Abstract
Discussion of how bedside to bench stem cell transplant studies inform human GVHD biology.
The last few years have seen a transformation in the application of biomarkers to the field of hematopoietic stem cell transplantation (HSCT), in particular, in the area of graft-versus-host disease (GVHD). In pioneering studies, Paczesny and colleagues [1] used a broad screening strategy to study individuals post-transplant to identify key molecules strongly linked to clinically well-defined acute GVHD, refining their list of candidate molecules by repetitive analyses of large patient cohorts from different centers to arrive at a handful of reliable markers strongly associated with the risk of developing GVHD and determining its severity and associated mortality. Out of this has emerged suppression of tumorigenicity 2 (ST2) as the single-most accurate predictor of GVHD incidence severity and associated mortality that can predict GVHD risk and determine treatment responsiveness and the probability of survival in recipients of both standard and reduced intensity conditioning [1, 2]. Of equal importance to refining the prediction of this major transplant outcome, biomarkers have also illuminated critical elements of GVHD mechanisms. Interestingly the strong correlation of biomarkers with GVHD lies with their relationship to tissue injury, rather than relating to immunologic markers of the T cell-mediated alloimmune attack.
The paper by Brissot and colleagues [3] in this issue is therefore of particular interest, as it identifies a new GVHD risk factor that is inherently immunologic and complementary to the recently described tissue injury markers. In an attempt to identify cytokines and chemokines that might associate with GVHD, they screened the plasma of HSCT recipients against an array of 42 cytokines by use of a bead-based multiplex protein array technology (Luminex, Thermo Fisher Scientific, Waltham, MA) or an ELISA, correlating their findings with GVHD disease and severity in 109 patients receiving a reduced intensity conditioning regimen. Out of this screen, only 1 relevant marker emerged: CX3CL1, a chemokine shed from endothelial cells.
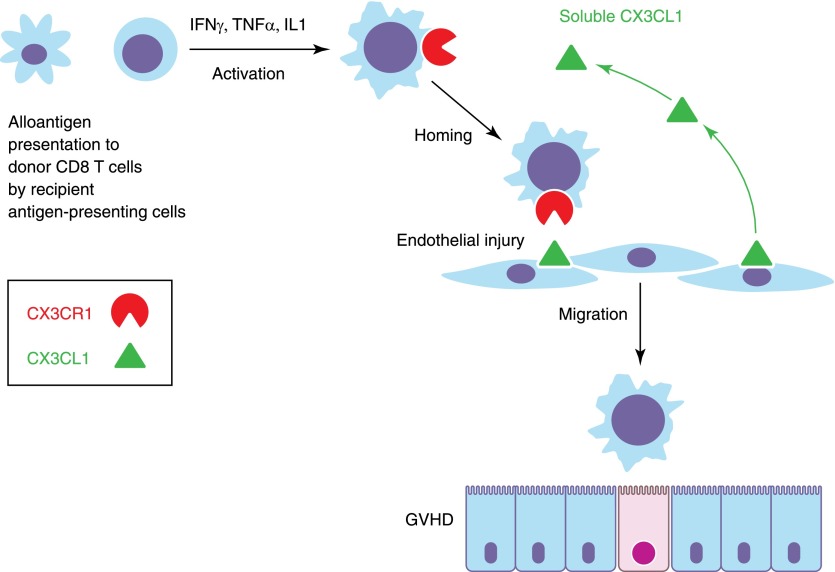
Between Days 0 and 50 after transplant, this cytokine correlated with incidence of grade II–IV acute GVHD. At later time-points, CX3CL1 lost its predictive significance, but presumably at that stage, GVHD had already occurred. The receptor for CX3CL1 (CX3CR1) occurs on CD8 T cells, and lymphocyte phenotyping by flow cytometry also revealed a significant association of CX3CR1 expression on CD8 cells with acute GVHD, where CX3CR1 expression was twice normal (60%) in GVHD patients. The importance of the CX3CL1/CX3CR1 axis in GVHD was strengthened by the finding that in GVHD tissue biopsies, there was both overexpression of CX3CL1 on endothelial cells and strong expression of CX3CR1 on contiguous mononuclear cells. As the first step in access of alloreactive T cells to the tissues is through the vascular endothelium (seen as vascular cuffing by T cells in early GVHD), the CX3CL1/CX3CR1 axis emerges as a critical step in T cell homing to the endothelium. Brissot and colleagues [3] propose that the conditioning regimen causes tissue injury, which increases CX3CL1 expression. This, in turn, induces CX3CR1 expression on CD8 T cells, setting off the homing of alloreacting donor cells to the tissues causing GVHD.
The implications of this work are profound. First, this bedside-to-bench approach has revealed an important component of the immune process that leads to acute GVHD. It should serve as a model of hypothesis-generating research in human stem cell transplantation (SCT), where only studies of multiple candidate molecules in large patient cohorts can hope to reveal patterns of association emerging out of the noise of competing predictors that characterize the polygenic diversity of human populations. The discovery of the CX3CR1/CX3CL1 axis fills in an important step in the path to acute GVHD, as illustrated in Fig. 1. Still unclear is what comes first: a damage response to the endothelium inducing up-regulation of the receptor on the T cells, creating the gateway for T cell arrival to the site of GVHD or alternatively, a genetically controlled variability in the propensity of donor CD8 T cells to up-regulate their CX3CR1? The latter question is of extreme interest, as it might be possible to predict GVHD by measuring CX3CR1 expression (in resting or activated CD8 T cells) by screening the donors. Likewise, recipients may also have varying propensities to generate and release CX3CL1 during tissue stress from conditioning regimens or the associated crossing of tissue boundaries by microorganisms during neutropenia. As ever, a crucial question that is raised by any GVHD mechanism is whether endothelial routing is also a key step in the induction of the graft-versus-leukemia effect in the bone marrow. Future studies clearly need to explore the genetic variations in expression of this critical receptor/ligand pair, the expression of CX3CR1 in donors, the factors that induce CX3CL1 in tissues, and the relationship of these findings with disease relapse.
Figure 1. Role of the CX3CL1/CX3CR1 axis in T cell homing to sites of GVHD.
Second, this new predictor for acute GVHD may further refine the increased accuracy of ST2 and other markers to identify whether an individual will develop clinically significant GVHD. CX3CL1 possesses many of the desiderata for an ideal biomarker—easy to assay in blood samples and able to predict outcome immediately post-SCT, allowing for appropriate maneuvering between GVHD prevention schedules. With an optimum set of markers, we can expect that predictive factors will become so reliable that they can be used prospectively to apply distinct but appropriate GVHD prevention strategies with great reliability.
Further studies will be needed to determine how universal and reliable a predictor the CX3CL1/CX3CR1 axis is in other transplant series under different conditioning regimen intensities, different degrees of donor matching, and alternative stem cell sources. Furthermore, more remains to be done to correlate CX3CL1 with established biomarkers. Will it further refine the already impressive ability of ST2 to predict acute GVHD with high precision and sensitivity, or will it disappear as an independent marker? In this regard, it will be of great importance to determine whether GVHD prediction can be pushed back before transplant by studying the donor’s T cells for CX3CR1 expression and the recipient's for the ligand.
Lastly, as the authors point out, the identification of this key point in the pathway of alloreacting T cells to reach target tissues opens the possibility of preventing acute GVHD with blocking agents to CX3CR1 or its ligand.
ACKNOWLEDGMENTS
J.B. is supported by the Intramural Research Program of the U.S. National Institutes of Health, National Heart, Lung, and Blood Institute.
Glossary
- CX3CR1
receptor for CX3CL1
- GVHD
graft-versus-host disease
- HSCT
hematopoietic stem cell transplantation
- SCT
stem cell transplantation
- ST2
suppression of tumorigenicity 2
Footnotes
SEE CORRESPONDING ARTICLE ON PAGE 227
REFERENCES
- 1.Vander Lugt M. T., Braun T. M., Hanash S., Ritz J., Ho V. T., Antin J. H., Zhang Q., Wong C. H., Wang H., Chin A., Gomez A., Harris A. C., Levine J. E., Choi S. W., Couriel D., Reddy P., Ferrara J. L., Paczesny S (2013) ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N. Engl. J. Med. 369, 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson R. P. Jr., Khawaja M. R., Perkins S. M., Elmore L., Mumaw C. L., Orschell C., Paczesny S (2014) Prognostic biomarkers for acute graft-versus-host disease risk following cyclophosphamide-fludarabine nonmyeloablative allotransplantation. Biol. Blood Marrow Transplant. 20, 1861–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brissot E., Bossard C., Malard F., Braudeau C., Chevallier P., Guillaume T., Delaunay J., Josien R., Gregoire M., Gaugler B., Mohty M. (2015) Involvement of the CX3CL1 (fractalkine)/CX3CR1 pathway in the pathogenesis of acute graft-versus-host disease. J. Leukoc. Biol. 97, 227–237. [DOI] [PubMed] [Google Scholar]