JAM-A and ALCAM mediate the transmigration of CD14+CD16+monocytes into the CNS of HIV seropositive individuals.
Keywords: junctional proteins, transmigration, AIDS
Abstract
Monocyte transmigration across the BBB is a critical step in the development of cognitive deficits termed HAND that affect 40–70% of HIV-infected individuals, even with successful antiretroviral therapy. The monocyte subsets that enter the CNS during HIV infection are not fully characterized. We examined PBMC from HIV-positive individuals from 2 distinct cohorts and enumerated monocyte populations, characterized their transmigration properties across an in vitro human BBB model, and identified surface proteins critical for the entry of these cells into the CNS. We demonstrated that the frequency of peripheral blood CD14+CD16+ and CD14lowCD16+ monocytes was increased in HIV-seropositive compared with -seronegative individuals, despite virologic control. We showed that CD14+CD16+ monocytes selectively transmigrated across our BBB model as a result of their increased JAM-A and ALCAM expression. Antibody blocking of these proteins inhibited diapedesis of CD14+CD16+ monocytes but not of T cells from the same HIV-infected people across the BBB. Our data indicate that JAM-A and ALCAM are therapeutic targets to decrease the entry of CD14+CD16+ monocytes into the CNS of HIV-seropositive individuals, contributing to the eradication of neuroinflammation, HAND, and CNS viral reservoirs.
Introduction
A spectrum of neurocognitive deficits, termed HAND, afflicts a large percentage of HIV-infected individuals and is not fully prevented or treated by cART [1]. The neuropathogenesis of HIV is propagated, in part, by the continual entry of monocytes into the CNS. HIV is detected in the CNS within the first 2 weeks of primary infection and is believed to enter upon the migration of infected peripheral blood monocytes into the brain parenchyma [2–4]. Once within the CNS, a neuroinflammatory cascade is initiated that results in the neuronal damage and loss associated with the cognitive deficits of HIV. Even with successful viral control by antiretroviral therapy, low-level neuroinflammation continues to persist in HIV-infected individuals [5, 6].
A mature subset of monocytes that expresses cell-surface CD16, the FcγIIIR, is highly susceptible to HIV and can be detected within the brains of HIV-positive people [7–9]. This CD16 subset can be divided into 2 monocyte populations defined as CD14+CD16+ and CD14lowCD16+ [10]. These cells can be a source of viral replication and represent a reservoir that persists and correlates with cognitive decline, even in cART-treated individuals [11, 12]. CD16+ cells comprise only 5–10% of the monocytes in seronegative people but are increased in the blood of HIV-infected individuals [13, 14]. We previously developed an in vitro monocyte maturation culture system to study CD16+ cells and determined that the CD14+CD16+ subset selectively transmigrated across our model of the human BBB [15]. We showed that the junctional proteins JAM-A (also known as CD321), ALCAM (also known as CD166), PECAM-1 (also known as CD31), and CD99 were increased on the monocytes from our culture system and mediated their diapedesis across the BBB [16].
In our present work, we analyzed peripheral blood from HIV-infected individuals from 2 distinct cohorts and demonstrated that CD16+ monocytes were present to a much greater extent than previously reported compared with uninfected subjects. These cells were consistently higher in seropositive individuals, irrespective of cART status, viral load, CD4 T cell count, and gender. We examined the transmigration of monocytes from the seropositive individuals across an in vitro model of the human BBB and determined that the CD14+CD16+ subset preferentially transmigrated compared with other subpopulations. These cells isolated from HIV-infected individuals expressed increased surface JAM-A, ALCAM, and PECAM-1 compared with those without HIV. JAM-A and ALCAM were critical for the diapedesis of CD14+CD16+ monocytes, but not of T cells, as antibody blocking specifically decreased the numbers that migrated across the BBB. Our findings indicate that JAM-A and ALCAM are novel, therapeutic targets to limit viral seeding of the brain and neuroinflammation mediated by ongoing monocyte entry into the CNS of HIV-infected individuals. Additionally, our work has implications for peripheral inflammatory disorders mediated by CD14+CD16+ monocytes that disproportionately affect seropositive people, such as cardiovascular disease [17].
MATERIALS AND METHODS
Blood samples and study participants
Blood from HIV-seronegative participants was obtained from deidentified donors or from leukopaks from the New York Blood Center (New York, NY, USA), according to established protocols at the Albert Einstein College of Medicine (Bronx, NY, USA). Blood from HIV-seropositive participants was obtained through the MHBB (U01MH083501 and U24MH100931), a research resource operating at the Icahn School of Medicine at Mount Sinai (New York, NY, USA), and the WIHS (Bronx, NY, USA). HIV-positive individuals in both studies were assayed for CD4 cell counts, plasma viral loads, and cART therapy at the time of blood draw. Patient demographic and virologic information are listed in Table 1.
TABLE 1.
Demographic and immunovirologic characteristics of HIV-infected participants
| MHBB (n = 56), mean ± sd | WIHS (n = 42), mean ± sd | |
|---|---|---|
| Patient demographics | ||
| Age, yr | 55 ± 7 | 48 ± 7 |
| % Female | 48 | 100 |
| % Racial/ethnic minority | 85 | 89 |
| Immunovirologic information | ||
| CD4 T cell count, cells/μl | 467.3 ± 318.9 | 553.7 ± 287.1 |
| Plasma HIV RNA, log copies/ml | 2.15 ± 1.26 | 2.36 ± 1.25 |
| % with Undetectable viral loads | 80 | 65 |
| % on cART | 100 | 64 |
Patients gave written, informed consent for the provision of blood for the purposes of HIV research before inclusion in the current study. The protocol under which these samples were obtained is approved by the Institutional Review Board at Montefiore Medical Center, the Albert Einstein College of Medicine, and the Mount Sinai Program for the Protection of Human Subjects Institutional Review Board.
M-CSF and soluble junctional protein analysis
Peripheral blood was collected into EDTA or heparin-coated collection tubes (BD Biosciences, San Jose, CA, USA). Plasma was separated from the blood by centrifugation. The plasma was collected, divided into aliquots, and stored at −80°C. There were no freeze-thaw cycles before analysis. Plasma was assayed for M-CSF and sALCAM by use of sandwich-capture ELISA, according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN, USA). To detect sJAM-A, we developed a sandwich ELISA by use of the following reagents from R&D Systems: goat anti-human JAM-A (1 μg/ml) at 4°C overnight as capture antibody, biotinylated goat anti-human JAM-A (0.1 μg/ml) at 37°C for 2 h as detection antibody, and recombinant human JAM-A Fc chimera for the standard curve. All samples were measured in duplicates. The limits of detection were 10, 80, and 63 pg/ml for M-CSF, sALCAM, and sJAM-A, respectively.
Cell isolation
After removing the plasma, PBMCs were isolated by use of Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden) and density gradient centrifugation. PBMCs were isolated within 4 h of blood draw. We performed our analyses on freshly isolated blood rather than cryopreserved samples, as CD16+ monocytes have been shown to decrease upon thawing.
Flow cytometry
PBMC cell-surface markers were analyzed by flow cytometry by use of fluorochrome-coupled mAb specific for human CD14 (clone M5E2), CD16 (clone 3G8), CD3 (clone HIT3a), CD19 (clone HIB19), CD56 (clone B159), CD66b (clone G10F5), HLA-DR (clone G46-6), JAM-A (clone M. Ab F11), ALCAM (clone 105902), PECAM-1 (clone WM59), CD99 (clone TU12), and CCR2 (clone 48607) or corresponding isotype-matched, negative control antibodies (all from BD Biosciences; except ALCAM and CCR2; R&D Systems). Antibodies were titrated to determine optimal concentrations for staining. PBMC (2–5 × 105) were washed once with FACS buffer [calcium- and magnesium-free PBS (Gibco, Grand Island, NY, USA), supplemented with 1% BSA (Thermo Scientific, Waltham, MA, USA)]. PBMCs were incubated in the dark on ice for 30 min with the appropriate antibodies. Following staining, PBMCs were washed once with FACS buffer and fixed with 2% paraformaldehyde.
Between 1 and 4 × 105 events were acquired with a BD FACSCanto II flow cytometer and analyzed by use of FlowJo software (v 10.0.6; TreeStar, Ashland, OR, USA). We acquired as many events as possible to ensure adequate numbers of cells for analysis, as monocytes represent only ∼5–10% of PBMCs, and CD16+ monocytes constitute an even smaller fraction of the PBMC. Forward- and side-scatter were used to determine monocyte gating (Fig. 1A). Monocytes were identified as CD14 and HLA-DR positive and CD3, CD19, CD56, and CD66b negative. Monocytes were defined according to surface CD16 expression (CD16+; Fig. 1B), followed by identification of each monocyte subset as CD14+CD16−, CD14+CD16+, and CD14lowCD16+ (Fig. 1C). T cells were identified as CD3 positive and CD19, CD56, CD66b, and CD14 negative. Neutrophils were identified as CD66b positive and CD3, CD19, and CD56 negative.
Figure 1. CD16+ monocytes are increased in the peripheral blood of HIV-infected individuals.
PBMCs from HIV-seropositive and -seronegative individuals were analyzed by flow cytometry. FACS plots from 1 representative individual is shown. (A) Forward- and side-scatter areas (FSC-A and SSC-A, respectively) characteristics were used to determine appropriate monocyte gating. Monocytes were identified as CD14- and HLA-DR-positive and CD3-, CD19-, CD56-, and CD66b-negative. (B) Monocytes were defined according to surface CD16 expression (CD16+). (C) CD16+ monocytes were discriminated further based on the relative expression of CD14. The CD16+ cells that expressed high levels of CD14 were denoted as CD14+CD16+ (blue), and those that had lower amounts were indicated as CD14lowCD16+ (red). The remainder of monocytes did not express CD16 and were identified as CD14+CD16− (green).
In vitro model of the human BBB
Human BMVECs were from Applied Cell Biology Research Institute (Kirkland, WA, USA). Cortical astrocytes were obtained as part of an ongoing, approved research protocol at the Albert Einstein College of Medicine. The in vitro human BBB model consists of coculturing the BMVEC and astrocytes on opposite sides of a tissue-culture insert (BD Falcon; Becton Dickinson, Franklin Lakes, NJ, USA) with 3 μm pores, as described previously [18, 19]. The astrocyte endfeet processes penetrated the insert through the pores to make contact with the BMVEC. The cocultures grow to confluence over 3 d to establish a tight barrier that expresses many markers consistent with the human BBB, including glucose transporter 1. Our in vitro model has high transendothelial electrical resistance and impermeability to albumin and tritiated inulin. The cocultures were used for transmigration assays 3 d after they were established.
Transmigration assay across the BBB model
PBMC (4 × 105) from HIV-seropositive individuals from the WIHS cohort were added to the top of each tissue coculture insert to assay transmigration across the BBB. Media alone, CCL2 (200 ng/ml; Invitrogen, Grand Island, NY, USA), or CXCL12 (100 ng/ml; R&D Systems) were added to the bottom of the coculture chamber. For blocking experiments, 20 μg/ml blocking antibody to JAM-A (clone J10.4; Santa Cruz Biotechnology, Santa Cruz, CA, USA), ALCAM (clone 81; Antigenix America, Huntington Station, NY, USA), or mouse IgG1 (MP Biomedical, Solon, OH, USA) was added to the top of the cocultures concurrently with the PBMC. Each transmigration condition was performed with 4 replicate cocultures. After 24 h, the cells that transmigrated were collected from the bottom of the chamber; immunostained for CD14-allophycocyanin, CD16-PE, and CD3-FITC; fixed with 2% paraformaldehyde; and quantified by flow cytometry. The percent of each leukocyte subset that transmigrated across the BBB, relative to its representation in the total population of cells being added to the coculture (i.e., the percentage of input cells), was determined. The optimal concentrations of CCL2 and CXCL12 and the duration of the transmigration assay were determined by dose response and kinetic analysis, respectively.
Transmigration assays were performed with cells isolated from women in the WIHS cohort, as we did not receive enough blood to perform these experiments with donors from the MHBB cohort. While we were unable to determine the effects of gender on transmigration by use of cells from HIV-positive individuals, we did not observe any differences in monocyte or T cell transmigration properties with cells derived from male or female HIV-seronegative donors (data not shown).
Statistical analysis
Statistical analyses were performed by use of Prism 6.0 software (GraphPad Software, San Diego, CA, USA). Two-tailed paired t-test or Wilcoxon signed-rank test was used to determine statistical significance (P ≤ 0.05); *P ≤ 0.05, **P ≤ 0.01, and ***P < 0.001 for all statistical analyses performed in this study.
RESULTS
Cohort characteristics
The study consisted of a total of 98 HIV-seropositive individuals from the MHBB and the Bronx/Manhattan WIHS cohorts (Table 1). The participants in the 2 cohorts did not differ with respect to race/ethnicity, CD4+ T cell count, or plasma viral load; however, the participants were significantly younger in the WIHS cohort (MHBB: 55 ± 7 yr; WIHS: 48 ± 7 yr; *P < 0.001). The MHBB cohort was 48% female, whereas the WIHS cohort was solely comprised of women. A portion of the WIHS participants was naive to cART.
CD16+ monocytes are increased in frequency in HIV-seropositive individuals
CD16+ monocytes are critical to the neuropathogenesis of HIV, as they promote neuroinflammation and facilitate infection of the CNS. To characterize these cells in the peripheral blood of HIV-seropositive and -seronegative individuals, PBMCs were isolated from fresh whole blood, stained for CD14 and CD16, and analyzed by flow cytometry. Monocytes were identified by forward- and side-scatter characteristics (Fig. 1A). Monocytes that expressed CD16 were greatly increased in frequency in the blood of HIV-infected individuals compared with individuals without HIV (Fig. 1B). CD16+ monocytes are heterogeneous and can be discriminated by their relative amounts of surface CD14. Some CD16+ monocytes express high amounts of CD14 (CD14+CD16+), whereas others have lower levels (CD14lowCD16+). Both CD16+ populations were present in greater frequencies in HIV-positive compared with HIV-negative people (Fig. 1C). For this reason, the CD14+CD16+ and CD14lowCD16+ monocyte subpopulations were pooled when characterizing the frequency of CD16+ monocytes.
The percent of CD16+ monocytes was significantly increased in the blood of HIV-positive individuals from the MHBB (37.7 ± 2.9%) and WIHS (33.7 ± 2.0%) cohorts compared with people without HIV (5.6 ± 0.8%; ***P < 0.001; Fig. 2A). The frequency of CD16+ monocytes did not differ among individuals receiving cART therapy (33.3 ± 2.6%) or in those naive to antiretrovirals (36.9 ± 3.1%; P > 0.05; Fig. 2B). There was no correlation between the percent of CD16+ monocytes and viral load (Fig. 2C; P > 0.05) or CD4 T cell count (Fig. 2D; P > 0.05). The percent of CD16+ monocytes did not vary with respect to gender (Fig. 2E). To identify a soluble factor that may contribute to the increase in the frequency of these monocytes, M-CSF, a monocyte maturation factor [20], was analyzed in the plasma of 20 HIV-negative and 80 -infected individuals. Compared with HIV-negative control subjects (65.5 ± 9.1 pg/ml), plasma M-CSF was increased significantly in HIV-positive participants from the MHBB (152.5 ± 24.3 pg/ml; **P < 0.01) and WIHS cohorts (138.9 ± 22.04 pg/ml; *P < 0.05; Fig. 2F).
Figure 2. The frequency of CD16+ monocytes does not correlate with markers of viral pathogenesis.
The frequency of CD16+ monocytes, relative to the total monocyte population, was determined in PBMCs isolated from 28 HIV-negative and 87 HIV-positive individuals from the MHBB (48 people) and the WIHS (39 people) cohorts. (A) CD16+ monocytes from HIV-seropositive individuals from the MHBB (squares) and WIHS (triangles) cohorts were quantified and compared with people who were seronegative (circles). The frequency of CD16+ monocytes was determined for (B) individuals who were naive to cART (open circles) and those receiving cART (solid circles). Correlations between the percent of CD16+ monocytes and (C) viral load or (D) CD4 T cell count were determined. (E) The percent of CD16+ monocytes was quantified for women (circles) and men (squares) in the MHBB and WIHS cohorts. (F) Plasma from 20 people without HIV (circles), 40 people from the MHBB (squares), and 40 people from the WIHS (triangles) cohorts was analyzed for M-CSF by ELISA. Data are represented as mean ± sem. Significance was determined by use of Wilcoxon signed-rank test or an unpaired two-tailed t-test; *P < 0.05, **P < 0.01; ***P < 0.001.
CD14+CD16+ monocytes from HIV-positive individuals selectively transmigrate across our model of the human BBB
The monocyte populations that enter into the CNS of HIV-infected individuals are not fully characterized. To determine the transmigration properties of each monocyte subset, PBMCs from seropositive subjects were added to our in vitro model of the human BBB and allowed to transmigrate in response to media alone or CCL2 for 24 h. Transmigrated cells were recovered and analyzed by flow cytometry. The percent of CD14+CD16−, CD14+CD16+, and CD14lowCD16+ monocytes that migrated across the BBB was determined.
CCL2 promoted a significant increase in the numbers of CD14+CD16+ monocytes that crossed the BBB (27.7 ± 2.8%), relative to that which occurred in media alone (9.7 ± 1.1%; ***P < 0.001; Fig. 3A and B; blue). CD14+CD16+ cells were primed to cross the BBB and comprised the majority of monocytes that transmigrated, even under baseline conditions, compared with the other subsets that transmigrated to a lesser extent (Fig. 3A and B). CCL2 was also chemotactic for CD14+CD16− cells (Fig. 3A; green), although the numbers of this population that migrated in response to the chemokine (1.7 ± 0.2%) were significantly less than CD14+CD16+ monocytes (27.7 ± 2.8%; ***P < 0.001; Fig. 3B). CD14lowCD16+ monocytes did not respond to CCL2 (Fig. 3B; red) as a result of low expression of the receptor for the chemokine, CCR2 (Fig. 3C).
Figure 3. CD14+CD16+ monocytes preferentially transmigrate across the BBB.
PBMCs from 27 HIV-infected individuals from the WIHS cohort were added to our in vitro model of the human BBB and allowed to transmigrate for 24 h in response to 200 ng/ml CCL2. After transmigration, the percent of monocytes that transmigrated, relative to the number of cells added to the BBB model, was calculated. (A) Dot plots demonstrate the monocyte populations before and after transmigration for 1 representative individual. (B) The transmigration of CD14+CD16− (green), CD14+CD16+ (blue), and CD14lowCD16+ (red) monocytes across the BBB in response to CCL2 (solid bars) or media alone (open bars) was determined. (C) CCR2 was analyzed on CD14+CD16+ (blue), CD14+CD16− (green), and CD14lowCD16+ (red) monocytes before transmigration across the BBB. Data are represented as mean ± sem. Significance was determined by two-tailed paired t-test; *P < 0.05, ***P < 0.001.
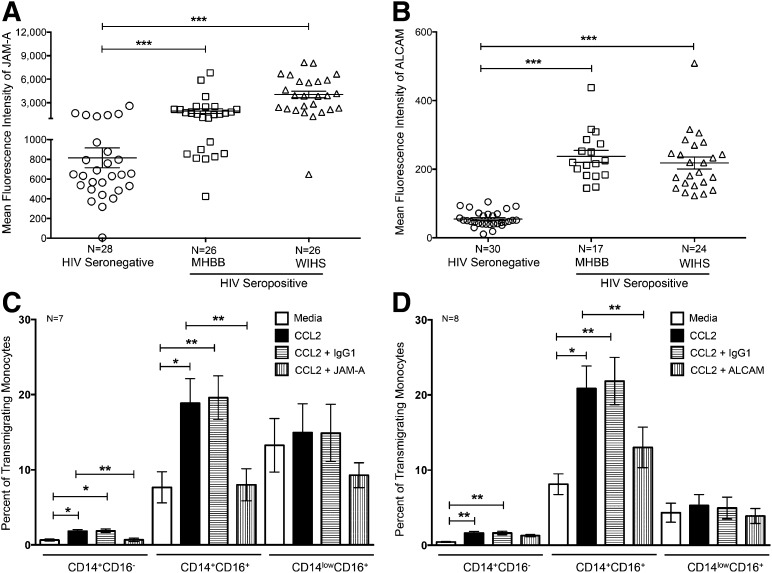
JAM-A and ALCAM are increased on CD14+CD16+ monocytes from HIV-infected people and are critical to the transmigration of these cells across the BBB
We determined previously that the junctional proteins that interact homotypically with endothelial cells and monocytes were increased on CD14+CD16+ monocytes during in vitro monocyte maturation [16]. To determine whether these junctional proteins were dysregulated during HIV infection, we characterized JAM-A, ALCAM, PECAM-1, and CD99 by flow cytometry on CD14+CD16+ monocytes from individuals with and without HIV. JAM-A (Fig. 4A) and PECAM-1 (Fig. 5A) were highly expressed on CD14+CD16+ monocytes from seronegative individuals, whereas ALCAM (Fig. 4B) and CD99 (Fig. 5B) were present to a lesser extent. There was a significant increase in JAM-A (Fig. 4A), ALCAM (Fig. 4B), and PECAM-1 (Fig. 5A) on CD14+CD16+ monocytes from HIV-infected individuals in the MHBB (JAM-A: 1944 ± 294.7; ALCAM: 237.5 ± 17.58; PECAM-1: 733.7 ± 87.6) and WIHS (JAM-A: 4053 ± 419.8; ALCAM: 218.2 ± 17.4; PECAM-1: 857.1 ± 81.8) cohorts compared with HIV-negative people (JAM-A: 814.6 ± 100.3, ***P < 0.001; ALCAM: 54.61 ± 4.1, ***P < 0.001; PECAM-1: 347.6 ± 40.2, ***P < 0.001). The increased expression of JAM-A, ALCAM, and PECAM-1 on CD14+CD16+ monocytes occurred in HIV-seropositive individuals, irrespective of antiretroviral therapy, viral load, CD4 T cell count, or gender (data not shown). There was no change in CD99 with respect to HIV status (Fig. 5B). JAM-A and ALCAM were also present on CD14+CD16− cells, although to a significantly lesser extent than on CD14+CD16+ monocytes [JAM-A, **P < 0.01; ALCAM, ***P < 0.001 (data not shown)]; in contrast, these proteins were barely detectable on CD14lowCD16+ monocytes (data not shown).
Figure 4. JAM-A and ALCAM mediate transmigration of CD14+CD16+ monocytes across the BBB.
PBMCs from 28–30 HIV-negative and 41–52 HIV-positive people (17–26 people MHBB; 24–26 people WIHS) were analyzed by flow cytometry. The mean fluorescence intensities of (A) JAM-A and (B) ALCAM were determined on CD14+CD16+ monocytes isolated from people without HIV (circles) and seropositive individuals from the MHBB (squares) and WIHS (triangles) cohorts. (C and D) PBMCs from 7–8 individuals with HIV were added to our in vitro model of the human BBB in the presence of JAM-A, ALCAM, or isotype-matched control antibodies and allowed to transmigrate for 24 h in response to media (open bars) or CCL2 (solid bars) in the presence or absence of blocking antibodies to (C) JAM-A and (D) ALCAM (vertical hatching) or isotype-matched control antibody (horizontal hatching). Data are represented as mean ± sem. Significance was determined by Wilcoxon signed-rank test or a two-tailed paired t-test; *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 5. PECAM-1 is increased on CD14+CD16+ monocytes in HIV-infected individuals.
PBMCs from 27 HIV-negative and 39 HIV-positive people (22 MHBB; 17 WIHS) were stained with antibodies to CD14, CD16, PECAM-1, and CD99 and analyzed by flow cytometry. After subtracting the contribution of the isotype-matched negative control antibody, the mean fluorescence intensity of (A) PECAM-1 and (B) CD99 was determined on CD14+CD16+ monocytes from people without HIV (circles) and in those with HIV from the MHBB (squares) and WIHS (triangles) cohorts. Data are represented as mean ± sem. Significance was determined by two-tailed paired t-test; **P < 0.01, ***P < 0.001.
To examine the contribution of these junctional proteins to monocyte migration across the BBB, transmigration assays were performed in the presence of blocking antibodies to JAM-A, ALCAM, or an isotype-matched control. Blocking JAM-A or ALCAM decreased the CCL2-mediated transmigration of CD14+CD16+ monocytes to baseline levels (Fig. 4C and D). An irrelevant isotype-matched, negative control antibody did not affect monocyte transmigration (Fig. 4C and D). The inhibitory effects of JAM-A- and ALCAM-blocking antibodies on CD14+CD16+ monocyte transmigration occurred in HIV-seropositive individuals, irrespective of antiretroviral therapy, viral load, or CD4 T cell count (data not shown). These studies demonstrate the importance of JAM-A and ALCAM in mediating the transmigration of CD14+CD16+ monocytes from HIV-infected individuals across the BBB. We were unable to determine whether JAM-A and ALCAM mediated monocyte diapedesis uniquely during HIV infection by use of cells from seronegative donors, as CD14+CD16+ monocytes were present in such few numbers in the PBMC of these individuals (see Fig. 2A). Whereas enumeration by flow cytometry was feasible, the numbers of these cells that transmigrated were so low that quantification of transmigration was not reliable (data not shown).
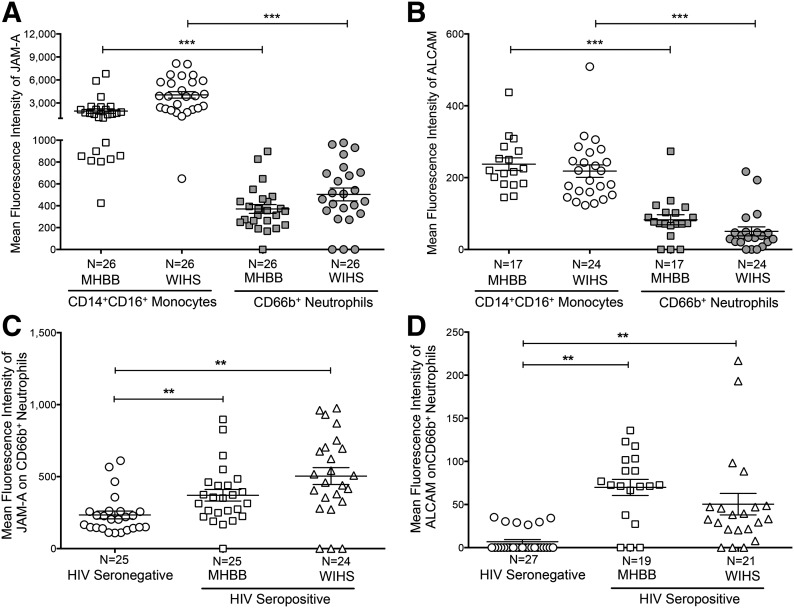
T cells and neutrophils express significantly less JAM-A and ALCAM than CD14+CD16+ monocytes
JAM-A and ALCAM may also be expressed on other leukocyte subsets. We characterized these junctional proteins on CD3+ T cells and CD66b+ neutrophils from the same individuals whose monocytes we analyzed. Whereas T cells from HIV-seropositive individuals expressed JAM-A (Fig. 6A and C) and ALCAM (Fig. 6B and D), these junctional proteins were present at significantly lower levels on the lymphocytes from individuals in the MHBB (JAM-A: 234.9 ± 14.1; ALCAM: 52 ± 3.4) and WIHS cohorts (JAM-A: 262.1 ± 15.0; ALCAM: 36 ± 1.8) compared with CD14+CD16+ monocytes (MHBB–JAM-A: 1944 ± 294.7, ***P < 0.001; ALCAM: 237.5 ± 17.6, ***P < 0.001; WIHS–JAM-A: 4053 ± 419.8, ***P < 0.001; ALCAM: 218.2 ± 17.4, ***P < 0.001). Neutrophils also expressed significantly less JAM-A (MHBB: 370.6 ± 40.1; WIHS: 503.8 ± 58.5) and ALCAM (MHBB: 82.8 ± 13.4; WIHS: 50.4 ± 12.5) on their cell surface, relative to CD14+CD16+ monocytes (MHBB–JAM-A: 1944 ± 294.7, ***P < 0.001; ALCAM: 237.5 ± 17.6, ***P < 0.001; WIHS–JAM-A: 4053 ± 419.8, ***P < 0.001; ALCAM: 218.2 ± 17.4, ***P < 0.001; Fig. 7A and B). There was no difference in JAM-A expression on T cells isolated from individuals with or without HIV (Fig. 6E). In contrast, ALCAM was increased significantly on T cells from those with HIV (MHBB: 52 ± 3.4; WIHS: 36 ± 1.8) compared with people without HIV (18.2 ± 2.5, **P < 0.01; Fig. 6F). JAM-A (MHBB: 370.6 ± 40.1; WIHS: 503.8 ± 58.5) and ALCAM (MHBB: 69.8 ± 9.4; WIHS: 50.4 ± 12.5) were increased on neutrophils during HIV infection (JAM-A: 233.7 ± 27, **P < 0.01; ALCAM: 6.9 ± 2.5, **P < 0.01; Fig. 7C and D).
Figure 6. CD3+ T cells express less JAM-A and ALCAM than CD14+CD16+ monocytes.
PBMCs from 14 HIV-negative and 41–52 HIV-positive individuals (17–26 MHBB; 24–26 WIHS) were analyzed by flow cytometry. (A and B) FACS data represented as histograms show the junctional protein expression from 1 representative individual. The mean fluorescent intensity of (A) JAM-A and (B) ALCAM (open peaks) or that obtained with the isotype-matched negative control antibodies (solid peaks) is indicated. (C and D) The mean fluorescence intensity of (C) JAM-A and (D) ALCAM was determined on CD14+CD16+ monocytes (open shapes) and CD3+ T cells (solid shapes) from HIV-infected individuals in the MHBB (squares) and WIHS (triangles) cohorts. (E) JAM-A and (F) ALCAM expression was determined on CD3+ T cells from HIV-negative (circles) and -positive people from the MHBB (squares) and WIHS (triangles) cohorts. Data are represented as mean ± sem. Significance was determined by a two-tailed paired t-test; **P < 0.01, ***P < 0.001.
Figure 7. CD66b+ neutrophils express less JAM-A and ALCAM than CD14+CD16+ monocytes.
PBMCs from HIV-negative and -positive individuals (from MHBB and WIHS) were isolated; stained for CD66b, CD14, CD16, JAM-A, and ALCAM; and analyzed by flow cytometry. After subtracting the contribution of the isotype-matched negative control antibodies, the mean fluorescence intensities of (A) JAM-A and (B) ALCAM were determined on CD14+CD16+ monocytes (open shapes) and CD66b+ neutrophils (solid shapes) from HIV-infected individuals in the MHBB (squares) and WIHS (circles) cohorts. (C) JAM-A and (D) ALCAM expression on CD66b+ neutrophils was determined on cells isolated from HIV-positive individuals in the MHBB (squares) and WIHS (triangles) cohorts and HIV-negative individuals (circles). Data are represented as mean ± sem. Significance was determined by a two-tailed paired t-test; **P < 0.01, ***P < 0.001.
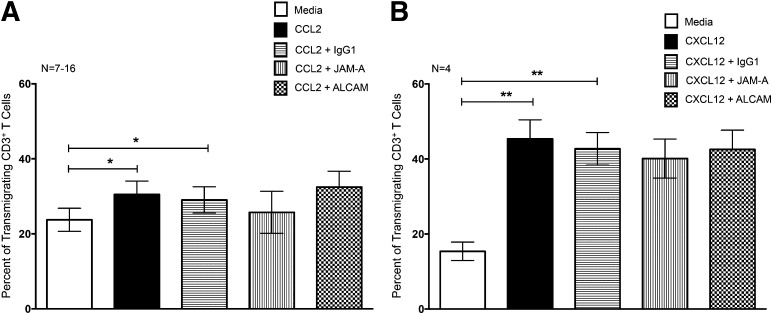
JAM-A and ALCAM do not mediate T cell transmigration across the BBB
To determine whether JAM-A and ALCAM mediated specifically monocyte migration, or if instead, these proteins were also involved in diapedesis for other cell types, we examined their contribution to T cell transmigration across the BBB for the same individuals for whom we determined monocyte migration. CCL2 promoted a small but significant increase in T cell transmigration across the BBB (30.5 ± 3.6%) compared with that which occurred for media alone (23.7 ± 3%; *P < 0.05; Fig. 8A). Antibodies to JAM-A or ALCAM did not affect the CCL2-mediated transmigration across the BBB (Fig. 8A). CXCL12, a potent lymphocyte chemoattractant [21], promoted a large increase in the number of T cells that transmigrated across the BBB (45.4 ± 5%), compared with media (15.4 ± 2.5%; **P < 0.01; Fig. 8B). These antibodies also failed to inhibit CXCL12-induced migration (Fig. 8B). These findings suggest that JAM-A and ALCAM are not critical for chemokine-mediated diapedesis of T cells across the BBB in the context of HIV.
Figure 8. JAM-A and ALCAM do not contribute to CD3+ T cell transmigration across the BBB.
PBMCs from 4–16 HIV-infected people were added to our in vitro model of the human BBB and allowed to transmigrate for 24 h in response to 200 ng/ml CCL2 or 100 ng/ml CXCL12. After transmigration, the cells were collected, stained for CD3, and quantified by flow cytometry. The percent of CD3+ T cells that transmigrated across the BBB in response to (A) CCL2 or (B) CXCL12 (solid bars) or media alone (open bars) was determined. Chemokine-mediated transmigration in response to (A) CCL2 or (B) CXCL12 was performed in the presence and absence of blocking antibodies to JAM-A (vertical hatching), ALCAM (checkered hatching), or isotype-matched negative controls (horizontal hatching). Data are represented as mean ± sem. Significance was determined by two-tailed paired t-test. *P < 0.05, **P < 0.01.
DISCUSSION
The neurologic consequences associated with HIV infection persist, despite the advances of cART in suppressing viral replication. Monocytes are key mediators that contribute to the neuropathogenesis of HIV, as these cells shepherd virus into the brain and initiate a cascade of neuroinflammatory events upon entry into the CNS parenchyma [9]. There are currently no successful therapeutic strategies targeting monocytes, despite overwhelming evidence for their role in HAND. This is, at least in part, as the monocyte subpopulation that mediates NeuroAIDS and the molecules that facilitate migration of these cells into the brain have not been fully characterized. We demonstrated that CD16+ monocytes were elevated in seropositive individuals despite virologic control and that increased, circulating M-CSF may contribute to the dysregulated frequency of these cells. We characterized CD14+CD16+ cells as the monocyte population that may contribute directly to HAND by selectively transmigrating across an in vitro model of the human BBB. We also demonstrated that the junctional proteins JAM-A and ALCAM specifically mediate diapedesis of CD14+CD16+ monocytes, but not of T cells, across the BBB.
Our finding that mature CD16+ monocytes are increased in HIV infection is in agreement with what we [16] and others [4, 7, 13, 14] determined previously. Our current work describes the novel finding that these CD16+ cells can comprise up to 80% of the total monocyte population in seropositive subjects. The identification of such high numbers of mature monocytes may be attributed, in part, to sensitive multicolor flow cytometry. For our analyses, we acquired up to 4 × 105 cells, as the total monocyte population (all CD14+ cells) represented only 7.5 ± 3.6% (n = 75) of the PBMC. At a frequency of 0.7 ± 0.5% (n = 75), CD16+ monocytes constituted an even smaller fraction of the PBMC, and therefore, we acquired as many events as possible to ensure adequate numbers of cells for analysis. While it is possible that the large numbers of CD16+ monocytes characterized in our study may also be attributed to the composition of our cohorts, which were predominantly of a Black and Hispanic racial/ethnic background, this is not likely. While genetic differences undoubtedly exist between these individuals and those of Caucasian descent, we believe their contribution to the frequency of CD16+ monocytes is minimal, as we did not identify any differences in the numbers of each monocyte population among individuals of different races/ethnicities in our seronegative participants (data not shown). Additional factors that may contribute to the high frequencies of CD16+ monocytes include inflammatory comorbidities, such as hypertension and cardiovascular disease, and the age of participants comprising our cohorts [17, 22–26].
We determined that these high percentages of CD16+ monocytes are as likely to occur in well-controlled individuals with virologic suppression and in those with extensive viral production. CD16+ monocytes also remained present in high frequencies, irrespective of the CD4+ T count. These data are in agreement with work from others [27]. Whereas we did not examine CD163 in this study, this cell-surface marker is indicative of monocyte activation and AIDS progression and may be a potential biomarker of HAND [28]. When CD163 monocyte expression was examined in a study by others, it correlated with viral load and CD4+ T cell counts below 450 cells/μl [27].
Our findings demonstrate that active viral replication is not necessary to maintain the presence of these cells in the peripheral blood. Increased frequencies of CD16+ monocytes have also been identified in elite controllers [29], further indicating that control of HIV replication alone is not enough to hinder the amount of these mature monocytes. This suggests that once the immune activation that results in increased frequencies of CD16+ monocytes is initiated, it is difficult to reverse, even upon quiescence of viral production. Other contributors, consequent to the immune dysregulation of chronic, persistent (albeit nonreplicating) HIV, may also serve as a continual stimulus to CD16+ monocyte production. Whatever their source, it is important to note that CD16+ monocytes are highly susceptible to HIV [8] compared with CD16− monocytes and represent a potential source of virus that is not eradicated with successful cART. Productively and latently infected CD16+ monocytes can be detected in infected individuals, even after years of suppressive antiretroviral therapy [12, 30]. The increased percentage of CD16+ monocytes present in HIV-infected individuals may therefore contribute to viral seeding of the brain and other organs as well.
After circulating in the blood, monocytes transmigrate across endothelial vessels and migrate into tissues. During pathogenic and inflammatory conditions, additional monocytes are recruited in response to elevated chemokines that direct the cells to their point of entry across the vasculature [31]. The transmigration properties of each monocyte subset had not been fully characterized, especially in the context of the BBB, which consists of highly specialized BMVECs [32, 33]. We determined that CD14+CD16+ cells comprised the majority of monocytes that transmigrated across our BBB model under both baseline conditions and in response to the potent monocyte chemoattractant CCL2 that is increased during HAND.
The interactions between the junctional proteins present on the monocyte and those on the endothelial cells are critical for extravasation [31]. Therefore, we characterized some of these molecules on the cell surface of CD14+CD16+ monocytes present in seropositive and seronegative individuals, and determined that JAM-A, ALCAM, and PECAM-1 were increased significantly during HIV infection. There was no change in CD99, suggesting that a global increase in cell-adhesion molecules did not occur during HIV infection but instead, that expression of only select mediators was changed. These findings were consistent among participants from both cohorts, despite interdonor variability. The increases in JAM-A, ALCAM, and PECAM-1 did not correlate with cART status, viral load, CD4 T cell count, and gender (data not shown), suggesting that these molecules may be dysregulated in many seropositive individuals with diverse states of health and virologic control.
In addition to being cell associated, these junctional molecules may be shed from the cell surface to generate soluble forms of the proteins [34–36]. In contrast to that which occurred with the cell-associated form of the junctional proteins, there was a significant decrease in sALCAM in the plasma of individuals with HIV (MHBB: 6524 ± 1475 pg/ml; WIHS: 4881 ± 1330 pg/ml) compared with uninfected people (16,849 ± 1667 pg/ml; Fig. 9A). There was no difference in sJAM-A with respect to HIV status (Fig. 9B). sALCAM and sJAM-A were shown to inhibit significantly leukocyte adhesion and transendothelial migration across endothelial cells [36–38]. The maintenance and decrease of sJAM-A and sALCAM, respectively, in the plasma may serve as additional mechanisms to enable the transmigration of CD14+CD16+ monocytes during HIV infection.
Figure 9. sALCAM is significantly decreased in the plasma of HIV-infected individuals.
Plasma was separated from whole blood isolated from HIV-positive individuals (squares and triangles) and HIV-negative people (circles) and (A) sALCAM and (B) sJAM-A analyzed by ELISA. Data are represented as mean ± sem. Significance was determined by two-tailed paired t-test. ***P < 0.001.
We hypothesize that the elevated levels of JAM-A and ALCAM on CD14+CD16+ monocytes in HIV-infected individuals promote more efficient migration of these cells into the brain. JAM-A and ALCAM were essential for CCL2-mediated transmigration of CD14+CD16+ monocytes across our BBB model, as antibody blockade inhibited diapedesis to baseline levels but not below. This suggests that JAM-A may be an attractive therapeutic target for decreasing transmigration of CD14+CD16+ monocytes during HIV infection. ALCAM may also be of importance, as our work demonstrates that the blocking of this protein specifically decreases the transmigration of CD14+CD16+ monocytes across the BBB. In addition to facilitating diapedesis, ALCAM mediates T cell activation by its heterotypic interaction on APCs with CD6 on T cells [39, 40]. This interaction is such a critical step in stabilizing the immunologic synapse that CD6 blockade is of current interest in autoimmune diseases [41]. The therapeutic targeting of ALCAM may have additional benefits in HIV-infected people, in addition to decreasing neuroinflammation, by minimizing the efficiency of T cell activation and their subsequent infection.
The targeting of JAM-A and ALCAM by administration of human mAb or by alternative therapeutic approaches may reduce or delay the general neuroinflammation that contributes to the pathogenesis of HAND. Whereas these proteins were increased on CD14+CD16+ monocytes in nearly every participant examined in this study, it is not likely that HAND will present in all of these individuals. The blocking of JAM-A and ALCAM may best result in clinically feasible therapy when administered to certain individuals at first onset of evidence of neurologic disease, neuroinflammation, and/or HAND. It is important to evaluate the risks associated with targeting host immune responses, such as monocyte entry into the CNS. Whereas monocyte diapedesis into the brain parenchyma is associated with the neuropathogenesis of HIV, this process is also essential for mounting immune responses to pathogens. A balance between inhibiting deleterious inflammation while permitting advantageous immune surveillance must be maintained. This is critical when developing interventional strategies to limit HAND, as evidenced by the multiple sclerosis studies and the α4 integrin inhibitor Natalizumab, which was so effective in limiting T cell CNS entry that the John Cunningham virus reactivated in some individuals who developed a fatal, progressive multifocal leukoencephalopathy [42–46]. In our studies, it is important to note that antibodies to JAM-A and ALCAM blocked only to baseline, suggesting that constitutive surveillance would not be affected by therapeutically targeting these proteins.
To determine whether the increase in junctional proteins was specific to CD14+CD16+ monocytes, we also characterized JAM-A and ALCAM on T cells and neutrophils from the same individuals whose monocytes we examined. Similar to our findings with CD14+CD16+ monocytes, ALCAM was increased significantly on T cells and neutrophils in those with HIV compared with seronegative people. JAM-A was increased significantly on neutrophils from those with HIV but not on T cells. It is worth noting that JAM-A was expressed on both T cells and neutrophils from HIV-seronegative individuals, whereas ALCAM was not present on either of these leukocytes. This is consistent with the findings of others, as ALCAM is rarely expressed on T cells and neutrophils and has only been described to be present during activation [47, 48]. These findings suggest that the dysregulation of junctional molecules during HIV infection may occur on multiple cell types as a result of persistent immune activation. Although T cells and neutrophils from infected individuals expressed JAM-A and ALCAM, these proteins were present at significantly lower levels than that on CD14+CD16+ monocytes.
As JAM-A and ALCAM are dysregulated on T cells and neutrophils during HIV infection, we characterized their contribution to diapedesis across the BBB. T cells comprised a larger percentage of PBMCs added to our coculture model of the BBB compared with monocytes, but JAM-A- and ALCAM-blocking antibodies did not affect the chemokine-mediated diapedesis of these lymphocytes. This may be a consequence of the stoichiometric differences in expression of the junctional proteins between monocytes and T cells. Whereas less implicated in diapedesis across the brain vasculature compared with T cells, neutrophils have been shown to enter into the CNS after infection by other neurotropic viruses [49, 50] and during inflammatory states [51–53]. In our studies, however, the transmigration of these cells under baseline conditions or in response to CCL2 or CXCL12 was not consistent (data not shown). Therefore, we could not assess the contribution of JAM-A and ALCAM to their diapedesis. T cells and neutrophils are not implicated in promoting the neuropathogenesis of HIV, and our findings indicate that JAM-A and ALCAM may specifically mediate the entry of CD14+CD16+ monocytes into the CNS parenchyma of HIV-infected individuals.
Although our studies were focused on monocyte transmigration across the BBB, our findings provide implications for the preferential entry of CD14+CD16+ monocytes into other tissues as well, as CCL2 is also elevated in many inflammatory processes. Additionally, elevated levels of JAM-A and ALCAM are implicated in facilitating leukocyte recruitment and tissue damage in other disorders, such as hypertension [54] and atherosclerosis [55], which occur with increased incidences in HIV-infected individuals. Our work is also of interest in the context of aging, which is becoming an important complication of HIV infection. CD16+ monocytes are implicated in promoting inflammation during the normal process of aging [22] and during “inflammaging”—premature aging that occurs in younger seropositive individuals as a result of chronic immune activation [24, 56]. This suggests that the increased frequencies of CD16+ monocytes in seropositive people may not only contribute to viral dissemination throughout the body and HAND, but they may also exacerbate the inflammation associated with other chronic diseases. The decrease of the entry of these monocytes into peripheral tissues by JAM-A and/or ALCAM blockade may also limit other inflammatory processes in HIV-infected individuals, providing additional therapeutic benefits.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health Grants MH075679 and MH090958 (to J.W.B.) and MH080663 (to S.M.); Mount Sinai Institute for NeuroAIDS Disparities pilot funds (R25 MH080663 to D.W.W.; MH083501 and MH100931 to S.M.; DA025567 to J.W.B.; AI035004 and AI142590 to K.A.; and AI070117 to D.W.W.); United Negro College Fund/Merck Graduate Science Dissertation Fellowship (to D.W.W.); and the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (AI051519). Data in this manuscript were collected by the WIHS Collaborative Study Group with Dr. Kathryn Anastos (New York City/Bronx Consortium) and the MHBB with Dr. Susan Morgello. The authors thank the patients and staff of the MHBB and the WIHS; Drs. Eliseo A. Eugenin, Peter J. Gaskill, Jackie Coley, Bezawit Megra, Mike Veenstra, Matias Jaureguiberry, and Brad Poulos; the New York Blood Center; and Dr. Lydia Tesfa for valuable contributions to this project.
Glossary
- ALCAM
activated leukocyte cellular adhesion molecule
- BBB
blood brain barrier
- BMVEC
brain microvascular endothelial cell
- cART
combined antiretroviral therapy
- HAND
HIV-associated neurocognitive disorder
- JAM-A
junctional adhesion molecule A
- MHBB
Manhattan HIV Brain Bank
- s
soluble
- WIHS
Women’s Interagency HIV Study
AUTHORSHIP
D.W.W. and J.W.B. conceived of and designed the experiments. D.W.W. performed the experiments and wrote the manuscript. D.W.W., K.A., S.M., and J.W.B. analyzed the data and edited the manuscript.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Antinori A., Arendt G., Becker J. T., Brew B. J., Byrd D. A., Cherner M., Clifford D. B., Cinque P., Epstein L. G., Goodkin K., Gisslen M., Grant I., Heaton R. K., Joseph J., Marder K., Marra C. M., McArthur J. C., Nunn M., Price R. W., Pulliam L., Robertson K. R., Sacktor N., Valcour V., Wojna V. E. (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69, 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peluso M. J., Meyerhoff D. J., Price R. W., Peterson J., Lee E., Young A. C., Walter R., Fuchs D., Brew B. J., Cinque P., Robertson K., Hagberg L., Zetterberg H., Gisslén M., Spudich S. (2013) Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J. Infect. Dis. 207, 1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valcour V., Chalermchai T., Sailasuta N., Marovich M., Lerdlum S., Suttichom D., Suwanwela N. C., Jagodzinski L., Michael N., Spudich S., van Griensven F., de Souza M., Kim J., Ananworanich J.; RV254/SEARCH 010 Study Group (2012) Central nervous system viral invasion and inflammation during acute HIV infection. J. Infect. Dis. 206, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulliam L., Sun B., Rempel H. (2004) Invasive chronic inflammatory monocyte phenotype in subjects with high HIV-1 viral load. J. Neuroimmunol. 157, 93–98. [DOI] [PubMed] [Google Scholar]
- 5.Anthony I. C., Ramage S. N., Carnie F. W., Simmonds P., Bell J. E. (2005) Influence of HAART on HIV-related CNS disease and neuroinflammation. J. Neuropathol. Exp. Neurol. 64, 529–536. [DOI] [PubMed] [Google Scholar]
- 6.Harezlak J., Buchthal S., Taylor M., Schifitto G., Zhong J., Daar E., Alger J., Singer E., Campbell T., Yiannoutsos C., Cohen R., Navia B.; HIV Neuroimaging Consortium (2011) Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 25, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer-Smith T., Croul S., Sverstiuk A. E., Capini C., L’Heureux D., Régulier E. G., Richardson M. W., Amini S., Morgello S., Khalili K., Rappaport J. (2001) CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J. Neurovirol. 7, 528–541. [DOI] [PubMed] [Google Scholar]
- 8.Ellery P. J., Tippett E., Chiu Y. L., Paukovics G., Cameron P. U., Solomon A., Lewin S. R., Gorry P. R., Jaworowski A., Greene W. C., Sonza S., Crowe S. M. (2007) The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J. Immunol. 178, 6581–6589. [DOI] [PubMed] [Google Scholar]
- 9.Williams D. W., Eugenin E. A., Calderon T. M., Berman J. W. (2012) Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J. Leukoc. Biol. 91, 401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D. N., Leenen P. J. M., Liu Y.-J., MacPherson G., Randolph G. J., Scherberich J., Schmitz J., Shortman K., Sozzani S., Strobl H., Zembala M., Austyn J. M., Lutz M. B. (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116, e74–e80. [DOI] [PubMed] [Google Scholar]
- 11.Kusao I., Shiramizu B., Liang C. Y., Grove J., Agsalda M., Troelstrup D., Velasco V. N., Marshall A., Whitenack N., Shikuma C., Valcour V. (2012) Cognitive performance related to HIV-1-infected monocytes. J. Neuropsychiatry Clin. Neurosci. 24, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiramizu B., Ananworanich J., Chalermchai T., Siangphoe U., Troelstrup D., Shikuma C., De Grutolla V., Sithinamsuwan P., Praihirunkit P., Rattanamanee S., Valcour V.; SEARCH 001.1 Study Group (2012) Failure to clear intra-monocyte HIV infection linked to persistent neuropsychological testing impairment after first-line combined antiretroviral therapy. J. Neurovirol. 18, 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulliam L., Gascon R., Stubblebine M., McGuire D., McGrath M. S. (1997) Unique monocyte subset in patients with AIDS dementia. Lancet 349, 692–695. [DOI] [PubMed] [Google Scholar]
- 14.Han J., Wang B., Han N., Zhao Y., Song C., Feng X., Mao Y., Zhang F., Zhao H., Zeng H. (2009) CD14(high)CD16(+) rather than CD14(low)CD16(+) monocytes correlate with disease progression in chronic HIV-infected patients. J. Acquir. Immune Defic. Syndr. 52, 553–559. [DOI] [PubMed] [Google Scholar]
- 15.Buckner C. M., Calderon T. M., Willams D. W., Belbin T. J., Berman J. W. (2011) Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell. Immunol. 267, 109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams D. W., Calderon T. M., Lopez L., Carvallo-Torres L., Gaskill P. J., Eugenin E. A., Morgello S., Berman J. W. (2013) Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS ONE 8, e69270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemkens L. G., Bucher H. C. (2014) HIV infection and cardiovascular disease. Eur. Heart J. 35, 1373–1381. [DOI] [PubMed] [Google Scholar]
- 18.Eugenin E. A., Berman J. W. (2003) Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods 29, 351–361. [DOI] [PubMed] [Google Scholar]
- 19.Weiss J. M., Berman J. W. (1998) Astrocyte expression of monocyte chemoattractant protein-1 is differentially regulated by transforming growth factor beta. J. Neuroimmunol. 91, 190–197. [DOI] [PubMed] [Google Scholar]
- 20.Hume D. A., MacDonald K. P. A. (2012) Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 119, 1810–1820. [DOI] [PubMed] [Google Scholar]
- 21.Li M., Ransohoff R. M. (2008) Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog. Neurobiol. 84, 116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hearps A. C., Martin G. E., Angelovich T. A., Cheng W.-J., Maisa A., Landay A. L., Jaworowski A., Crowe S. M. (2012) Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 11, 867–875. [DOI] [PubMed] [Google Scholar]
- 23.Wrigley B. J., Shantsila E., Tapp L. D., Lip G. Y. H. (2013) CD14++CD16+ monocytes in patients with acute ischaemic heart failure. Eur. J. Clin. Invest. 43, 121–130. [DOI] [PubMed] [Google Scholar]
- 24.Hearps A. C., Martin G. E., Rajasuriar R., Crowe S. M. (2014) Inflammatory co-morbidities in HIV+ individuals: learning lessons from healthy ageing. Curr. HIV/AIDS Rep. 11, 20–34. [DOI] [PubMed] [Google Scholar]
- 25.Baker J. V., Hullsiek K. H., Singh A., Wilson E., Henry K., Lichtenstein K., Onen N., Kojic E., Patel P., Brooks J. T., Hodis H. N., Budoff M., Sereti I.; CDC SUN Study Investigators (2014) Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS 28, 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thienemann F., Sliwa K., Rockstroh J. K. (2013) HIV and the heart: the impact of antiretroviral therapy: a global perspective. Eur. Heart J. 34, 3538–3546. [DOI] [PubMed] [Google Scholar]
- 27.Fischer-Smith T., Tedaldi E. M., Rappaport J. (2008) CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res. Hum. Retroviruses 24, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdo T. H., Weiffenbach A., Woods S. P., Letendre S., Ellis R. J., Williams K. C. (2013) Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 27, 1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnan S., Wilson E. M. P., Sheikh V., Rupert A., Mendoza D., Yang J., Lempicki R., Migueles S. A., Sereti I. (2014) Evidence for innate immune system activation in HIV type 1-infected elite controllers. J. Infect. Dis. 209, 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giri M. S., Nebozyhn M., Raymond A., Gekonge B., Hancock A., Creer S., Nicols C., Yousef M., Foulkes A. S., Mounzer K., Shull J., Silvestri G., Kostman J., Collman R. G., Showe L., Montaner L. J. (2009) Circulating monocytes in HIV-1-infected viremic subjects exhibit an antiapoptosis gene signature and virus- and host-mediated apoptosis resistance. J. Immunol. 182, 4459–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenwood J., Heasman S. J., Alvarez J. I., Prat A., Lyck R., Engelhardt B. (2011) Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol. Appl. Neurobiol. 37, 24–39. [DOI] [PubMed] [Google Scholar]
- 32.Ballabh P., Braun A., Nedergaard M. (2004) The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 16, 1–13. [DOI] [PubMed] [Google Scholar]
- 33.Abbott N. J., Rönnbäck L., Hansson E. (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda K., Quertermous T. (2004) Molecular isolation and characterization of a soluble isoform of activated leukocyte cell adhesion molecule that modulates endothelial cell function. J. Biol. Chem. 279, 55315–55323. [DOI] [PubMed] [Google Scholar]
- 35.Bazzoni G. (2003) The JAM family of junctional adhesion molecules. Curr. Opin. Cell Biol. 15, 525–530. [DOI] [PubMed] [Google Scholar]
- 36.Koenen R. R., Pruessmeyer J., Soehnlein O., Fraemohs L., Zernecke A., Schwarz N., Reiss K., Sarabi A., Lindbom L., Hackeng T. M., Weber C., Ludwig A. (2009) Regulated release and functional modulation of junctional adhesion molecule A by disintegrin metalloproteinases. Blood 113, 4799–4809. [DOI] [PubMed] [Google Scholar]
- 37.Ostermann G., Fraemohs L., Baltus T., Schober A., Lietz M., Zernecke A., Liehn E. A., Weber C. (2005) Involvement of JAM-A in mononuclear cell recruitment on inflamed or atherosclerotic endothelium: inhibition by soluble JAM-A. Arterioscler. Thromb. Vasc. Biol. 25, 729–735. [DOI] [PubMed] [Google Scholar]
- 38.Masedunskas A., King J. A., Tan F., Cochran R., Stevens T., Sviridov D., Ofori-Acquah S. F. (2006) Activated leukocyte cell adhesion molecule is a component of the endothelial junction involved in transendothelial monocyte migration. FEBS Lett. 580, 2637–2645. [DOI] [PubMed] [Google Scholar]
- 39.Gimferrer I., Calvo M., Mittelbrunn M., Farnós M., Sarrias M. R., Enrich C., Vives J., Sánchez-Madrid F., Lozano F. (2004) Relevance of CD6-mediated interactions in T cell activation and proliferation. J. Immunol. 173, 2262–2270. [DOI] [PubMed] [Google Scholar]
- 40.Zimmerman A. W., Joosten B., Torensma R., Parnes J. R., van Leeuwen F. N., Figdor C. G. (2006) Long-term engagement of CD6 and ALCAM is essential for T-cell proliferation induced by dendritic cells. Blood 107, 3212–3220. [DOI] [PubMed] [Google Scholar]
- 41.Pinto M., Carmo A. M. (2013) CD6 as a therapeutic target in autoimmune diseases: successes and challenges. BioDrugs 27, 191–202. [DOI] [PubMed] [Google Scholar]
- 42.Tada H., Rappaport J., Lashgari M., Amini S., Wong-Staal F., Khalili K. (1990) Trans-activation of the JC virus late promoter by the tat protein of type 1 human immunodeficiency virus in glial cells. Proc. Natl. Acad. Sci. USA 87, 3479–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dubois V., Dutronc H., Lafon M. E., Poinsot V., Pellegrin J. L., Ragnaud J. M., Ferrer A. M., Fleury H. J. (1997) Latency and reactivation of JC virus in peripheral blood of human immunodeficiency virus type 1-infected patients. J. Clin. Microbiol. 35, 2288–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y., Bord E., Tompkins T., Miller J., Tan C. S., Kinkel R. P., Stein M. C., Viscidi R. P., Ngo L. H., Koralnik I. J. (2009) Asymptomatic reactivation of JC virus in patients treated with natalizumab. N. Engl. J. Med. 361, 1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jilek S., Mathias A., Canales M., Lysandropoulos A., Pantaleo G., Schluep M., Du Pasquier R. A. (2013) Natalizumab treatment alters the expression of T-cell trafficking marker LFA-1 α-chain (CD11a) in MS patients. Mult. Scler. 20, 837–842. [DOI] [PubMed] [Google Scholar]
- 46.Ransohoff R. M. (2007) Natalizumab for multiple sclerosis. N. Engl. J. Med. 356, 2622–2629. [DOI] [PubMed] [Google Scholar]
- 47.Bowen M. A., Patel D. D., Li X., Modrell B., Malacko A. R., Wang W. C., Marquardt H., Neubauer M., Pesando J. M., Francke U., Haynes B. F., Aruffo A. (1995) Cloning, mapping, and characterization of activated leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J. Exp. Med. 181, 2213–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fessler M. B., Malcolm K. C., Duncan M. W., Worthen G. S. (2002) A genomic and proteomic analysis of activation of the human neutrophil by lipopolysaccharide and its mediation by p38 mitogen-activated protein kinase. J. Biol. Chem. 277, 31291–31302. [DOI] [PubMed] [Google Scholar]
- 49.Hosking M. P., Liu L., Ransohoff R. M., Lane T. E. (2009) A protective role for ELR+ chemokines during acute viral encephalomyelitis. PLoS Pathog. 5, e1000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan X. T., Tumpey T. M., Kunkel S. L., Oakes J. E., Lausch R. N. (1998) Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Invest. Ophthalmol. Vis. Sci. 39, 1854–1862. [PubMed] [Google Scholar]
- 51.Gorina R., Lyck R., Vestweber D., Engelhardt B. (2014) β2 Integrin-mediated crawling on endothelial ICAM-1 and ICAM-2 is a prerequisite for transcellular neutrophil diapedesis across the inflamed blood-brain barrier. J. Immunol. 192, 324–337. [DOI] [PubMed] [Google Scholar]
- 52.Allen C., Thornton P., Denes A., McColl B. W., Pierozynski A., Monestier M., Pinteaux E., Rothwell N. J., Allan S. M. (2012) Neutrophil cerebrovascular transmigration triggers rapid neurotoxicity through release of proteases associated with decondensed DNA. J. Immunol. 189, 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wojkowska D. W., Szpakowski P., Ksiazek-Winiarek D., Leszczynski M., Glabinski A. (2014) Interactions between neutrophils, Th17 cells, and chemokines during the initiation of experimental model of multiple sclerosis. Mediators Inflamm. 2014, 590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H., Oliveira-Sales E. B., McBride F., Liu B., Hewinson J., Toward M., Hendy E. B., Graham D., Dominiczak A. F., Giannotta M., Waki H., Ascione R., Paton J. F., Kasparov S. (2012) Upregulation of junctional adhesion molecule-A is a putative prognostic marker of hypertension. Cardiovasc. Res. 96, 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmitt M. M. N., Megens R. T. A., Zernecke A., Bidzhekov K., van den Akker N. M., Rademakers T., van Zandvoort M. A., Hackeng T. M., Koenen R. R., Weber C. (2014) Endothelial junctional adhesion molecule-a guides monocytes into flow-dependent predilection sites of atherosclerosis. Circulation 129, 66–76. [DOI] [PubMed] [Google Scholar]
- 56.Martin G. E., Gouillou M., Hearps A. C., Angelovich T. A., Cheng A. C., Lynch F., Cheng W.-J., Paukovics G., Palmer C. S., Novak R. M., Jaworowski A., Landay A. L., Crowe S. M. (2013) Age-associated changes in monocyte and innate immune activation markers occur more rapidly in HIV infected women. PLoS One 8, e55279. [DOI] [PMC free article] [PubMed] [Google Scholar]