (4.4) |
| IV (%) | 21 (18.4) | 48 (42.1) | 69 (60.5) | 12 (10.5) | 25 (21.9) | 37 (32.5) | 106 (93.0) |
| Recurrent Dis (%) | 1 (0.9) | 2 (1.7) | 3 (2.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (2.6) |
| Histology | |||||||
| Adenocarcinoma (%) | 24 (21.0) | 37 (32.5) | 61 (53.5) | 10 (8.8) | 13 (11.4) | 23 (20.2) | 84 (73.7) |
| Squamous (%) | 0 (0.0) | 10 (8.8) | 10 (8.8) | 0 (0.0) | 11 (9.6) | 11 (9.6) | 21 (18.4) |
| Adenosquamous (%) | 0 (0.0) | 1 (0.9) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.9) |
| Large cell (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.9) | 1 (0.9) | 2 (1.7) | 2 (1.7) |
| NOS/other (%) | 0 (0.0) | 4 (3.5) | 4 (3.5) | 1 (0.9) | 1 (0.9) | 2 (1.7) | 6 (5.3) |
| Response to induction | |||||||
| CR/PR (%) | 0/10 (8.8) | 0/16 (14.00 | 0/26 (22.8) | 0/2 (1.7) | 0/6 (5.3) | 0/8 (7.0) | 0/34 (29.8) |
| SD (%) | 14 (12.3) | 36 (31.6) | 50 (43.9) | 10 (8.8) | 20 (17.5) | 30 (26.3) | 80 (70.2) |
| Induction cycles | |||||||
| Median | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Range | 1–6 | 2–6 | 1–6 | 4–6 | 2–6 | 2–6 | 1–6 |
| Prior therapy | |||||||
| Radiation (%) | 8 (7.0) | 11 (9.6) | 19 (16.7) | 3 (2.6) | 10 (8.8) | 13 (11.4) | 32 (28.1) |
| Surgery (any) (%) | 14 (12.3) | 31 (27.2) | 45 (39.5) | 8 (7.0) | 15 (13.2) | 23 (20.2) | 68 (59.6) |
| Systemic (%) | 24 (21.0) | 52 (45.6) | 76 (66.7) | 12 (10.5) | 26 (22.8) | 38 (33.3) | 114 (100.0) |
| Mutations (yes/no) | |||||||
| EGFR | 1/9 | 7/14 | 8/23 | 2/6 | 1/7 | 3/13 | 11/36 |
| KRAS | 1/4 | 3/15 | 4/19 | 1/4 | 1/5 | 2/9 | 6/28 |
Curative and palliative included.
ECOG, Eastern Cooperative Oncology Group; PS, performance status; NOS, not otherwise specified; CR/PR, complete response/partial response; SD, stable disease; EGFR, epidermal growth factor receptor; KRAS, V-Ki-rase2 Kirsten rat sarcoma viral oncogene homolog.
adverse events
Imetelstat was generally well tolerated, with ∼32% and 26% of patients requiring dose reductions and delays, respectively. Grade 3/4 neutropenia and thrombocytopenia were higher in the imetelstat arms, occurring in 17.3% and 12.5% versus 0% and 0% and 34.6% and 12.5% versus 0% and 0% for imetelstat (alone and with bevacizumab) versus control arms, respectively (Table 2). However, neutropenic fever and bleeding episodes (epistaxis, hemoptysis, pulmonary hemorrhage and intracranial hemorrhage) were infrequent (<2% each). All grade anemia adverse events were also more frequent with imetelstat (17.3% and 25% versus 3.8% and 8.3%, respectively), but only one patient experienced grade 3 anemia with imetelstat plus bevacizumab.
Table 2.
Hematologic and laboratory adverse events by treatment arm and severity (>5% total incidence)
| System organ class preferred term | NCI CTCAE Grade | Observation (N = 26) | Imetelstat only (N = 52) | Bevacizumab only (N = 12) | Imetelstat plus bevacizumab (N = 24) | Total (N = 114) |
|---|---|---|---|---|---|---|
| Thrombocytopenia | All grades | 0 | 25 (48.1%) | 1 (8.3%) | 13 (54.2%) | 39 (34.2%) |
| 3–4 | 0 | 18 (34.6%) | 0 | 3 (12.5%) | 21 (18.4%) | |
| Neutropenia | All grades | 0 | 12 (23.1%) | 1 (8.3%) | 10 (41.7%) | 23 (20.2%) |
| 3–4 | 0 | 9 (17.3%) | 0 | 3 (12.5%) | 12 (10.5%) | |
| Anemia | All grades | 1 (3.8%) | 9 (17.3%) | 1 (8.3%) | 6 (25.0%) | 17 (14.9%) |
| 3–4 | 0 | 0 | 0 | 1 (4.2%) | 1 (<1%) | |
| Alanine aminotransferase increased | All grades | 0 | 6 (11.5%) | 0 | 3 (12.5%) | 9 (7.9%) |
| 3–4 | 0 | 3 (5.8%) | 0 | 0 | 3 (2.6%) | |
| Aspartate aminotransferase increased | All grades | 0 | 6 (11.5%) | 0 | 3 (12.5%) | 9 (7.9%) |
| 3–4 | 0 | 0 | 0 | 0 | 0 | |
| Hypokalemia | All grades | 0 | 4 (7.7%) | 0 | 4 (16.7%) | 8 (7.0%) |
| 3–4 | 0 | 0 | 0 | 0 | 0 | |
| Hyponatremia | All grades | 0 | 4 (7.7%) | 1 (8.3%) | 3 (12.5%) | 8 (7.0%) |
| 3–4 | 0 | 0 | 0 | 0 | 0 | |
| Hyperglycemia | All grades | 2 (7.7%) | 1 (1.9%) | 1 (8.3%) | 3 (12.5%) | 7 (6.1%) |
| 3–4 | 1 (3.8%) | 0 | 0 | 0 | 1 (<1%) | |
| Hypoalbuminemia | All grades | 2 (7.7%) | 2 (3.8%) | 0 | 2 (8.3%) | 6 (5.3%) |
| 3–4 | 0 | 0 | 0 | 0 | 0 |
The most frequent treatment-related non-hematologic adverse events involved the gastrointestinal (57.9%), musculoskeletal (45.6%), respiratory (39.5%), nervous (42.9%), tegumentary (26.3%), and psychiatric systems (21.9%). Constitutional symptoms, metabolic abnormalities, and infections occurred in 50.9%, 35.1%, and 30.7%, respectively, but only 7%, 3.5%, and 3.5%, respectively, were of grade 3/4 severity (Table 3). Both hematologic and non-hematologic adverse events were consistently more frequent in patients receiving imetelstat plus bevacizumab compared with imetelstat alone (anemia, neutropenia, thrombocytopenia, fatigue, nausea/vomiting, hypertension, dizziness, and headaches). However, imetelstat affected patients receiving bevacizumab differentially (lower pulmonary hemorrhage and proteinura, similar epistaxis and hypertension and higher fatigue, nausea/vomiting, headache, and myelusuppression).
Table 3.
Non-hematologic adverse events (>10% all grade incidence)
| System organ class preferred term | NCI CTCAE Grade | Observation (N = 26) | Imetelstat only (N = 52) | Bevacizumab only (N = 12) | Imetelstat plus bevacizumab (N = 24) | Total (N = 114) |
|---|---|---|---|---|---|---|
| Gastrointestinal disorders | All grades | 10 (38.5%) | 32 (61.5%) | 6 (50.0%) | 18 (75.0%) | 66 (57.9%) |
| 3–4 | 0 | 1 (1.9%) | 0 | 1 (4.2%) | 2 (1.8%) | |
| Nausea | All grades | 2 (7.7%) | 21 (40.4%) | 3 (25.0%) | 12 (50.0%) | 38 (33.3%) |
| 3–4 | 0 | 0 | 0 | 0 | 0 | |
| Vomiting | All grades | 1 (3.8%) | 12 (23.1%) | 2 (16.7%) | 7 (29.2%) | 22 (19.3%) |
| 3–4 | 0 | 0 | 0 | 0 | 0 | |
| Constipation | All grades | 2 (7.7%) | 6 (11.5%) | 0 | 6 (25.0%) | 14 (12.3%) |
| 3–4 | 0 | 0 | 0 | 0 | 0 | |
| Constitutional symptoms | All grades | 7 (26.9%) | 32 (61.5%) | 3 (25.0%) | 16 (66.7%) | 58 (50.9%) |
| 3–4 | 1 (3.8%) | 5 (9.6%) | 2 (16.7%) | 1 (4.2%) | 9 (7.9%) | |
| Fatigue | All grades | 3 (11.5%) | 22 (42.3%) | 3 (25.0%) | 10 (41.7%) | 38 (33.3%) |
| 3–4 | 0 | 3 (5.8%) | 1 (8.3%) | 0 | 4 (3.5%) | |
| Peripheral edema | All grades | 0 | 7 (13.5%) | 1 (8.3%) | 6 (25.0%) | 14 (12.3%) |
| 3–4 | 0 | 0 | 0 | 0 | 0 | |
| Infections and infestations | All grades | 6 (23.1%) | 14 (26.9%) | 3 (25.0%) | 14 (58.3%) | 37 (32.5%) |
| 3–5a | 0 | 3 (5.8%) | 0 | 1 (4.2%) | 4 (3.5%) | |
| Metabolism and nutrition disorders | All grades | 5 (19.2%) | 19 (36.5%) | 5 (41.7%) | 11 (45.8%) | 40 (35.1%) |
| 3–4 | 1 (3.8%) | 1 (1.9%) | 1 (8.3%) | 1 (4.2%) | 4 (3.5%) | |
| Decreased appetite | All grades | 2 (7.7%) | 7 (13.5%) | 3 (25.0%) | 1 (4.2%) | 13 (11.4%) |
| 3–4 | 0 | 0 | 1 (8.3%) | 0 | 1 (<1%) | |
| Musculoskeletal and connective tissue disorders | All grades | 9 (34.6%) | 23 (44.2%) | 7 (58.3%) | 13 (54.2%) | 52 (45.6%) |
| 3–4 | 0 | 1 (1.9%) | 0 | 0 | 1 (<1%) | |
| Back pain | All grades | 3 (11.5%) | 9 (17.3%) | 0 | 5 (20.8%) | 17 (14.9%) |
| 3–4 | 0 | 0 | 0 | 0 | 0 | |
| Nervous system disorders | All grades | 7 (26.9%) | 22 (42.3%) | 5 (41.7%) | 16 (66.7%) | 50 (43.9%) |
| 3–5b | 2 (7.7%) | 2 (3.8%) | 1 (8.3%) | 0 | 5 (4.4%) | |
| Dizziness | All grades | 1 (3.8%) | 7 (13.5%) | 1 (8.3%) | 7 (29.2%) | 16 (14.0%) |
| 3–4 | 0 | 1 (1.9%) | 0 | 0 | 1 (<1%) | |
| Headache | All grades | 2 (7.7%) | 5 (9.6%) | 0 | 8 (33.3%) | 15 (13.2%) |
| 3–4 | 0 | 0 | 0 | 0 | 0 | |
| Psychiatric disorders | All grades | 3 (11.5%) | 14 (26.9%) | 2 (16.7%) | 6 (25.0%) | 25 (21.9%) |
| 3–4 | 0 | 0 | 1 (8.3%) | 0 | 1 (<1%) | |
| Respiratory disorders | All grades | 7 (26.9%) | 21 (40.4%) | 6 (50.0%) | 11 (45.8%) | 45 (39.5%) |
| 3–4 | 0 | 3 (5.8%) | 0 | 1 (4.2%) | 4 (3.5%) | |
| Cough | All grades | 3 (11.5%) | 9 (17.3%) | 1 (8.3%) | 4 (16.7%) | 17 (14.9%) |
| 3–4 | 0 | 0 | 0 | 0 | 0 | |
| Epistaxis | All grades | 0 | 6 (11.5%) | 4 (33.3%) | 5 (20.8%) | 15 (13.2%) |
| 3–4 | 0 | 1 (1.9%) | 0 | 1 (4.2%) | 2 (1.8%) | |
| Dyspnea | All grades | 3 (11.5%) | 6 (11.5%) | 1 (8.3%) | 2 (8.3%) | 12 (10.5%) |
| 3–4 | 0 | 0 | 0 | 0 | 0 | |
| Skin and subcutaneous tissue disorders | All grades | 1 (3.8%) | 14 (26.9%) | 5 (41.7%) | 10 (41.7%) | 30 (26.3%) |
| 3–4 | 0 | 0 | 0 | 0 | 0 | |
| System Organ Class Preferred Term | NCI CTCAE Grade | Observation (N = 26) | Imetelstat Only (N = 52) | Bevacizumab Only (N = 12) | Imetelstat plus Bevacizumab (N = 24) | Total (N = 114) |
One fatal intracranial hemorrhage in the imetelstat-only arm was reported as unrelated to imetelstat by the investigator.
There was one fatal pneumonia in the observation arm and one fatal sepsis in the imetelstat-only arm that was reported as unrelated to imetelstat by the investigator.
Overall, 17 patients discontinued treatment due to imetelstat-related adverse events as determined by the investigator (10 in the imetelstat arm and 7 in the imetelstat plus bevacizumab arm). The most frequent causes for discontinuation were thrombocytopenia (6 patients), infusion reactions, and fatigue (2 patients each).
efficacy
The median number of imetelstat maintenance cycles was 3. ORR (additional improvement after the best response to induction) was 3.0% (2/66) in the imetelstat arm and 0% (0/35) in the control arm (P = 0.32), with 51.5% (34/66) and 54.3% (19/35) of patients achieving stable disease, respectively.
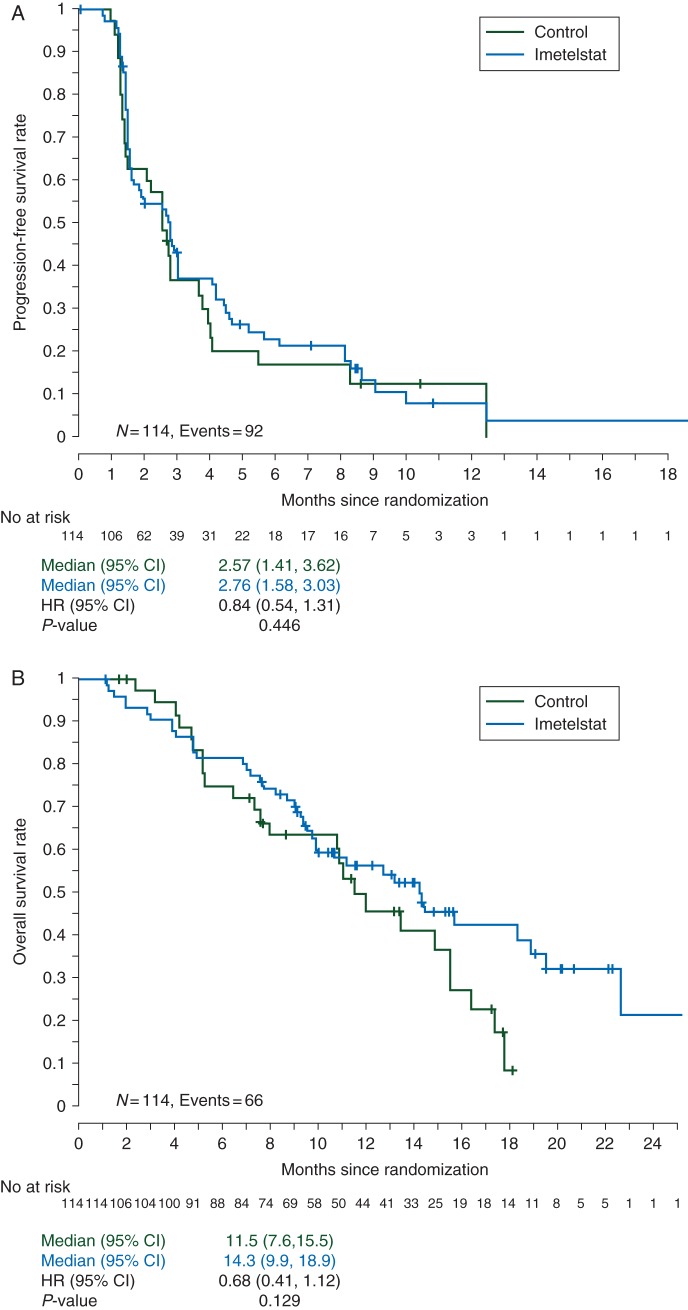
Analysis of PFS showed a non-significant improvement in favor of the imetelstat arm (HR = 0.844; 95% CI 0.54–1.31; P = 0.446). Median PFS was 2.76 months (95% CI 1.58–3.03) for imetelstat and 2.57 months (95% CI 1.41–3.62) for control (Figure 2A). A non-significant trend for improvement in OS was observed in favor of imetelstat (Figure 2B). Median survival time (MST) for imetelstat was 14.3 months (95% CI 9.9–18.9) and 11.5 months (95% CI 7.6–15.5) for the control arm (HR = 0.68; 95% CI 0.41–1.12; P = 0.129).
Figure 2.
(A) Kaplan–Meier (K–M) plots for progression-free survival (PFS) in the intent-to treat population, broken down by the treatment arm. There was no significant difference in PFS between arms. (B) K–M plots for overall survival (OS) in the intent-to-treat population, broken down by the treatment arm. There was no significant difference in OS between arms.
No subgroup analyzed showed significantly superior PFS or MST with imetelstat. In patients receiving imetelstat plus bevacizumab versus bevacizumab alone, median PFS was 4.5 versus 3.8 months (HR = 0.95; 95% CI 0.42–2.14; P = 0.907) and MST was 14.2 versus 11.0 months (HR = 0.54; 95% CI 0.22–1.31; P = 0.167). Imetelstat did not appear effective in patients with squamous tumors, compared with observation, with a median PFS of 1.6 versus 1.7 months (HR = 1.13; 95% CI 0.41–3.07; P = 0.816) and a MST of 10.7 versus 12.0 months (HR = 1.66; 95% CI 0.57–4.85; P = 0.351). However, patients who had non-squamous tumors and received imetelstat with or without bevacizumab had the best MST and HR versus controls (18.4 versus 11.6 months; 95% CI 9.9–22.7; HR = 0.6; 95% CI 0.33–1.09; P = 0.092).
TL analysis and correlation with efficacy
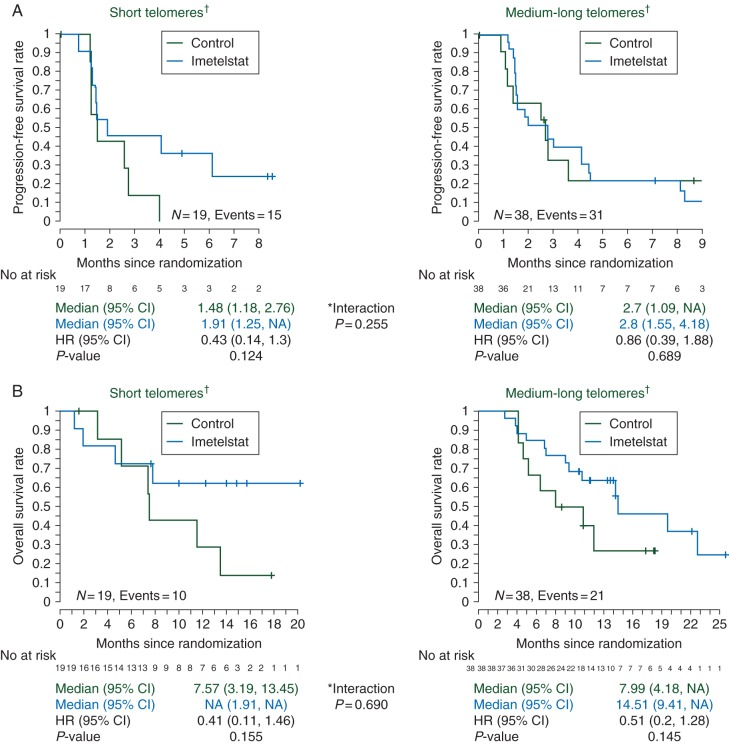
TL data by quantitative PCR (qPCR) were available for 57 patients. Results in the group with the shortest 1/4 and 1/3 of TL were similar, with consistent but attenuated results in the shortest 1/2 group, suggesting that a smaller patient subset (shortest 1/3 TL group) might be the one with the most potential to benefit.
In the 19 patients with the shortest 1/3 TL, imetelstat maintenance minimally increased mPFS from 1.5 months for the control arm to 1.9 months for the imetelstat-treated arm (HR = 0.43; 95% CI 0.14–1.3; P = 0.124 [un-stratified log-rank]). Among the 38 patients with the longest 2/3 TL, the HR was 0.86 (95% CI 0.39–1.88). Short TL by qPCR was associated with a worse mPFS (1.5 months), compared with patients with long TL (2.7 months) in the control arm (Figure 3A). Similarly, using telomerase fluorescence in situ hybridization (TeloFISH) (59 samples in total), a non-significant trend toward improved PFS among patients with the shortest 1/3 TL (HR = 0.45; 95% CI 0.14–1.48; P = 0.177) was observed.
Figure 3.
(A) K–M plots for PFS broken down by the treatment arm and telomere length (TL) measured by quantitative PCR (qPCR). (B) K–M plots for OS broken down by the treatment arm and TL measured by qPCR. No significant difference between arms was observed in either case.
The OS observed in the imetelstat patients was also superior to that shown in the control arm, when broken down by qPCR TL, although not statistically significant (Figure 3B). Similarly, OS improvements with imetelstat was also observed by TeloFISH in both the patients with short and long TL tumors (HR = 0.44; 95% CI 0.11–1.87; P = 0.256 and HR = 0.58; 95% CI 0.25–1.36; P = 0.200, respectively).
discussion
The treatment of advanced NSCLC, particularly non-squamous NSCLC, has seen tremendous changes and paradigm shifts over the past decade, with resulting survival improvements not restricted to the molecular revolution initiated with the discovery of epidermal growth factor receptor mutations and other genetic alterations that have led to the new concept of personalized (lung) cancer therapy [18]. Changes have also been seen in the more traditional ‘one-size-fits-all’ chemotherapy strategies, where the use of bevacizumab and/or pemetrexed and the concept of maintenance chemotherapy are now well accepted and widely applied.
It is the latter concept that our trial attempted to address, by utilizing the novel agent imetelstat, which based on preliminary evidence showed that, following chemotherapy, tumor regrowth (or progression) may rely primarily on the proliferation of a cellular subcompartment with progenitor cell or ‘stem cell’-like properties. Proliferation of this putative ‘stem cell’ subpopulation is associated with telomerase upregulation, making telomerase inhibitors, like imetelstat, a promising anti-cancer-targeted therapy [19, 20].
Because TL gets critically shorter with each cell division and does not cause immediate cell death (it is not a rapidly acting mechanism), it is logical to combine a telomerase inhibitor with cytotoxic chemotherapy, which has faster anti-tumor effects, and continue the telomerase inhibitor in a continuation maintenance approach. However, our phase I trial showed dose-limiting myelosuppression when combining imetelstat with chemotherapy, necessitating a switch maintenance approach.
Although our present trial did not meet the primary end point of improving PFS with the addition of maintenance imetelstat and thus should be considered a negative trial, some of the results obtained are hypotheses-generating and suggest that further investigations are warranted. First, our biomarker analysis is consistent with the hypothesis that clinical benefit from telomerase inhibition should be greater in patients with tumors possessing shorter telomeres. We showed a numerical but not statistically significant improvement in both the mPFS and MST of patients with the shortest 1/3 TL (measured by qPCR) receiving imetelstat. However, although imetelstat did not improve PFS for those with the longest TL, again supporting the predictive power of the shortest 1/3 TL, this power is lost with the observation of a similarly higher MST in patients with the longest TL on imetelstat. Whether the latter is the consequence of the crossover design or from subsequent therapies that could have predominantly benefited the longest TL group of patient is a hypothesis that requires confirmatory studies. Secondly, the OS achieved among patients who had non-squamous tumors, including 36 patients who remained on maintenance bevacizumab, almost reached statistical significance (18.4 months, P = 0.092, n = 93). This result compares favorably with the many trials where bevacizumab has been part of the maintenance treatment regimen, including ATLAS [21] and PointBreak [22], and in light of these results, the door opens to a scenario where the maintenance doublet of bevacizumab plus imetelstat could follow a period of ‘triplet induction’ chemotherapy, including paclitaxel–carboplatin–bevacizumab or pemetrexed–carboplatin–bevacizumab.
In conclusion, maintenance imetelstat failed to improve PFS in an advanced NSCLC patient population with diverse TL responding to first-line therapy. There was a trend toward a survival improvement, particularly in patients receiving bevacizumab and those with short telomeres. Prospective confirmation of short TL as a predictive biomarker will be required for further clinical development of imetelstat.
funding
Clinical trial support was provided by Geron Corporation to conduct this study. This work was supported by grants from the National Institutes of Health SPORE (P50 CA70907) and the National Institutes of Health Cancer Center support grant (P30 CA142543-04).
disclosure
EB is a previous and BB is a present employee of Geron Corporation and have received travel funding as well as stock options. VP received travel funds for this study. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank Jerry Shay for his contributions in the preclinical studies leading up to this clinical trial and his helpful suggestions regarding its design. The authors also thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance in the development of this manuscript.
references
- 1.McEachern MJ, Krauskopf A, Blackburn EH. Telomeres and their control. Annu Rev Genet. 2000;34:331–358. doi: 10.1146/annurev.genet.34.1.331. [DOI] [PubMed] [Google Scholar]
- 2.Harley CB. Telomerase is not an oncogene. Oncogene. 2002;21:494–502. doi: 10.1038/sj.onc.1205076. [DOI] [PubMed] [Google Scholar]
- 3.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 4.Harley CB, Sherwood SW. Telomerase, checkpoints and cancer. Cancer Surv. 1997;29:263–284. [PubMed] [Google Scholar]
- 5.Asai A, Oshima Y, Yamamoto Y, et al. A novel telomerase template antagonist (GRN163) as a potential anticancer agent. Cancer Res. 2003;63:3931–3939. [PubMed] [Google Scholar]
- 6.Herbert BS, Gellert GC, Hochreiter A, et al. Lipid modification of GRN163, an N3′→P5′ thio-phosphoramidate oligonucleotide, enhances the potency of telomerase inhibition. Oncogene. 2005;24:5262–5268. doi: 10.1038/sj.onc.1208760. [DOI] [PubMed] [Google Scholar]
- 7.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 8.Herbert B, Pitts AE, Baker SI, et al. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc Natl Acad Sci USA. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 10.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 11.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 12.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 13.Zukin M, Barrios CH, Rodrigues Pereira J, et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group Performance Status of 2. J Clin Oncol. 2013;31:2849–2853. doi: 10.1200/JCO.2012.48.1911. [DOI] [PubMed] [Google Scholar]
- 14.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 15.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Common Terminology Criteria for Adverse Events v.4.0 (CTCAE) 2011. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm .
- 18.Johnson BE, Kris MG, Berry LD, et al. A multicenter effort to identify driver mutations and employ targeted therapy in patients with lung adenocarcinomas: The Lung Cancer Mutation Consortium (LCMC) J Clin Oncol. 2013;31 (suppl; abstr 8019) [Google Scholar]
- 19.Joseph I, Tressler R, Bassett E, et al. The telomerase inhibitor imetelstat depletes cancer stem cells in breast and pancreatic cancer cell lines. Cancer Res. 2010;70:9494–9504. doi: 10.1158/0008-5472.CAN-10-0233. [DOI] [PubMed] [Google Scholar]
- 20.Castelo-Branco P, Zhang C, Lipman T, et al. Neural tumor-initiating cells have distinct telomere maintenance and can be safely targeted for telomerase inhibition. Clin Cancer Res. 2011;17:111–121. doi: 10.1158/1078-0432.CCR-10-2075. [DOI] [PubMed] [Google Scholar]
- 21.Johnson BE, Kabbinavar F, Fehrenbacher L, et al. ATLAS: randomized, double-blind, placebo-controlled, phase IIIB trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2013;31:3926–3934. doi: 10.1200/JCO.2012.47.3983. [DOI] [PubMed] [Google Scholar]
- 22.Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:4349–4357. doi: 10.1200/JCO.2012.47.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.