Summary
Children and adults residing at high altitude (HA) compared to low altitude (LA) have larger lung volumes; however, it is unknown whether this response to chronic hypoxia begins early in life. Our objective was to determine whether infants and toddlers at HA have larger lung volumes compared to infants and toddlers at LA. Oxygen saturation (SaO2), functional residual capacity (FRC), as well as serum levels of vascular endothelial growth factor (VEGF) and erythropoietin (EPO) were measured in infants and toddlers from HA (N = 50; 3,440 m) and LA (N = 35; 440 m). There were no significant differences in somatic size for HA and LA subjects; however, HA subjects had significantly lower SaO2 (88.5% vs. 96.7%; P < 0.0001). Subjects at HA had significantly greater FRC compared to subjects at LA (group mean: 209 and 157 ml; P < 0.0001), adjusting for body length. Male infants at HA had a significantly greater FRC compared to males at LA (57 ml; P-value < 0.001); however, the increase in FRC for females at HA compared to LA was not significant (20 ml; P-value = 0.101). VEGF and EPO were significantly higher for subjects at HA compared to LA with no gender differences. In summary, infants and toddlers at HA have lower oxygen saturations, higher serum levels of VEGF and EPO, and higher FRC compared to subjects at LA; however, chronic hypoxia appears to generate a more robust response in lung growth in male compared to female infants early in life.
Keywords: chronic hypoxia, functional residual capacity, oxygen saturation, vascular endothelial growth factor, erythropoietin
Introduction
Children, adolescents, and adults residing at high altitude (HA) since birth exhibit increased lung growth in response to chronic hypoxia; subjects at HA have larger lung volumes and greater pulmonary diffusing capacities compared to subjects residing at sea-level.1–5 This response occurs in subjects whose ancestors have resided at HA for thousands of years, as well as those with only a few generations at HA, such as Europeans; therefore, physiologic adaption to HA can occur within an individual lifetime and not limited to native populations.3,6,7
Although infants and toddlers residing at HA are also exposed to chronic hypoxia, there have been no measurements of lung volumes in these very young subjects to determine whether increased lung growth begins early in life versus occurring at an older age when there are significant increases in physical activity and metabolic requirements. If lung growth is stimulated by chronic hypoxia early in life, then understanding the mechanisms for increased lung growth in infants may provide important insights for developing therapeutic interventions for infants born with an arrest in alveolar development following extreme premature birth or infants born with congenital hypoplastic lungs.
We hypothesized that chronic hypoxia from residing at HA would stimulate lung growth early in life. In addition, we hypothesized that infants exposed to a chronic hypoxic environment from early in life would have elevated serum levels of growth factors that are downstream of hypoxic inducible factor (HIF), which regulates many important physiologic responses to hypoxia. We measured serum levels of two downstream growth factors of hypoxia-inducible factor (HIF), vascular endothelial growth factor (VEGF), which is critical for lung development, and erythropoietin (EPO), which modulates erythrogenesis.8–10
Materials and Methods
Subjects
Healthy infants and toddlers between 1 and 24 months of age were recruited for this study. The HA subjects were born and raised at an elevation of 3,440 meters above sea level (masl) in La Quiaca-Jujuy-Argentina. The low altitude (LA) subjects were recruited in Tucumán, Argentina at an elevation of 440 masl. The HA and LA cities are both located in the northwest part of Argentina. Subjects from both study sites had similar ancestry, based upon family surnames and ethnicity. Subjects were excluded if they were born premature (<37 weeks gestation) or had a history of respiratory problems. Subjects were recruited between 2008 and 2011.
The protocol was approved by the Bioethics committee from the Faculty of Medicine of the National University of Tucuman, Argentina, the Bioethics Committee from the Ministry of Health from Jujuy, Argentina and the Institutional Review Board from Indiana University, Indianapolis IN, USA. Written consent was obtained from parents of infants evaluated.
Study Design
Infants from HA were evaluated in the Hospital Jorge Uro in La Quiaca, while infants from LA were evaluated in the Hospital del Niño Jesus, Tucumán. Weight and length were measured using a scale and stadiometer. Subjects received sedation with chloral hydrate (50–75 mg/kg, PO) and measurements were obtained while sleeping in the supine position. Heart rate, respiratory rate, and percentage oxygen saturation (SaO2) were recorded during sleep.
Functional Residual Capacity (FRC) was measured during tidal breathing using the constant bias flow open circuit nitrogen washout technique, as previously described.11–15 At end-expiration, a valve switched the inspired gas from room air to 100% oxygen, and the bias flow of oxygen and the expired gas entered a 500 ml Plexiglas mixing chamber where the mixed expired N2 concentration was continuously measured with a calibrated N2 analyzer (Med Science, St. Louis, MO) at the outlet of the chamber. The constant bias flow of oxygen was set with a precision flow meter (Timeter Instruments, Michigan City, IN) and the analog signal of the fractional nitrogen concentration was digitized by a 12bitA-Dconverter, displayed on the computer monitor in real time, and stored for subsequent integration of the mixed expired nitrogen signal during the washout. The nitrogen analyzer was calibrated with room air and 100% oxygen and the volume of nitrogen expired during the washout was calculated from the integrated mixed expired nitrogen signal during the washout. The washout circuit was calibrated by washing out known volumes of room air using a calibrated syringe. For each subject, four washout maneuvers were performed with a 5-min interval between each maneuver to allow re-equilibration with room air. FRC was calculated as the expired nitrogen volume divided by the difference of the initial and final nitrogen concentrations (0.79% and 0.00%, respectively), corrected for dead space volume of mask and switching valve (12 and 30 ml for the smaller and larger facemask, respectively), and expressed at body temperature and pressure, saturated conditions (BTPS). The individual's FRC was expressed as the mean of FRC values within 10%. The same nitrogen washout equipment for measuring FRC was used for HA and LA and calibration was performed prior to each HA and LA study.
Five milliters of venous blood was obtained and serum samples from HA and LA subjects were frozen for subsequent analysis of VEGF and EPO by Elisa (R&D system, Minneapolis, MN).
Analysis
Mean and standard deviations (SD) were used to present continuous variables and unpaired t-tests were used to compare demographics between groups. Analysis of respiratory measurements and serum growth factors was performed using analysis of covariance (ANCOVA) adjusting for body length and included an interaction term between Altitude and gender. The ANCOVA model with an interaction term allows evaluation of gender specific altitude effect. VEGF and EPO levels were log transformed for analysis.
Results
Demographics
We evaluated a total of 88 infants and toddlers; however, three infants did not sleep following chloral hydrate and measurements were not obtained. FRC was measured in 50 subjects from HA and 35 subjects from LA. There were no significant differences for gender, age, weight, or body length between the HA and LA groups (Table 1). When HA and LA groups were divided by gender, there were no significant differences in demographics for HA versus LA males and no significant differences in HA versus LA females.
Table 1. Demographics.
| N | Gender (females/males) | Age (months) | Weight (kg) | Length (cm) | |
|---|---|---|---|---|---|
| Low altitude | 35 | 18/17 | 10.7 (±7.3) | 8.9 (±2.3) | 70.2 (±9.3) |
| High altitude | 50 | 27/23 | 12.5 (±7) | 8.7 (±1.8) | 72.0 (±7.7) |
| P = value | 0.26 | 0.79 | 0.32 | ||
|
| |||||
| N | Age (months) | Weight (kg) | Length (cm) | ||
|
| |||||
| Low altitude, females | 18 | 11.4 (±7.8) | 8.8 (±2.4) | 70.7 (±9.9) | |
| High altitude, females | 27 | 12.6 (±7.1) | 8.6 (±1.8) | 71.9 (±7.9) | |
| P-value | 0.61 | 0.83 | 0.64 | ||
| Low altitude, males | 17 | 9.8 (±6.9) | 9 (±2.3) | 69.6 (±9.1) | |
| High altitude, males | 23 | 12.3 (±7.1) | 8.9 (±1.8) | 72.1 (±7.7) | |
| P-value | 0.27 | 0.9 | 0.36 | ||
Mean (±SD).
Oxygen Saturation and Respiratory Rate
Infants and toddlers at HA had significantly lower oxygen saturations while sleeping compared to subjects at LA (88.5% vs. 96.7%; P < 0.0001) and oxygen saturation was not related to body length. This difference in oxygen saturation was present for males and females; there were no significant gender differences at HA or LA.
Respiratory rate (breaths per minute [BPM]) decreased with increasing body length (P < 0.0001) and after adjusting for body length, there was no significant difference for subjects at HA versus LA (29.7 BPM vs. 29.5 BPM; P = 0.13). Males had a very small, but statistically significant higher respiratory rate compared to females (30.6 BPM vs. 28.8 BPM; P < 0.05); however, the interaction term for gender by altitude was not significant (P = 0.265).
Functional Residual Capacity
Individual values of FRC versus body length for male and female subjects at HA and LA are presented in Figure 1. All four groups demonstrated a significant increase in FRC with increasing body length. When groups were compared adjusting for body length, subjects at HA had significantly greater FRC compared to subjects at LA (group mean: 209 ml vs. 157 ml; P < 0.0001). There was also a significant interaction term for altitude by gender (P < 0.036). When analyzed by ANCOVA for gender specific effect adjusting for body length, males at HA had a significantly greater FRC compared to males at LA (group mean: 223 ml vs. 144 ml; P-value < 0.001); however, the difference in FRC for females at HA compared to females at LA did not achieve significance (group mean: 196 ml vs. 168 ml; P-value = 0.101). Males at HA also had a significantly greater FRC compared to females at HA (group mean: 223 ml vs. 196 ml; P = 0.021); however, there was not a significant difference in FRC for females at LA compared to males at LA (group mean: 168 ml vs. 144 ml; P = 0.411).
Fig. 1.
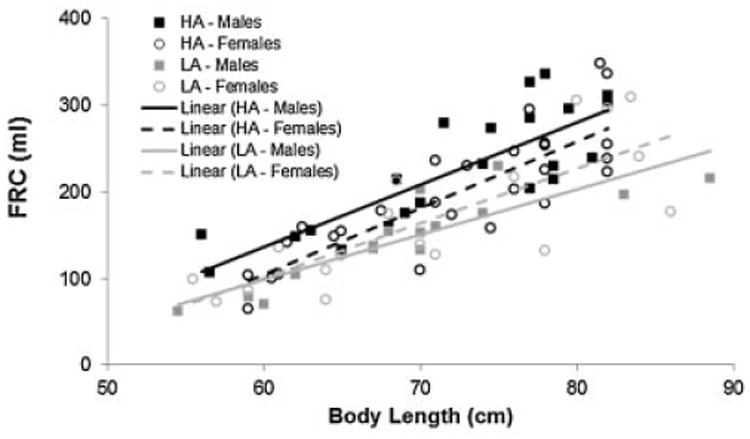
Functional residual capacity (FRC) for infants and toddlers versus body length (cm) for males and females at high altitude (HA) and low altitude (LA). FRC adjusted for body length was significantly greater for infants and toddlers at HA compared to LA (P < 0.0001). When analyzed by gender, males at HA had significantly greater lung volumes compared to males at LA (P < 0.0001), as well as females at HA (P < 0.021). However, there were no significant differences in lung volumes for females at HA compared to females at LA (P = 0.1008), nor females at LA versus males at LA (P = 0.4109).
Serum VEGF and EPO
Mean values of log VEGF and log EPO for subjects at HA and LA are presented in Figure 2A and B. Log VEGF was significantly higher for infants and toddlers at HA compared to LA (0.137; P < 0.0026) after adjusting for body length; gender was not significant (P = 0.371). Log EPO was also significantly higher for infants and toddlers at HA compared to LA (0.34; P < 0.032) after adjusting for body length; gender was not significant (P = 0.361). Serum levels of VEGF and EPO were not associated with FRC adjusted for body length and gender.
Fig. 2.
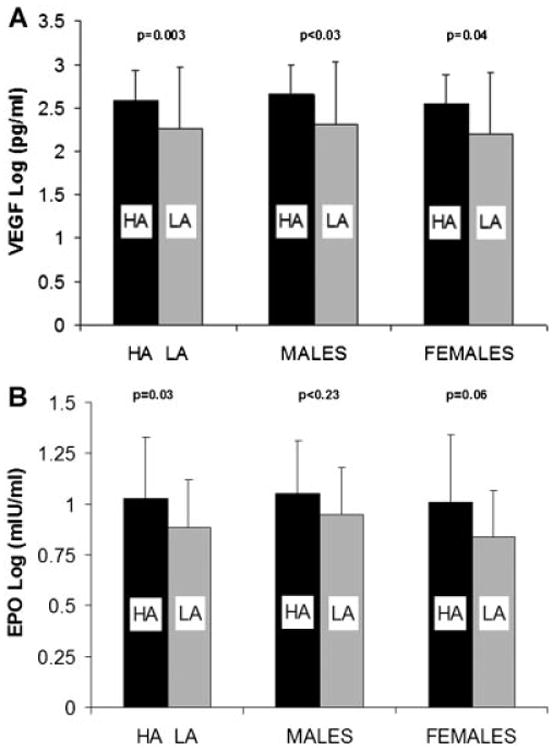
Comparison of vascular endothelial growth factor (VEGF) and erythropoietin (EPO) for infants and toddlers at high altitude (HA; black bars) compared to low altitude (LA; grey bars) for males and females. A: Serum levels of log VEGF were significantly higher for infants and toddlers at HA compared to LA. The effect of HA was present for both males and females analyzed separately, while there were no significant differences between males and females at HA or LA. B: Serum levels of log EPO were significantly higher for infants and toddlers at HA compared to LA. The effect of HA was present for males, while the difference for females approached statistical significance (P = 0.06). There were no significant differences between males and females at either HA or LA.
Discussion
Our study is the first to demonstrate that infants and toddlers residing at HA have increased lung volumes compared to subjects residing at LA. Previous studies have demonstrated that older children and adults born and raised at HA have larger lung volumes than subjects at sea level;1–5 however, our findings indicate that this response to chronic hypoxia starts very early in life. In addition, our results suggest a gender difference in the response to chronic hypoxia early in life. We also found that serum levels of VEGF and EPO were significantly higher in infants and toddlers at HA compared to subjects at LA. These results indicate that chronic hypoxia from residing at HA increases hypoxia inducible growth factors and lung growth very early in life.
For infants and toddlers born and residing at HA, FRC increased with increasing body length; however, after adjusting for body length, infants and toddlers at HA had significantly greater FRC compared to subjects at LA. Our findings in infants and toddlers are consistent with the increased lung volumes found in older children and adults at HA compared to those at LA, thus indicating that the effects of chronic hypoxia upon lung growth begin very early in life.1–5 Increased lung growth early in life is more likely to be secondary to an increase in alveolar number, rather than an increase in alveolar size; however, we are not aware of any human histologic data either in children or adults comparing lung parenchymal structure in subjects at HA versus LA. There are animal studies that demonstrate increased lung volume, pulmonary diffusing capacity, and surface area for gas exchange for animals raised at HA compared to animals raised at LA, and acinar structural changes that developed at HA persisted for years after animals were returned to LA.16–19 The infants we evaluated ranged in age from 1 to 24 months and we did not evaluate newborns. Therefore, we were not able to determine whether infants at HA had larger FRC at birth, although extrapolation of our data to a younger age suggests that lung volume might still be higher at an even younger age than we assessed. We are not aware of any previous studies assessing lung volumes in infants or newborns at HA; however, Mortola et al.20 compared the compliance of the respiratory system (CRS) of newborns at HA and LA. These investigators reported a 35% greater CRS in newborns at HA compared to newborns at LA, which is similar in magnitude to the increase in FRC that we found in our infants and toddlers at HA compared to LA. Therefore, the increased CRS as a newborn at HA may be secondary to an increased FRC that may have occurred in utero. HA infants may have a normal specific compliance (CRS/FRC), which would be consistent with the findings in adults that the pulmonary pressure volume curves obtained from adults at HA and LA do not differ when normalized for lung volume.21 Cumulatively, these studies indicate that subjects at HA have increased lung volumes very early in life and this effect may be initiated in utero.
We also found an interaction between Altitude and gender. When analyzed by gender, males at HA had greater lung volumes than males at LA; however, there was not a significant difference in lung volumes for females at HA versus females at LA. Males at HA also had larger lung volumes than females at HA; however, there was no gender difference in FRC at LA. Among older children and adults residing at sea-level, males have larger lung volumes than females after adjusting for body length; however, this gender difference does not appear to be present very early in life,22–24 which is consistent with our current findings at LA. While the FRC of female infants and toddlers tended to be higher at HA compared to LA, the difference was not statistically significant. As older female children and adults at HA have larger lung volumes than females at LA, chronic hypoxia does stimulate lung growth in females.1–5 A much larger sample size would probably have enabled us to detect a significant increase in FRC for female infants and toddlers at HA; however, the effect of chronic hypoxia upon lung volumes early in life was much more robust in males than females at HA, which does not appear to occur at LA. Adults at HA exhibit larger lung volumes relative to their airway size when compared to subjects at LA, which has been referred to as dysanaptic lung growth.21 Gender differences also produce dysanaptic lung growth, as females have smaller airways than males with the same lung volumes.25 Our findings suggest that chronic hypoxia at HA accelerates gender differences in dysanaptic lung growth very early in life.
As expected, our subjects at HA had significantly lower oxygen saturations compared to subjects at LA, which is expected and consistent with previous studies of infants and children at HA.26–29 However, neither our study nor previous studies found difference in oxygen saturation between male and female infants at HA. While the chronic hypoxia resulted in higher serum levels of VEGF and EPO compared to subjects at LA, there were not gender differences in the VEGF and EPO. Healthy newborns at sea level have been reported to have VEGF concentrations in cord blood that are higher than the levels in maternal serum. In addition, infant serum levels of VEGF at 1 and 4 days of age are higher than levels in cord blood, which suggests VEGF is produced by the neonate.30 Therefore, our observed higher serum levels of VEGF in infants at HA compared to infants at LA reflects an overall systemic response by the infant to chronic hypoxia and may account for the absence of significant correlations between these growth factors and FRC. Both VEGF and EPO are downstream of hypoxia inducible factor (HIF), which is an important regulator of the response to hypoxia, as well as an important modulator of lung growth.8–10 Rats exposed to chronic hypoxia have increased serum levels of VEGF, as well as increased VEGF within the lung tissue compared to rats exposed to normoxia.31 We were not able to directly assessed VEGF in the lungs of our infants, which may account for our not finding a significant correlation between serum VEGF and FRC.
Estrogen and androgen receptors are expressed in the lung and sex hormones are important determinants of lung growth and development during fetal and postnatal life. Estrogens, which have stimulatory effects on lung maturation,32,33 can induce angiogenesis by the promotion of VEGF expression,34–36 and can modulate alveologenesis.37–40 While serum levels of estrogen and androgen are very low during infancy, these hormones are present and we are not aware of studies that have evaluated whether sex hormones or their receptors are altered early in life under conditions of chronic hypoxia. While we found that chronic hypoxia produced an increase in the lung volumes for male, but not female infants at HA, our study is not able to determine whether the increase in lung volume is associated with increased angiogenesis and increased pulmonary surface area for diffusion early in life.
There are several limitations to our study. Our measurements of FRC were obtained using the bias flow open-circuit washout technique that we have previously demonstrated provides similar FRC values as a closed circuit helium equilibration technique in this very young age group.11 This methodology provided the advantages of using a few, simple components that were easily transported between the LA site and the remote HA site in the Andes. The technique does not monitor tidal volume during the washout, as there is no pneumotachometer in the circuit; however, not using a pneumotachometer eliminates the potential problems with adjusting for differences in the physical properties of the gases at HA versus LA. Our measurements were obtained while subjects were sleeping with chloral hydrate sedation, which is commonly used in this very young age group, who cannot actively cooperate in performing pulmonary function tests. Chloral hydrate does not appear to significantly affect FRC, although it can have a small affect upon respiratory rate and tidal volume.41–43
In summary, we found that infants and toddlers at HA have lower oxygen saturations, higher serum levels of VEGF and EPO, and higher FRC compared to subjects at LA. In addition, although males and females infants and toddlers at sea level do not differ in FRC, we found that male, but not female infants and toddlers at HA demonstrated significantly larger FRC than similar gender subjects at LA. Chronic hypoxia from residing at HA appears to generate a more robust response in lung growth in males compared to females early in life. Detailed studies of newborns followed longitudinally are required to evaluate the relative contributions of the in utero and postnatal environments upon lung growth and development. Understanding the mechanisms that contribute to the increased lung volumes with chronic hypoxia, as well as gender differences early in life, may provide strategies to target compensatory lung growth in infants with lung disease,44 as well as account for gender related differences in lung diseases from infancy to adulthood.45,46
Acknowledgments
This study was supported by NIH—Fogarty International Center Research Grant (1R03TW007807) “Lung Growth in Infants and Toddlers Residing at High Altitude” and NIH R01 HL54062 “Growth of Airways and Lung Parenchyma in Normal Infants.”
Funding source: NIH and Fogarty International Center Research Grant, Numbers: 1R03TW007807, R01 HL54062.
Footnotes
Conflict of interest: None.
References
- 1.Droma T, McCullough RG, McCullough RE, Zhuang JG, Cymerman A, Sun SF, Sutton JR, Moore LG. Increased vital and total lung capacities in Tibetan compared to Han residents of Lhasa (3,658 m) Am J Phys Anthropol. 1991;86:341–351. doi: 10.1002/ajpa.1330860303. [DOI] [PubMed] [Google Scholar]
- 2.Frisancho A. Developmental adaptation to high altitude hypoxia. Int J Biometeorol. 1997;21:135–146. doi: 10.1007/BF01553707. [DOI] [PubMed] [Google Scholar]
- 3.Frisancho AR, Frisancho HG, Albalak R, Villain M, Vargas E, Soria R. Developmental, genetic, and environmental components of lung volumes at high altitude. Am J Hum Biol. 1997;9:191–203. doi: 10.1002/(SICI)1520-6300(1997)9:2<191::AID-AJHB5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Greksa LP, Spielvogel H, Caceres E. Total lung capacity in young highlanders of Aymara ancestry. Am J Phys Anthropol. 1994;94:477–486. doi: 10.1002/ajpa.1330940404. [DOI] [PubMed] [Google Scholar]
- 5.Mueller WH, Schull VN, Schull WJ, Soto P, Rothhammer F. A multinational Andean genetic and health program: growth and development in an hypoxic environment. Ann Hum Biol. 1978;5:329–352. doi: 10.1080/03014467800002981. [DOI] [PubMed] [Google Scholar]
- 6.DeGraff AC, Jr, Grover RF, Johnson RL, Jr, Hammond JW, Jr, Miller JM. Diffusing capacity of the lung in Caucasians native to 3,100 m. J Appl Physiol. 1970;29:71–76. doi: 10.1152/jappl.1970.29.1.71. [DOI] [PubMed] [Google Scholar]
- 7.de Bisschop C, Kiger L, Marden MC, Ajata A, Huez S, Faoro V, Martinot JB, Naeije R, Guénard H. Pulmonary capillary blood volume and membrane conductance in Andeans and low-landers at high altitude: a cross-sectional study. Nitric Oxide. 2010;23:187–193. doi: 10.1016/j.niox.2010.05.288. [DOI] [PubMed] [Google Scholar]
- 8.Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med. 2011;183:152–156. doi: 10.1164/rccm.201009-1393PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon D, Ponka P, Prchal JT. Hypoxia 5. Hypoxia and hematopoiesis. Am J Physiol Cell Physiol. 2011;300:C1215–C1222. doi: 10.1152/ajpcell.00044.2011. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell PH. Hypoxia-inducible factor as a physiological regulator. Exp Physiol. 2005;90:791–797. doi: 10.1113/expphysiol.2005.030924. [DOI] [PubMed] [Google Scholar]
- 11.Tepper RS, Asdell S. Comparison of helium dilution and nitrogen washout measurements of functional residual capacity in infants and very young children. Pediatr Pulmonol. 1992;13:250–254. doi: 10.1002/ppul.1950130414. [DOI] [PubMed] [Google Scholar]
- 12.Morris MG, Gustafsson P, Tepper R, Gappa M, Stocks J. The bias flow nitrogen washout technique for measuring the functional residual capacity in infants. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. Eur Respir J. 2001;17:529–536. doi: 10.1183/09031936.01.17305290. [DOI] [PubMed] [Google Scholar]
- 13.Gerhardt T, Hehre D, Feller R, Reifenberg L, Bancalari E. Serial determination of pulmonary function in infants with chronic lung disease. J Pediatr. 1987;110:448–456. doi: 10.1016/s0022-3476(87)80516-4. [DOI] [PubMed] [Google Scholar]
- 14.Tepper RS, Merth IT, Newth CJ, Gerhardt T, Stocks J, Sly P, Tepper R, Morgan W. Infant Respiratory Function Testing. New York: John Wiley & Sons; 1999. Measurement of functional residual capacity in infants by helium dilution and nitrogen washout techniques; pp. 165–190. [Google Scholar]
- 15.Castile RG, Iram D, McCoy KS. Gas trapping in normal infants and in infants with cystic fibrosis. Pediatr Pulmonol. 2004;37:461–469. doi: 10.1002/ppul.10446. [DOI] [PubMed] [Google Scholar]
- 16.Hsia CC, Carbayo JJ, Yan X, Bellotto DJ. Enhanced alveolar growth and remodeling in Guinea pigs raised at high altitude. Respir Physiol Neurobiol. 2005;147:105–115. doi: 10.1016/j.resp.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Hsia CCW, Johnson RL, Jr, McDonough P, Dane DM, Hurst MD, Fehmel JL, Wagner HE, Wagner PD. Residence at 3,800-m altitude for 5 mo in growing dogs enhances lung diffusing capacity for oxygen that persists at least 2.5 years. J Appl Physiol. 2007;102:1448–1455. doi: 10.1152/japplphysiol.00971.2006. [DOI] [PubMed] [Google Scholar]
- 18.Ravikumar P, Bellotto DJ, Johnson RL, Hsia CCW. Permanent alveolar remodeling in canine lung induced by high-altitude residence during maturation. J Appl Physiol. 2009;107:1911–1917. doi: 10.1152/japplphysiol.00552.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson RL, Jr, Cassidy SS, Grover RF, Schutte JE, Epstein RH. Functional capacities of lungs and thorax in beagles after prolonged residence at 3,100 m. J Appl Physiol. 1985;59:1773–1782. doi: 10.1152/jappl.1985.59.6.1773. [DOI] [PubMed] [Google Scholar]
- 20.Mortola JP, Rezzonico R, Fisher JT, Villena-Cabrera N, Vargas E, Gonzales R, Pena F. Compliance of the respiratory system in infants born at high altitude. Am Rev Respir Dis. 1990;142:43–48. doi: 10.1164/ajrccm/142.1.43. [DOI] [PubMed] [Google Scholar]
- 21.Brody JS, Lahiri S, Simpser M, Motoyama EK, Velasquez T. Lung elasticity and airway dynamics in Peruvian natives to high altitude. J Appl Physiol. 1977;42:245–251. doi: 10.1152/jappl.1977.42.2.245. [DOI] [PubMed] [Google Scholar]
- 22.Dezateux C, Stocks J, Dundas I, Fletcher ME. Impaired airway function and wheezing in infancy. Am J Respir Crit Care Med. 1999;159:403–410. doi: 10.1164/ajrccm.159.2.9712029. [DOI] [PubMed] [Google Scholar]
- 23.Tepper RS, Reister T. Forced expiratory flows and lung volumes in normal infants. Pediatr Pulmonol. 1993;15:357–361. doi: 10.1002/ppul.1950150608. [DOI] [PubMed] [Google Scholar]
- 24.Stocks J, Quanjer P. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J. 1995;8:492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 25.Sheel AW, Guenette JA, Yuan R, Holy L, Mayo JR, McWilliams AM, Lam S, Coxson HO. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J Appl Physiol. 2009;107:1622–1628. doi: 10.1152/japplphysiol.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamponia MJ, Babaali H, Yugar F, Gilman RH. Reference values for pulse oximetry at high altitude. Arch Dis Child. 1998;78:461–465. doi: 10.1136/adc.78.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuland DS, Steinhoff MC, Gilman RH, Bara M, Olivares EG, Jabra A, Finkelstein D. Prevalence and prediction of hypoxemia in children with respiratory infections in the Peruvian Andes. J Pediatr. 1991;119:900–906. doi: 10.1016/s0022-3476(05)83040-9. [DOI] [PubMed] [Google Scholar]
- 28.Niermeyer S, Shaffer EM, Thilo E, Corbin C, Moore LG. Arterial oxygenation and pulmonary arterial pressure in healthy neonates and infants at high altitude. J Pediatr. 1993;123:767–772. doi: 10.1016/s0022-3476(05)80857-1. [DOI] [PubMed] [Google Scholar]
- 29.Niermeyer S, Yang P, Shanmina Drolkar, Zhuang J, Moore L. Arterial oxygen saturation in Tibetan and Han infants born in Lhasa, Tibet. N Engl J Med. 1995;333:1248–1252. doi: 10.1056/NEJM199511093331903. [DOI] [PubMed] [Google Scholar]
- 30.Malamitsi-Puchner A, Tziotis J, Protonotariou E, Xyni K, Sarandakou A, Creatsas G. Heparin-binding angiogenic factors (basic fibroblast growth factor and vascular endothelial growth factor) in early neonatal life. Pediatr Res. 1999;45:877–880. doi: 10.1203/00006450-199906000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Christou H, Yoshida A, Arthur V, Morita T, Kourembanas S. Increased vascular endothelial growth factor production in the lungs of rats with hypoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol. 1998;18:768–776. doi: 10.1165/ajrcmb.18.6.2980. [DOI] [PubMed] [Google Scholar]
- 32.Boucher E, Provost PR, Devillers A, Tremblay Y. Levels of dihydrotestosterone, testosterone, androstenedione, and estradiol in canalicular, saccular, and alveolar mouse lungs. Lung. 2010;188:229–233. doi: 10.1007/s00408-010-9231-x. [DOI] [PubMed] [Google Scholar]
- 33.Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol. 2007;293:L272–L278. doi: 10.1152/ajplung.00174.2007. [DOI] [PubMed] [Google Scholar]
- 34.Applanat MP, Buteau-Lozano H, Herve MA, Corpet A. Vascular endothelial growth factor is a target gene for estrogen receptor and contributes to breast cancer progression. Adv Exp Med Biol. 2008;617:437–444. doi: 10.1007/978-0-387-69080-3_42. [DOI] [PubMed] [Google Scholar]
- 35.Fraser HM, Wilson H, Silvestri A, Morris KD, Wiegand SJ. The role of vascular endothelial growth factor and estradiol in the regulation of endometrial angiogenesis and cell proliferation in the marmoset. Endocrinology. 2008;149:4413–4420. doi: 10.1210/en.2008-0325. [DOI] [PubMed] [Google Scholar]
- 36.Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci USA. 2000;97:10972–10977. doi: 10.1073/pnas.200377097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patrone C, Cassel TN, Pettersson K, Piao YS, Cheng G, Ciana P, Maggi A, Warner M, Gustafsson JA, Nord M. Regulation of postnatal lung development and homeostasis by estrogen receptor beta. Mol Cell Biol. 2003;23:8542–8552. doi: 10.1128/MCB.23.23.8542-8552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massaro D, Clerch LB, Massaro GD. Estrogen receptor-{alpha} regulates pulmonary alveolar loss and regeneration in female mice: morphometric and gene expression studies. Am J Physiol Lung Cell Mol Physiol. 2007;293:L222–L228. doi: 10.1152/ajplung.00384.2006. [DOI] [PubMed] [Google Scholar]
- 39.Massaro D, Massaro GD. Estrogen receptor regulation of pulmonary alveolar dimensions: alveolar sexual dimorphism in mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L866–L870. doi: 10.1152/ajplung.00396.2005. [DOI] [PubMed] [Google Scholar]
- 40.Massaro D, Massaro GD. Estrogen regulates pulmonary alveolar formation, loss, and regeneration in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1154–L1159. doi: 10.1152/ajplung.00228.2004. [DOI] [PubMed] [Google Scholar]
- 41.Jackson EA, Rabbette PS, Dezateux C, Hatch DJ, Stocks J. The effect of triclofos sodium sedation on respiratory rate, oxygen saturation, and heart rate in infants and young children. Pediatr Pulmonol. 1991;10:40–45. doi: 10.1002/ppul.1950100109. [DOI] [PubMed] [Google Scholar]
- 42.Tepper RS, Morgan WJ, Cota K, Wright A, Taussig LM. Physiologic growth and development of the lung during the first year of life. Am Rev Respir Dis. 1986;134:513–519. doi: 10.1164/arrd.1986.134.3.513. [DOI] [PubMed] [Google Scholar]
- 43.Turner DJ, Morgan SE, Landau LI, LeSouef PN. Methodological aspects of flow-volume studies in infants. Pediatr Pulmonol. 1990;8:289–293. doi: 10.1002/ppul.1950080414. [DOI] [PubMed] [Google Scholar]
- 44.Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175:978–985. doi: 10.1164/rccm.200611-1660PP. [see comment] [Review] [78 refs] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carey MA, Card JW, Voltz JW, Arbes SJ, Jr, Germolec DR, Korach KS, Zeldin DC. It's all about sex: gender, lung development and lung disease. Trends Endocrinol Metab. 2007;18:308–313. doi: 10.1016/j.tem.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hampl V, Bibova J, Ostadalova I, Povysilova V, Herget J. Gender differences in the long-term effects of perinatal hypoxia on pulmonary circulation in rats. Am J Physiol Lung Cell Mol Physiol. 2003;285:L386–L392. doi: 10.1152/ajplung.00389.2002. [DOI] [PubMed] [Google Scholar]


