Abstract
Background/aims:
Although disturbed sleep is associated with cognitive deficits, the association between sleep disturbance and Alzheimer’s disease pathology is unclear. In this pilot study, we examined the extent to which sleep duration, sleep quality, and sleep-disordered breathing are associated with β-amyloid (Aβ) deposition in the brains of living humans.
Methods:
We studied 13 older adults (8 with normal cognition and 5 with mild cognitive impairment). Participants completed neuropsychological testing, polysomnography, and Aβ imaging with [11C]-Pittsburgh compound B.
Results:
Among participants with mild cognitive impairment, higher apnea–hypopnea index and oxygen desaturation index were associated with greater Aβ deposition, globally and regionally in the precuneus. There were no significant associations between sleep-disordered breathing and Aβ deposition among cognitively normal participants. There were no significant associations between sleep duration or sleep fragmentation and Aβ deposition.
Conclusion:
These preliminary results suggest that among older adults with mild cognitive impairment, greater sleep-disordered breathing severity is associated with greater Aβ deposition.
Keywords: Sleep, sleep apnea, mild cognitive impairment, Alzheimer’s disease, amyloid, positron emission tomography
Introduction
Several studies suggest an association between disturbed sleep and Alzheimer’s disease (AD). Sleep is known to be disrupted in individuals with AD, who have greater sleep fragmentation and poorer sleep efficiency than cognitively normal older adults.1–3 Sleep disturbance is also common in individuals with mild cognitive impairment (MCI),4 who are at elevated risk of AD.5 While early studies comparing the sleep of those with AD to that of normal adults implied that AD pathology caused sleep disruption, several newer studies raise the possibility that poor sleep actually promotes AD neuropathology. For example, sleep deprivation has been shown to enhance amyloid plaque deposition in a mouse model of AD,6 and in cognitively normal humans, poor sleep quality has been linked to amyloid burden as measured by cerebrospinal fluid (CSF) amyloid-β (Aβ42) peptide.7 Furthermore, we recently showed that reports of shorter sleep duration and poorer sleep quality among community-dwelling older adults were associated with greater Aβ deposition, measured by positron emission tomography imaging with [11C]-Pittsburgh compound B (PET-PIB).8
In addition to diminished quality sleep, sleep-disordered breathing (SDB) has been associated with the development of MCI and dementia.9 SDB is characterized by recurrent respiratory events during sleep that result in hypoxia and sleep fragmentation and is present in more than half of older adults.10 Osorio et al.11 recently demonstrated a trend toward an association between SDB and CSF Aβ levels in a sample of cognitively normal participants with the apolipoprotein E (APOE) ϵ4 allele. Despite this knowledge, little is known about the association between objectively measured sleep quality or SDB and β-amyloid deposition in living subjects. We conducted a pilot study of the association between sleep disturbance, measured by polysomnography (PSG), and Aβ deposition, measured by PET-PIB. We studied cognitively normal individuals and those with MCI.
Methods
Participants
Participants were eight cognitively normal adults aged ≥55 years and five with MCI. Since our original aim was to investigate the association between sleep/wake variables and Aβ deposition independently of SDB, we excluded individuals reporting a history of SDB or excessive daytime sleepiness. Participants were recruited from other studies or the community. During telephone screening, they provided demographic information and health history and completed the Epworth Sleepiness Scale12 (ESS) and the 15-item Geriatric Depression Scale13 (GDS). Participants were excluded if they reported a prior diagnosis of sleep apnea; history of clinical stroke, AD, or Parkinson’s disease; use of a sleeping aid, benzodiazepine, or anticholinergic medication; or had an ESS score >10. In addition, cognitively normal participants were excluded if they had a current psychiatric disorder or a 15-item GDS ≥6. Because up to half of persons with MCI have neuropsychiatric symptoms, including depressive symptoms,14 we permitted persons with MCI to participate even if they had elevated GDS scores. During an in-person study visit, eligible individuals completed a series of neuropsychological tests, a medical history form, and the full 30-item GDS;13 informants completed the Clinical Dementia Rating (CDR) Scale.15 Cognitively normal participants were excluded if they had a 30-item GDS score ≥10. Subjects were classified as cognitively normal or having MCI16 or dementia17 by either a board-certified neuropsychologist (J.B.) or by the consensus of clinical investigators at the Johns Hopkins Alzheimer’s DiseaseResearchCenter. Participants were required to have a CDR score of 0.5 to qualify for MCI diagnosis; cognitively normal participants were required to have a CDR of 0. All MCI subtypes were eligible. Participants provided written informed consent. This study was approved by the Johns Hopkins Medical Institutions Institutional Review Board.
Neuropsychological testing
During a research visit separate from the ones at which PSG and neuroimaging were conducted, participants completed a battery of neuropsychological tests. These were administered by a psychometrist under the supervision of a board-certified neuropsychologist (J.B.) and included the Mini-Mental State Examination,18 Wechsler Memory Scale—Revised Logical Memory subtest (immediate and delayed recall),19 Boston Naming Test,20 Delis-Kaplan Executive Function System (D-KEFS) Trail-Making Test,21 Wechsler Adult Intelligence Scale (4th edition) Letter-Number Sequencing subtest,22 and the Paced Auditory Serial Addition Test (PASAT).23
Polysomnography
Participants completed two consecutive nights of attended PSG with a standard montage (Embla N7000 amplifiers with RemLogic 1.1 software). The first night was for adaptation; only second night data were used. During sleep studies, participants went to bed at their standard bedtimes but were asked to remain in bed for 8 h. Data were scored by polysomnographic technologists using standard criteria, supervised by a board-certified sleep medicine physician. Total sleep time (TST) was defined as amount of time spent asleep (minutes) in bed. Sleep fragmentation was measured by wake after sleep onset (WASO; number of minutes awake after initial sleep onset) and arousal index (AI; number of arousals/hour of sleep). Arousals were defined as an abrupt increase in electroencephalogram (EEG) frequency lasting 3 s following at least 10 s of sleep. SDB was quantified by the apnea–hypopnea index (AHI; number of apneas + hypopneas/hour of sleep). Apneas were defined as cessation of respiration for ≥10 s; hypopneas were defined as 30% decrement in airflow with ≥4% decrease in SaO2. We also calculated a non-obstructive AHI, which included both central and mixed respiratory events (number of non-obstructive apneas + hypopneas/hour of sleep). Oxygen desaturation index (ODI) was calculated as number of desaturations ≥3% per hour during sleep.
β-amyloid imaging
Participants received PET scans of their brains with the PIB radiotracer. Scanning and processing details have been described elsewhere.24 PET images were co-registered to 1.5-T magnetic resonance imaging (MRI) scans. The parametric images of distribution volume ratios (DVRs) were derived from dynamic PET images using a simplified reference tissue model and linear regression with spatial constraint algorithm.24 The volume of interest (VOI) DVR was obtained by applying VOI to DVR images. We studied two outcomes: (1) the cortical DVR (cDVR), a global measure of Aβ deposition based on a VOI that included frontal, temporal, occipital, and parietal cortex gray matter, and both cingulate and precuneus; and (2) precuneus DVR (pDVR), one of the regions with the earliest Aβ deposition. Investigators measuring and interpreting PET results were blinded to the primary predictors (i.e. SDB status, other PSG variables).
Statistical analysis
We compared participants with normal cognition and those with MCI using Mann–Whitney tests for continuous variables and Fisher’s exact test for categorical variables. We generated scatterplots and computed Spearman correlation coefficients to determine the association between SDB and Aβ deposition in each group. Two-sided tests were used for all analyses, which were performed using Stata MP 12.1 (StataCorp, College Station, TX, USA).
Results
Of the 13 participants, 5 met criteria for MCI; 3 were of the amnestic multiple-domain subtype, 1 had non-amnestic single-domain MCI, and 1 had non-amnestic multiple-domain MCI. Cognitively normal participants had a mean ± standard deviation age of 69.4 ± 5.6 and those with MCI averaged 75.2 ± 11.3 years (Table 1). Cognitively normal participants also were more likely to be women than those with MCI, but these differences were not statistically significant. There was a trend toward a greater number of depressive symptoms, as measured by elevated GDS score, in MCI subjects (p = 0.06). All cognitively normal participants had GDS scores <10, the cutoff for mild depression on the GDS; one MCI subject had a score ≥10, but it was 18, which is in the mild range.25 No participant reported taking an opioid medication or having a history of congestive heart failure—both of which are risk factors for central respiratory events. Cognitively normal participants had a lower cDVR (1.2 ± 0.2 vs 1.5 ± 0.2, p < 0.05) and prDVR (1.3 ± 0.2 vs 1.7 ± 0.3, p < 0.05) than those with MCI. These amyloid burden levels are consistent with what has been observed in other samples.26,27 As expected, participants with normal cognition had better performance on all neuropsychological tests (all p < 0.05; Table 2).
Table 1.
Participant characteristics (mean ± SD, n (%)).
| Normal (n = 8) | MCI (n = 5) | |
|---|---|---|
| Age | 69.4 ± 5.6 | 75.2 ± 11.3 |
| Female | 5 (62.5) | 1 (20.0) |
| Non-white | 2 (25.0) | 1 (20.0) |
| Education | 15.5 ± 2.3 | 14.6 ± 2.4 |
| 30-item GDS | 1.5 ± 1.5 | 6.8 ± 6.8 |
| BMI (kg/m2) | 25.5 ± 3.5 | 26.2 ± 8.2 |
| cDVR | 1.2 ± 0.2 | 1.5 ± 0.2* |
| prDVR | 1.3 ± 0.2 | 1.7 ± 0.3* |
BMI: body mass index; cDVR: cortical distribution volume ratio; GDS: Geriatric Depression Scale; MCI: mild cognitive impairment; prDVR: precuneus distribution volume ratio.
p < 0.05.
Table 2.
Neuropsychological test performance, by cognitive status.
| Neuropsychological test | Normal (n = 8) | MCI (n = 5) |
|---|---|---|
| Mini-MentalState Examination | 29.0 ± 0.9 | 26.8 ± 0.8** |
| Logical Memory Immediate Recall | 14.1 ± 2.5 | 7.2 ± 3.0** |
| Logical Memory Delayed Recall | 13.3 ± 2.9 | 4.4 ± 2.8** |
| Boston Naming Testa | 29.4 ± 0.7 | 24.3 ± 2.8** |
| TMT Number Sequencing (seconds) | 34.4 ± 11.8 | 59.0 ± 19.1* |
| TMT Letter Sequencing (seconds) | 35.0 ± 15.1 | 68.8 ± 23.6* |
| TMT Number-Letter Switching (seconds) | 78.4 ± 32.2 | 250.4 ± 119.5** |
| WAIS-IV Letter-Number Sequencing | 20.4 ± 3.9 | 11.4 ± 7.2* |
| PASAT Trial 1 (%) | 82.3 ± 11.9 | 37.3 ± 27.2* |
| PASAT Trial 2 (%) | 57.9 ± 17.2 | 35.3 ± 21.7* |
MCI: mild cognitive impairment; TMT: Delis-Kaplan Executive Function System Trail-Making Test; WAIS-IV: Wechsler Adult Intelligence Scale, 4th Edition; PASAT: Paced Auditory Serial Addition Test.
One participant with multiple-domain MCI was missing data for the Boston Naming Test.
p < 0.05, **p < 0.01.
PSG revealed that participants with MCI had shorter TST, greater WASO, and higher AI, compared to those with normal cognition, but these differences were not statistically significant (Table 3). There was a trend toward a higher AHI (31.0 ± 22.6 vs 7.6 ± 8.2, p = 0.06) and ODI (27.1 ± 20.0 vs 7.4 ± 4.9, p = 0.06) among participants with MCI, compared to cognitively normal participants. One participant (12.5%) with normal cognition and four (80%) with MCI had at least moderate sleep apnea (AHI ≥ 15, p < 0.05). Non-obstructive respiratory events, as measured by the non-obstructive AHI, were more frequent in the MCI group than in the normal group (19.1 ± 22.7 vs 0.4 ± 0.9, p < 0.05).
Table 3.
Polysomnographic indices.
| Normal (n = 8) | MCI (n = 5) | |
|---|---|---|
| Total sleep time (min) | 398.5 ± 34.8 | 374.4 ± 32.7 |
| Wake after sleep onset (min) | 73.4 ± 30.1 | 99.4 ± 32.8 |
| Arousal index | 12.2 ± 7.3 | 14.6 ± 12.4 |
| AHI | 7.6 ± 8.2 | 31.0 ± 22.6 |
| Non-obstructive AHI | 0.4 ± 0.9 | 19.1 ± 22.7* |
| Oxygen desaturation index | 7.4 ± 4.9 | 27.1 ± 20.0 |
AHI: apnea–hypopnea index; MCI: mild cognitive impairment.
p < 0.05.
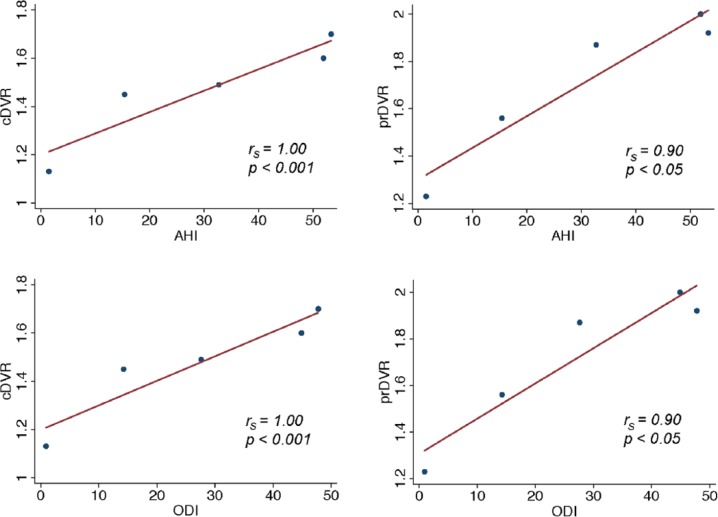
Among cognitively normal participants, there were no significant associations between TST, WASO, AI, and cDVR (Table 4). Similarly, no associations were found between AHI, non-obstructive AHI, or ODI and cDVR in this group. Among MCI participants, however, there were robust positive associations between AHI and cDVR and between ODI and cDVR, such that greater AHI and ODI were associated with greater amyloid burden (Figure 1, rs = 1.00, p < 0.001 for both). There was no association between TST, WASO, AI, or non-obstructive AHI and cDVR in those with MCI.
Table 4.
Spearman correlations (rs) between sleep variables and β-amyloid burden.
| Normal (n = 8), rs |
MCI (n = 5), rs |
|||
|---|---|---|---|---|
| cDVR | pDVR | cDVR | pDVR | |
| Total sleep time (minutes) | −0.52 | −0.41 | 0.10 | 0.30 |
| Wake after sleep onset (minutes) | 0.45 | 0.32 | −0.10 | −0.30 |
| Arousal index | −0.36 | −0.17 | 0.10 | −0.30 |
| AHI | −0.29 | −0.34 | 1.00*** | 0.90* |
| Non-obstructive AHI | −0.55 | −0.51 | 0.80 | 0.90* |
| Oxygen desaturation index | −0.33 | −0.42 | 1.00*** | 0.90* |
AHI: apnea–hypopnea index; cDVR: cortical distribution volume ratio; prDVR: precuneus distribution volume ratio.
p < 0.05, ***p < 0.001.
Figure 1.
Association between PSG indices and PIB DVR.
PSG: polysomnography; PIB: [11C]-Pittsburgh compound B; DVR: distribution volume ratio; AH: apnea–hypopnea index; ODI: oxygen desaturation index; cDVR: cortical PIB distribution volume ratio: prDVR: precuneus PIB distribution volume ratio.
Among participants with normal cognition, there was no significant association between any PSG indices and prDVR. Among those with MCI, greater AHI, non-obstructive AHI, and ODI each were associated with greater amyloid burden in the precuneus, measured by the prDVR (Figure 1 and Table 4, rs = 0.90, p < 0.05 for all); there was no association between TST, WASO, or AI and prDVR in this group.
Discussion
We studied the association between disturbed sleep and PET-PIB-measured Aβ deposition in small samples of older adults with normal cognition or MCI. Among participants with MCI, greater SDB severity (AHI) and greater hypoxemia (ODI) were strongly associated with greater Aβ deposition, measured globally in the cortex and regionally in the precuneus. A significant number of the disordered breathing events in the MCI subjects were non-obstructive in nature, and the severity of non-obstructive SDB was also significantly associated with pDVR, but not cDVR. We did not observe an association between SDB indices and Aβ deposition in participants with normal cognition. In addition, we did not observe significant associations between sleep duration or sleep fragmentation and Aβ deposition in either group.
Our findings have several implications. First, the association between SDB/hypoxemia and Aβ deposition among MCI participants, but not normal elders, suggests that SDB and hypoxemia may contribute to amyloid deposition and accelerate AD among those with MCI. This complements studies linking SDB to diagnoses of MCI and dementia9 and could have implications for slowing the progression of AD, given the prevalence of SDB and availability of SDB therapies. Second, despite screening for SDB history and daytime sleepiness, most MCI participants had moderate to severe occult SDB, compared to one of eight with normal cognition. Though this may be due to poor recall of apnea history among MCI participants, the frequency of SDB in these individuals prompts questions about the prevalence of SDB in the broader MCI population and the extent to which untreated SDB contributes to MCI prevalence. Also, we observed a higher proportion of non-obstructive respiratory events in MCI participants, but given our small MCI sample, this finding needs replication in a larger cohort.
Our findings are consistent with those recently reported by Osorio et al.,11 suggesting associations between SDB severity and CSF evidence of amyloid deposition in cognitively normal older adults positive for the APOE ϵ4 allele. Similarly, we observed an association between SDB severity and greater Aβ burden in the brain among those with MCI (i.e. at higher risk for AD), but not among normals. It may be that the effects of SDB/hypoxia on Aβ aggregation are most pronounced after significant Aβ accumulation has already occurred, leading to an acceleration of further Aβ deposition.28
To our knowledge, there are only two other studies examining the relationship between objectively measured sleep quality or SDB and Aβ. Ju et al.7 used actigraphy to measure sleep quality and CSF Aβ to infer Aβ deposition, while Osorio et al.11 used home sleep testing to assess the association between SDB and CSF Aβ. Major strengths of this pilot study include the use of PSG and PET imaging, gold standard methods for measuring sleep and Aβ deposition in living humans, respectively. However, our study also has clear limitations. The first is our very small sample of persons with MCI and normal cognition. This combined with the low SDB prevalence and severity in cognitively normal participants may have obscured an association between SDB and amyloid in this population. We previously reported an association between subjective measures of lower sleep amount and poorer sleep quality with Aβ burden as measured by PET-PIB.8 In this study, we did not observe an association between sleep amount and fragmentation measured by PSG, but the study was likely underpowered to detect such an association. In addition, our exclusion of individuals with excessive daytime sleepiness and clinical history of SDB might have obscured potential associations in cognitively normal subjects. Furthermore, although there were no statistically significant differences in age, sex, or depressive symptoms between the MCI and normal cognition groups, the absence of these differences may be due to the small sample size in this pilot study. Thus, we cannot rule out that confounding by these variables accounts for the different patterns of results observed between groups. Moreover, this was a cross-sectional study, impeding assessment of temporal SDB–amyloid associations and precluding determination of potential causal direction. It is also possible that there is no direct causal link between SDB and amyloid, but that both arise from a third, shared disease process. Prospective studies in humans with larger samples and additional experimental work in animal models is needed to further our understanding of the SDB–AD association.
Footnotes
Funding: This study was supported by a Synapses, Circuits and Cognitive Disorders Award from the Brain Science Institute at Johns Hopkins University and a grant from the National Institute on Aging (AG038893; to Gwenn S Smith). Dr Adam P Spira is supported in part by a Mentored Research Scientist Development Award (1K01AG033195) from the National Institute on Aging. Dr Dean F Wong was partly supported during part of this time by NIH NIDA career award (K24 DA000412; 2000–2011). Dr Mark N Wu was supported by NIH grants K08NS059671 and R01NS079584, as well as a Burroughs-Wellcome Fund Career Award for Medical Scientists.
Declaration of conflicting interest: Dr Gwenn S Smith has received research support from the NIH (NIMH, NIA), including AG038893.
Dr Yun Zhou has received payment from contracts with Avid and GE Healthcare for [18F] amyloid imaging radiopharmaceuticals through JohnsHopkinsUniversity.
Dr Anil Mathur has received payment from contracts with Avid and GE Healthcare for [18F] amyloid imaging radiopharmaceuticals through JohnsHopkinsUniversity.
Dr Anil Kumar is a salaried employee of JohnsHopkinsUniversity, funded in part from its contracts with Avid and GE Healthcare for [18F] amyloid imaging.
Dr James R Brašić has received payment from contracts with Avid and GE Healthcare for [18F] amyloid imaging radiopharmaceuticals through JohnsHopkins University and has received research support from the Brain and Behavior Research Foundation and the Essel Foundation.
Dr Dean F Wong was partly supported during part of this time by NIH NIDA career award (K24 DA000412; 2000–2011) and has received payment from contracts with Avid and GE Healthcare for [18F] amyloid imaging radiopharmaceuticals through JohnsHopkinsUniversity.
Dr Mark N Wu was supported by NIH grants K08NS059671 and R01NS079584, as well as a Burroughs-Wellcome Fund Career Award for Medical Scientists. He is co-principal investigator on the Synapses, Circuits and Cognitive Disorders Award from the Brain Science Institute at JohnsHopkinsUniversity that funded this research.
References
- 1. Prinz PN, Peskind ER, Vitaliano PP, et al. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc 1982; 30(2): 86–93. [DOI] [PubMed] [Google Scholar]
- 2. Prinz PN, Vitaliano PP, Vitiello MV, et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol Aging 1982; 3(4): 361–370. [DOI] [PubMed] [Google Scholar]
- 3. Vitiello MV, Prinz PN, Williams DE, et al. Sleep disturbances in patients with mild-stage Alzheimer’s disease. J Gerontol 1990; 45(4): M131–M138. [DOI] [PubMed] [Google Scholar]
- 4. Beaulieu-Bonneau S, Hudon C. Sleep disturbances in older adults with mild cognitive impairment. Int Psychogeriatr 2009; 21(4): 654–666. [DOI] [PubMed] [Google Scholar]
- 5. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256(3): 240–246. [DOI] [PubMed] [Google Scholar]
- 6. Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009; 326(5955): 1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ju YE, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol 2013; 70(5): 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spira AP, Gamaldo AA, An Y, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol 2013; 70(12): 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011; 306(6): 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ancoli-Israel S, Kripke DF, Klauber MR, et al. Sleep-disordered breathing in community-dwelling elderly. Sleep 1991; 14(6): 486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Osorio RS, Ayappa I, Mantua J, et al. The interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer’s disease in cognitively normal elderly individuals. Neurobiol Aging 2014; 35(6): 1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johns M. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991; 14: 540–545. [DOI] [PubMed] [Google Scholar]
- 13. Wancata J, Alexandrowicz R, Marquart B, et al. The criterion validity of the Geriatric Depression Scale: a systematic review. Acta Psychiatr Scand 2006; 114(6): 398–410. [DOI] [PubMed] [Google Scholar]
- 14. Geda YE, Roberts RO, Knopman DS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry 2008; 65(10): 1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43(11): 2412–2414. [DOI] [PubMed] [Google Scholar]
- 16. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256(3): 183–194. [DOI] [PubMed] [Google Scholar]
- 17. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV-TR). 4th ed. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
- 18. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12(3): 189–198. [DOI] [PubMed] [Google Scholar]
- 19. Wechsler D. Wechsler Memory Scale–Revised (WMS-R). San Antonio, TX: The Psychological Corporation, 1987. [Google Scholar]
- 20. Kaplan EF, Goodglass H, Weintraub S. Boston Naming Test. 2nd ed. Philadelphia, PA: Lea & Febiger, 1983. [Google Scholar]
- 21. Delis D, Kaplan E, Kramer J. Delis-Kaplan executive function system. San Antonio, TX: The Psychological Corporation, 2001. [Google Scholar]
- 22. Wechsler D. Wechsler Adult Intelligence Scale—Fourth Edition (WAIS-IV). San Antonio, TX: The Psychological Corporation, 2008. [Google Scholar]
- 23. Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills 1977; 44(2): 367–373. [DOI] [PubMed] [Google Scholar]
- 24. Zhou Y, Resnick SM, Ye W, et al. Using a reference tissue model with spatial constraint to quantify [11C]Pittsburgh compound B PET for early diagnosis of Alzheimer’s disease. Neuroimage 2007; 36(2): 298–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yesavage JA. Geriatric Depression Scale and scoring guidelines, http://www.stanford.edu/~yesavage/GDS.english.long.html (accessed 25 June 2014).
- 26. Hatashita S, Yamasaki H. Clinically different stages of Alzheimer’s disease associated by amyloid deposition with [11C]-PIB PET imaging. J Alzheimers Dis 2010; 21(3): 995–1003. [DOI] [PubMed] [Google Scholar]
- 27. Wolk DA, Klunk W. Update on amyloid imaging: from healthy aging to Alzheimer’s disease. Curr Neurol Neurosci Rep 2009; 9(5): 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol 2014; 10(2): 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]