Molecular mechanisms identified by Pyk2 regulation and T cell receptor-induced functions in human T cells
Keywords: Akt, p85 phosphorylation
Abstract
TCR-induced signaling controls T cell activation that drives adaptive immunity against infections, but it can also induce dysfunctional T cell responses that promote pathologic disease. The PI3K pathway regulates many downstream effector responses after TCR stimulation. However, the molecular mechanisms that induce PI3K function downstream of the TCR are not fully understood. We have previously shown that Pyk2 is activated downstream of the TCR in a PI3K-independent manner. Although Pyk2 controls adhesion, proliferation, and cytokine production in T cells, the mechanisms by which it controls these processes are not known. In this study, we generated Pyk2-deficient human T cells to elucidate further the role that this kinase plays in TCR-induced effector functions and signaling. We observed that Pyk2 localized with the p85 regulatory subunit of PI3K at the LAT complex and that PI3K-dependent signaling was impaired in Pyk2-deficient T cells. Likewise, functions downstream of PI3K, including IFN-γ production and proliferation, were also suppressed in human T cells deficient in Pyk2. Collectively, these data demonstrate that Pyk2 is a critical regulator of PI3K function downstream of the TCR.
Introduction
T cells serve critical functions in the clearance of pathogens and the development of pathologic diseases in humans. To fight infectious organisms efficiently or to induce autoimmune or inflammatory diseases, the TCR expressed on the T cells must recognize its cognate peptide-bound MHC presented on an APC or infected target cell [1]. This interaction activates the TCR and initiates intracellular signaling. LAT and SLP-76 are critical adaptor proteins that serve as nucleation sites for intracellular signaling cascades activated by the TCR [1, 2]. PI3K is one protein that is recruited to the LAT/SLP-76 complex [3–7]. PI3K signaling activates kinases, including the Akt and Erk1/Erk2, and these proteins promote proinflammatory cytokine production, survival, and proliferation [8–16].
PI3K is comprised of a regulatory subunit and a catalytic subunit. To become activated, the regulatory subunit’s SH2 domains bind to membrane-associated phosphoproteins [17]. Upon TCR induction, p85-PI3K has been reported to bind to phosphorylated LAT and/or SLP-76, which facilitate PI3K activation [3–7]. However, it is not clear if p85-PI3K binds directly to these adaptor proteins, as none of the reported phosphotyrosine-binding sites on these proteins contains the consensus-binding motif for the SH2 domain(s) of p85-PI3K [18]. The catalytic activity of PI3K is enhanced when the regulatory subunit is tyrosine phosphorylated, and this phosphorylation is correlated with enhanced T cell proliferation [19, 20]. PI3K phosphorylation and function are induced via the activities of Lck and/or Fyn [20–22], although it is unknown if p85-PI3K is a direct Lck and/or Fyn substrate downstream of the TCR. Therefore, the precise mechanisms that control PI3K activity after TCR stimulation are not known.
TCR induction also leads to the Src family kinase-dependent, PI3K-independent phosphorylation of Pyk2 [23, 24]. We recently found that Pyk2 regulates TCR signaling events that drive T cell adhesion [25]. With the use of a combination of TCR and costimulatory receptor agonists, other groups have reported that Pyk2 is a critical regulator of adhesion, proliferation, and cytokine production in primary mouse and Jurkat T cells [26–28]. However, the mechanisms by which Pyk2 controls these processes were not fully investigated, and these studies did not clarify if Pyk2 regulates TCR-dependent function in the absence of costimulation. This is an intriguing question to address, as Pyk2 displays unusual TCR-inducible phosphorylation kinetics relative to other TCR signaling proteins in human T cells [23, 29]. Thus, we investigated if Pyk2 controlled TCR-induced effector responses in human T cells. We also characterized how TCR signaling was altered in the absence of Pyk2.
In this manuscript, we investigated the function of Pyk2 in TCR-dependent signaling and T cell function. We observed that proliferation and IFN-γ production, but not IL-2 release and CD69 up-regulation, were impaired after TCR stimulation in Pyk2-deficient human T cells. Interestingly, proximal signaling events that led to LAT phosphorylation were normal in these cells, whereas SLP-76 phosphorylation and PI3K-dependent signaling were impaired whenthe expression or catalytic function of Pyk2 was reduced. Thus, Pyk2 is a critical regulator of select PI3K-mediated functions induced downstream of TCR stimulation.
MATERIALS AND METHODS
Ethics statement
All experiments using primary human T cells were conducted in accordance with the Declaration of Helsinki. Discarded blood products were obtained from the DeGowin Blood Center at the University of Iowa (Iowa City, IA, USA). Anonymous blood donors had provided written consent for their unused blood products to be used in research projects. This consent form has been reviewed and approved by the Institutional Review Board at the University of Iowa. The cells provided to the investigators in this study were completely de-identified.
Plasmids
The sequences for the luciferase and Pyk2-specific miRNAs have been described previously [25]. These sequences were cloned into the pENTR-miR30 expression vector as described previously [30] or into the EcoRI and XhoI cut sites in the MCSV-LMP retroviral packing vector (donated by Dr. Bruce Hostager, University of Iowa).
Virus production
293T cells were maintained in complete DMEM (10% FBS, 2 mM L-glutamine, 50 U/ml penicillin-50 μg/ml streptomycin). Cells were cultured at 1 × 106 cells/ml in 6-well tissue-culture plates and then transfected with 2 μg of the MCSV-LMP vectors that contained the luciferase or Pyk2-specific miRNAs, 2 μg of the pCL-ECO packaging vector, and 2 μg of the vesicular stomatitis virus glycoprotein envelope vector by use of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). After 48 h, the viral supernatants were concentrated by use of the Lenti-X concentrating solution (Clontech, Mountain View, CA, USA), per the manufacturer’s instructions. This concentrated virus was used to transduce HuT78 T cells.
Transduction or transfection of HuT78 T cells
HuT78 T cells were grown at a density of 5–7 × 105 cells/ml in complete IMDM (20% FBS, 2 mM L-glutamine, 50 U/ml penicillin-50 μg/ml streptomycin). To generate HuT78 T cell lines that stably expressed the miRNAs, 0.5 × 106 cells were infected with the concentrated retroviruses in the presence of 8 μg/ml polybrene (Sigma-Aldrich, St. Louis, MO, USA) for 24 h at 37°C and then transferred to fresh complete IMDM containing 1.0 μg/ml puromycin (Santa Cruz Biotechnologies, Santa Cruz, CA, USA). The resulting pools of transduced cells were derived from multiple cellular clones that stably expressed these miRNAs. For transient transfections, 12 × 106 cells were transfected with 7 μg Luc or Pyk2 miRNA-containing pENTR-miR30 expression vectors by use of the Lonza Nucleofector Solution R and the Amaxa program V-001. This process was repeated 6–7 h later.
Primary CD4 T cell isolation and transfection
CD4+ T cells were isolated from PBMCs and activated as described previously [31]. After 3–5 days, the stimulatory beads and IL-2 were removed. CD4+ hAPBTs (10 × 106) were then transfected with 5 μg Luc or Pyk2-specific miRNA plasmid by use of the Lonza Nucleofector Kit R and the Amaxa program X-001. These cells were incubated in complete RPMI media for 3 days and then used in experiments where indicated.
Cell viability assays
Equal numbers of control or Pyk2-deficient HuT78 T cells, obtained from different transfections or different stable cell-line passages, were resuspended in complete IMDM, 1 day after transfection. Cell viability was determined by trypan blue exclusion, and live and dead cells were counted by use of a hemocytometer. Likewise, equal numbers of control or Pyk2-deficient CD4 hAPBTs were resuspended in complete RPMI in the presence of 0 or 1 μg/ml immobilized anti-CD3 antibody. Three days later, viable and nonviable cells in each of these samples were counted as described above. To mitigate the differences between human donors and transfections, these values were normalized as described in the figure legends.
Flow cytometry
Control and Pyk2-deficient CD4 hAPBTs were stimulated by cross-linked anti-CD3 and anti-CD4 (clone RPA-T4; BioLegend, San Diego, CA, USA) antibodies, as described [31]. After 18 h, these cells were washed with FACS buffer (PBS with 5% FBS and 0.05% sodium azide). Surface staining was conducted by use of PE-Cy5-labeled anti-CD69 antibody (BioLegend) or the labeled isotype control. Samples were collected by use of an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA). The MFI of each sample was determined by use of CFlow 6 software. To lessen the variability in CD69 expression between human donors, the data were normalized by use of the following formula:
 |
The average percentage ± sem from 3 experiments was then calculated and graphed.
Cytokine production
Cells (3–5 × 105) were stimulated by use of immobilized anti-CD3 antibody for 24 h [31]. Culture supernatants were collected, and IL-2 and IFN-γ production was measured by use of a standard tetramethylbenzidine peroxidase ELISA, as described previously [32]. The ELISA antibodies were purchased from eBioscience (San Diego, CA, USA). The streptavidin-HRP was from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). The data were normalized by use of the formula below, and the mean of 4–5 independent experiments ± sem was calculated, by use of the following formula:
 |
Cellular imaging
HuT78 T cells (3.5 × 105) or CD4+ hAPBTs were stimulated on glass chamber slides and coated with 5 μg/ml anti-CD3 mAb, as described previously [31, 33]. The cells were fixed, permeabilized, and stained, and the images were captured by use of the Leica AM TIRF microscope, found in the University of Iowa’s Central Microscopy Core, as described [31]. The primary antibodies, specific for Pyk2, phospho-LAT Y226, or p85-PI3K, were acquired from Abcam (Cambridge, United Kingdom), BD Biosciences, and Millipore (Billerica, MA, USA), respectively. The colocalization analyses were performed as described [31], and the graphs show 50–75 cells taken from 2–3 independent experiments (25 cells/experiment).
Immunoblotting
Anti-TCR antibody-stimulated HuT78 and CD4 hAPBT lysates were separated by SDS-PAGE, and immunoblotting was conducted on the LI-COR Odyssey infrared imager, as described previously [25, 31, 34]. The primary antibodies used in immunoblotting assays were as follows: anti-phospho-Akt S473 (Invitrogen), anti-phospho-Akt T308 (Cell Signaling Technology, Beverly, MA, USA), anti-phospho-LAT Y226 (BD Biosciences), anti-phospho-SLP-76 Y128 (BD Biosciences), anti-phospho-Erk1/Erk2 (Invitrogen), anti-phospho-GSK3α/β S21/S9 (Cell Signaling Technology), antiphosphotyrosine (clone 4G10; Millipore), anti-p85-PI3K (Millipore), anti-LAT (Millipore), anti-SLP-76 (Cell Signaling Technology), anti-FAK (Millipore), anti-Pyk2 (Abcam), anti-Akt (Cell Signaling Technology), and anti-p42/p44 (Cell Signaling Technology). The immunoblot band intensity was quantified by use of Odyssey v3.0 software. The data were normalized relative to actin or GAPDH expression, as described previously [25, 31, 34].
Immunoprecipitations
HuT78 T cells or CD4 hAPBTs were stimulated by use of soluble anti-TCR antibodies, as described [25, 31, 34]. Immunoprecipitations were conducted by use of anti-Pyk2 (clone C-19; Santa Cruz Biotechnology) or the stimulatory antibody alone [31, 32, 34].
Pyk2 and PI3K inhibition
For immunoblotting experiments, CD4 hAPBTs were resuspended at 3 ×107 cells/ml and pretreated with various doses of the FAK/Pyk2 inhibitor PF431396 (Tocris Bioscience, Bristol, United Kingdom) for 1 h at 37°C and stimulated by use of anti-TCR antibodies as described [25, 31]. To detect differences in IFN-γ production, 1 × 106 cells were pretreated for 1 h with PF431396 or for 15 min with 100 nM wortmannin (Calbiochem) or 10 μM LY294002 (Calbiochem). Cells were then stimulated in the presence of these inhibitors, as indicated above, to detect differences in IFN-γ production.
Statistical analysis
All statistics were performed in Microsoft Excel by use of a two-tailed t-test assuming equal variance.
RESULTS
Proliferation defects in Pyk2-deficient T cells
We have described a miRNA that selectively inhibits Pyk2 expression in the human Jurkat E6.1 T cell line [25]. However, TCR signaling in the human HuT78 T cell line more closely mimics hAPBTs [8, 32]. Therefore, we introduced this miRNA into HuT78 T cells. We first generated stable HuT78 T cell lines that expressed the control, Luc-specific miRNA, or Pyk2-specific miRNA. Relative to the control cells, these Pyk2-deficient cells were viable and proliferated normally in culture (Supplemental Fig. 1A). However, we noted that the expression of the Pyk2-related protein, FAK, was decreased by 50% in these cells. This decrease was not observed upon short-term knockdown of Pyk2 in Jurkat cells [25]. The expression of the regulatory subunits of PI3K (p85 and p55), Akt, SLP-76, and LAT was also increased in these Pyk2-deficient HuT78 T cells (Supplemental Fig. 1B and C). Thus, long-term suppression of Pyk2 expression appears to lead to compensatory changes in other TCR signaling molecules.
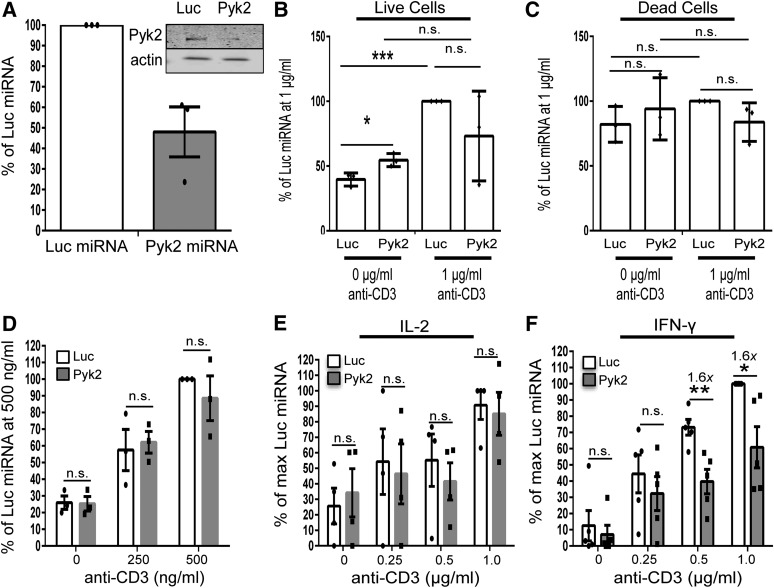
To overcome these compensatory changes, we next transiently suppressed Pyk2 expression in HuT78 T cells. Pyk2 expression was inhibited up to 75–80% by Day 4 after transfection with the Pyk2-specific miRNA (Fig. 1A and B). Unlike the stable Pyk2-deficient HuT78 T cells, these cells expressed normal levels of FAK, LAT, SLP-76, Akt, and Erk1/Erk2 before and after TCR stimulation (Supplemental Fig. 1B and C). In contrast to the stable, Pyk2-deficient HuT78 T cells, the transient knocking down of Pyk2 expression in HuT78 T cells or Jurkat T cells inhibited their proliferation (Fig. 1C and unpublished observation). CD4+ hAPBTs, in which Pyk2 expression was reduced by ∼50%, were also generated (Fig. 2A and Supplemental Fig. 2A). We then tracked anti-CD3 antibody-induced proliferation. As seen in Fig. 2B, compared with the control CD4+ hAPBTs, TCR-induced proliferation was significantly impaired in the Pyk2-deficient CD4+ hAPBTs. This defect was not a result of increased cell death induced by anti-CD3 antibody stimulation (Fig. 2C). Thus, Pyk2 appears to be a critical regulator of human T cell proliferation.
Figure 1. HuT78 T cell proliferation is defective in the absence of Pyk2.
(A) Representative immunoblot analysis comparing Pyk2 expression in Luc or Pyk2 miRNA-treated HuT78 T cells over time. (B) Pyk2 expression relative to the Day 3 Luc miRNA-transfected cell sample was calculated. Each dot represents the quantified data from 1 experiment, and the bar graphs depict the average of the 2 independent experiments ± sd. (C) Live and dead cells were counted by trypan blue exclusion. The average percent change relative to the Luc miRNA-treated cells at Day 2 ± sem was calculated. *P < 0.05; ***P < 0.001.
Figure 2. Pyk2 repression inhibits select downstream effector functions upon TCR activation.
(A) Control and Pyk2-deficient CD4 hAPBTs were generated. Pyk2 expression in whole-cell lysates was analyzed by immunoblotting. The relative expression of Pyk2 compared with the actin-loading control was calculated. The graph shows quantified differences in Pyk2 expressions from 3 independent transfections ± sem, with each dot representing the average Pyk2 expression for an individual experiment. (B and C) Control or Pyk2-deficient CD4 hAPBTs were incubated in the presence or absence of 1 μg/ml anti-CD3 for 3 days. Live and dead cells were counted by trypan blue exclusion. (B) Live cell data represented as the fold change over the 0 μg/ml control ± sem, and each dot shows the calculated value for each experimental replicate. (C) Dead cell data represented as the fold change over the 0 μg/ml Luc miRNA control ± sem. Each dot shows an experimental replicate. (F) Control and Pyk2-deficient CD4 hAPBTs were stimulated using various doses of soluble anti-TCR antibodies for 4 h. CD69 surface expression was then assessed by flow cytometry. Data are depicted as the average fold change relative to the 500 ng/ml Luc miRNA sample ± sem, with each of the dots depicting the relative expression obtained from each human donor. *P < 0.05; **P < 0.01; ***P < 0.001.(E and F) Control or Pyk2-deficient CD4 hAPBTs were stimulated by use of various doses of immobilized anti-CD3 for 24 h. IL-2 (E) and IFN-γ (F) production was assessed by ELISA. The data were normalized to percentages of the maximum cytokine secretion of the Luc miRNA cells, and the mean of at least 3 independent experiments ± sem is shown, with each dot representing the data obtained from an individual human donor.
Effects of Pyk2 suppression on cytokine production and CD69 up-regulation in human T cells
We next asked if Pyk2-deficient cells had reduced TCR-induced effector responses. To that end, we generated control or Pyk2-deficient CD4+ hAPBTs and stimulated these cells with anti-CD3 antibodies. We then examined if the early activation marker CD69 was normally upregulated after TCR induction in the Pyk2-deficient CD4 hAPBTs. As shown in Figure 2D, the TCR-mediated upregulation of CD69 was not affected by Pyk2 suppression. We also examined the production of the cytokines IL-2 and IFN-γ. Following anti-CD3 antibody stimulation, Pyk2-deficient CD4+ hAPBTs produced normal levels of IL-2 (Fig. 2E), whereas IFN-γ production was impaired (Fig. 2F). Likewise, Pyk2-deficient Jurkat cells also produced normal levels of IL-2 upon TCR activation (unpublished observations). Thus, Pyk2 does not regulate TCR-inducible IL-2 secretion in CD4+ hAPBTs, whereas maximal TCR-mediated IFN-γ production is dependent on Pyk2. Therefore, select TCR-inducible functions are impaired in the Pyk2-deficient CD4+ hAPBTs.
Pyk2 partially colocalizes with phosphorylated LAT in human T cells
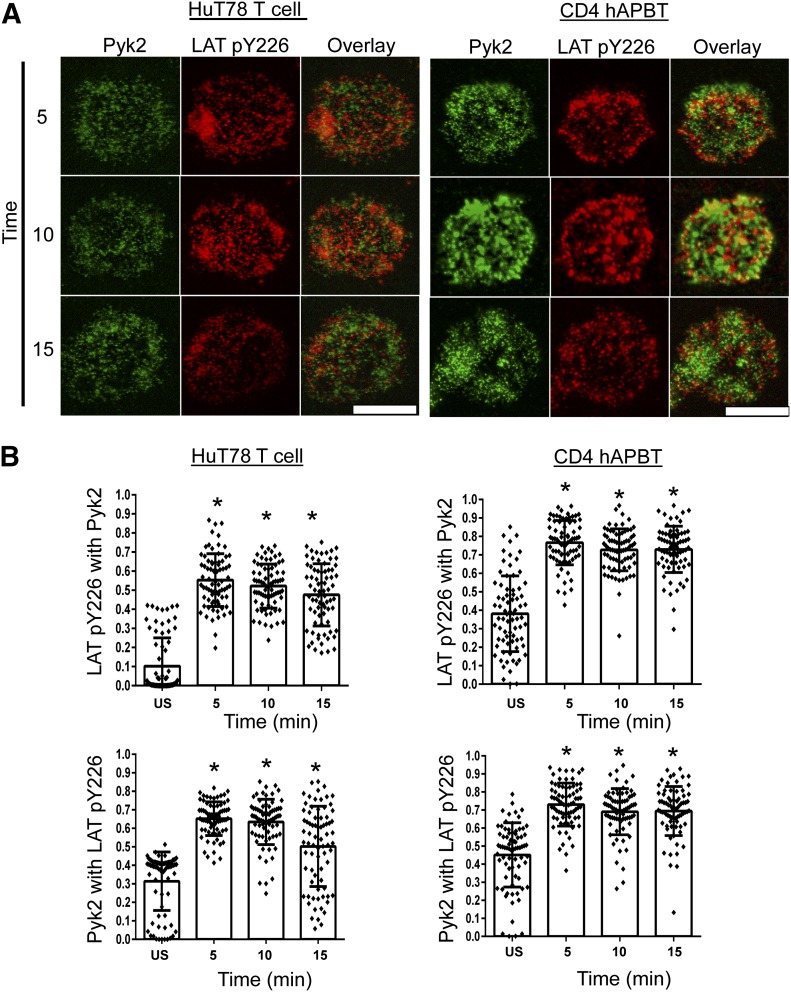
When T cells bind to peptide-loaded APCs or to anti-CD3 antibody-coated beads, Pyk2 is recruited to the T cell membrane, where it localizes to the T cell-APC or T cell-bead interface [35–37]. To investigate further the subcellular localization of Pyk2 in human T cells, we coated anti-CD3 antibody onto glass chamber slides and stimulated HuT78 T cells or CD4+ hAPBTs on these surfaces. We then examined whether Pyk2 colocalized with phosphorylated LAT by use of TIRF microscopy, as LAT directly or indirectly recruits many cytoplasmic proteins to the T cell membrane. As shown in Fig. 3A, Pyk2 and phosphorylated LAT were present in membrane-associated microclusters after TCR stimulation. We next quantified the levels of colocalization between the Pyk2 and phosphorylated LAT microclusters. For unknown reasons, the background colocalization obtained from staining with the secondary antibodies alone was calculated to be ∼30% in HuT78 T cells and CD4 hAPBTs, and this observation was consistent across multiple experiments (US samples; see Figs. 3 and 5). Taking this background staining into account, 30–50% of the Pyk2 and phosphorylated LAT clusters were colocalized in the HuT78 T cells or CD4 hAPBTs after TCR activation, and this colocalization above background was statistically significant (Fig. 3B). These data demonstrate that a fraction of membrane-associated Pyk2 colocalizes with LAT after TCR induction; however, Pyk2 is also recruited to membrane regions that do not contain phosphorylated LAT.
Figure 3. Pyk2 partially colocalizes with phosphorylated LAT in human T cells.
(A) HuT78 T cells (left) and CD4 hAPBTs (right) were stimulated with anti-CD3 antibody coated onto glass chamber slides for 5, 10, or 15 min. Immunofluorescent staining was conducted by use of anti-Pyk2 and anti-phospho-LAT Y226 (LAT pY226) antibodies, followed by the appropriate secondary antibodies. The negative control (US) samples were stimulated for 5 min and stained by use of the secondary antibodies alone. Data are representative of 3 independent experiments. Original scale bars, 5 μm. (B) The Manders correlation coefficients (M1 and M2) were calculated for 75 cells (25 from each independent experiment). In the graph, each dot represents an individual cell, and the mean coefficient value ± sd is also shown. *P < 0.0001 relative to the negative control.
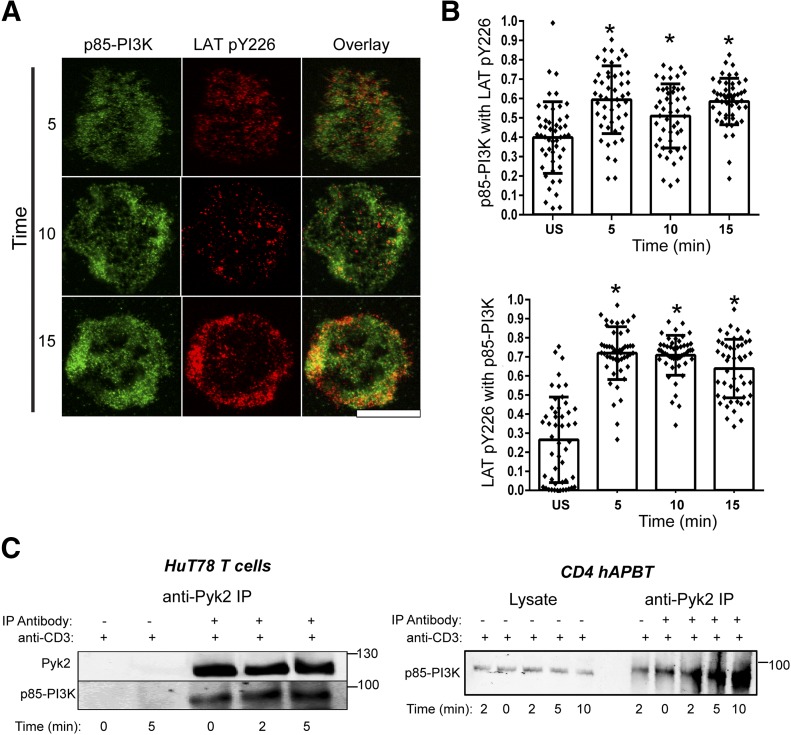
Figure 5. p85-PI3K associates with phosphorylated LAT and Pyk2.
(A) HuT78 T cells stimulated by use of anti-CD3 antibody coated on glass chamber slides for 5, 10, or 15 min. Cells were also stimulated for 5 min and stained by use of the secondary antibodies alone (US). Data are representative of 2 independent experiments. Original scale bar, 5 μm. (B) The Manders correlation coefficients were calculated for 50 cells (25 from each independent experiment). The calculated coefficient for each cell is depicted by the dots, and the average coefficient value ± sd is shown in the bar graph and error bars. *P < 0.005 compared with the negative control. (C) HuT78 T cells (left) and CD4 hAPBTs (right) were stimulated with soluble anti-TCR antibodies for various times and lysed. Pyk2 was immunoprecipitated (IP) from these cells, and its association with p85-PI3K was assessed by immunoblotting. Data are representative of at least 3 independent experiments.
Early TCR-mediated signaling is not altered in Pyk2-deficient HuT78 T cells
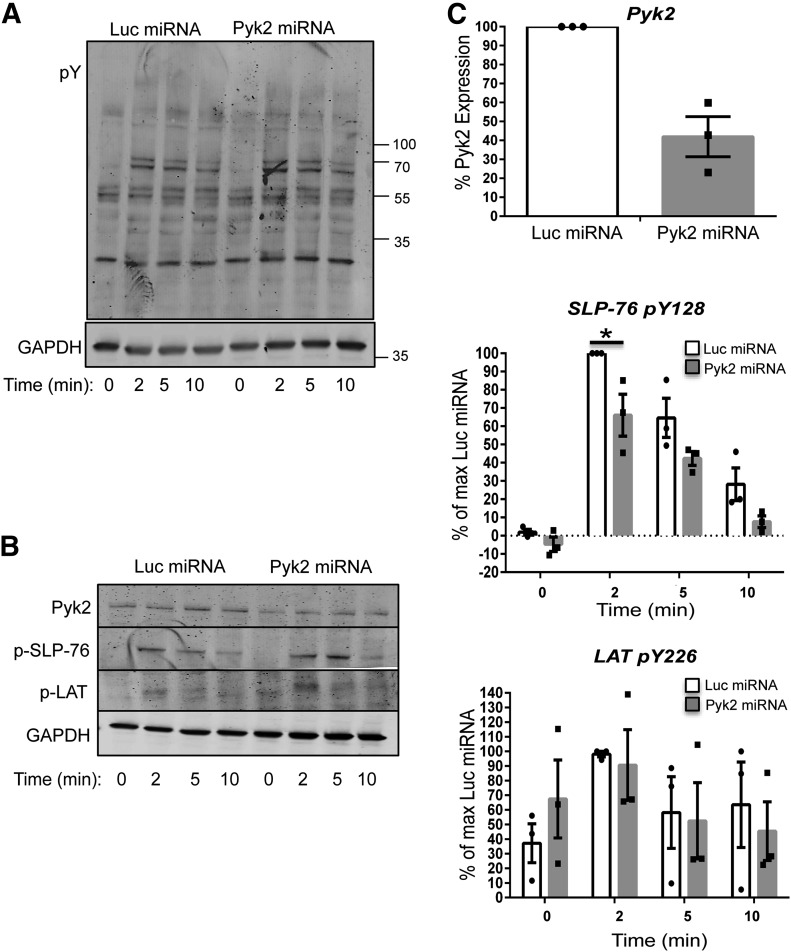
Pyk2 is rapidly phosphorylated after TCR stimulation and associates with many proximal signaling molecules, including Lck, Fyn, ZAP-70, and LAT (Fig. 3 and refs. [23, 24, 28, 38]). Thus, Pyk2 may regulate early TCR-mediated signaling events. To address this possibility, control and Pyk2-deficient HuT78 T cells were generated by transient transfection. In these experiments, Pyk2 expression was inhibited by ∼60% (Fig. 4B and C and Supplemental Fig. 2B). The control and Pyk2-deficient HuT78 T cells were then stimulated for various times with soluble anti-CD3 antibodies, and differences in tyrosine phosphorylation were detected by immunoblotting. Early TCR-mediated signaling appeared to remain intact in the Pyk2-deficient HuT78 T cells (Fig. 4A). Likewise, no detectable differences in LAT Y226 phosphorylation were observed (Fig. B and C). By contrast, the TCR-inducible phosphorylation of SLP-76 Y128 was reduced by 20–35% in the Pyk2-deficient HuT78 T cells (Fig. B and C and Supplemental Fig. 2B). These data demonstrate that Pyk2 suppression does not globally inhibit proximal signaling events but suggest that Pyk2 is a positive regulator of SLP-76 phosphorylation downstream of the TCR.
Figure 4. Pyk2-deficient cells have normal proximal TCR-induced signaling.
Control and Pyk2-deficient HuT78 T cells were stimulated with anti-CD3 for various times. (A) Global changes in tyrosine phosphorylation were assessed by antiphosphotyrosine (pY) immunoblotting. Data are representative of 3 independent experiments. pY, . (B and C) The expression of Pyk2 and the site-specific phosphorylation of LAT Y226 (p-LAT) and SLP-76 Y128 (p-SLP-76) were examined by immunoblotting. Data are representative of 3 independent experiments. (C)_The change in the phosphorylation of each of these proteins relative to the maximum Luc sample was calculated. The mean expression of Pyk2 across all experimental time-points was also calculated. In the graphs, dots represent values calculated from an individual stimulation, and the mean of 3 experiments ± sem is shown in the bar graphs and error bars. *P < 0.05 relative to the Luc miRNA control at that time-point.
Pyk2 associates with p85-PI3K and controls PI3K activation downstream of the TCR
As Pyk2 was not required to induce the most proximal TCR signaling events, we next looked at more downstream signaling pathways. The TCR-inducible phosphorylation of Pyk2 occurs independently of PI3K activity [23, 24], and previous studies have demonstrated that Pyk2 controls PI3K activation in multiple cell lineages [39, 40]. Thus, it was possible that Pyk2 regulated PI3K signaling downstream of the TCR. SLP-76 associates with p85-PI3K after TCR induction, and SLP-76 and p85-PI3K are recruited to the plasma membrane via the interaction of SLP-76 with LAT [3–7]. Consistent with this observation, we found that p85-PI3K significantly colocalizes above background levels with phosphorylated LAT in HuT78 T cells after TCR stimulation (Fig. 5A and B). It is not likely that Pyk2 binds directly to LAT, as it has no SH2 domains that could bind to LAT phosphotyrosine residues. LAT was not detected in Pyk2 immunoprecipitates, derived from HuT78 T cells (data not shown), further suggesting that Pyk2 associates with LAT via intermediate proteins.
Pyk2 colocalizes with phosphorylated LAT (Fig. 3), and Pyk2 also associates with p85-PI3K in other cell lineages [41, 42]. Therefore, we next determined whether Pyk2 interacted with p85-PI3K in human T cells. To that end, we stimulated HuT78 T cells and CD4 hAPBTs with anti-TCR antibodies for various times and performed Pyk2 immunoprecipitations. As shown in Fig. 5C, p85-PI3K constitutively interacted with Pyk2 in HuT78 T cells and CD4 hAPBTs, and this association was enhanced after TCR activation. We saw little to no association of Pyk2 with the p85 PI3K subunit in immunoprecipitations performed with no anti-Pyk2 antibodies [Fig. 5C; lanes labeled by plus (+) signs by anti-CD3 and minus (−) signs by Pyk2 immunoprecipitates]. As the stimulatory anti-CD3 antibody was present in these samples, this shows that the precipitated Pyk2/p85-PI3K complex is not an artifact of nonspecific binding to an antibody or a result of these proteins being present in complexes pulled down by the stimulatory antibody. Thus, Pyk2 associates with p85-PI3K at the LAT complex, where it could potentially control PI3K activation after TCR induction.
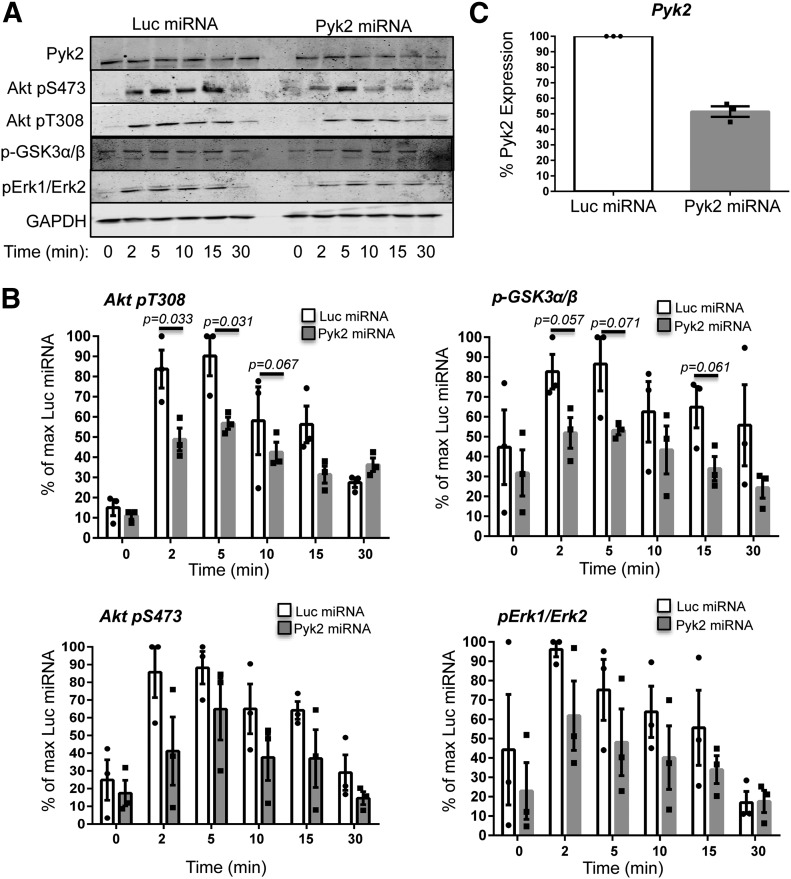
We next determined if PI3K-dependent signaling was altered in Pyk2-deficient HuT78 T cells. Previous work has demonstrated that the TCR-inducible phosphorylation of Akt and Erk1/Erk2 is reduced when PI3K activity is inhibited [8, 9, 11, 13, 16]. Therefore, we compared the kinetics and magnitude of the TCR-mediated phosphorylation of Akt T308, Akt S473, and Erk1/Erk2 phosphorylation in the control or Pyk2-deficient HuT78 T cells. After TCR stimulation, Akt T308 phosphorylation was 2- to 3-fold higher in the controls than in the Pyk2-deficient cells (Fig. A and B and Supplemental Fig. 3A). Likewise, the TCR-dependent phosphorylation of Akt S473 and Erk1/Erk2 was reduced in the absence of Pyk2 (Fig. 6A and B and Supplemental Fig. 3A). GSK3α/β S21/S9 also had lower TCR-inducible phosphorylation in the Pyk2-deficient cells, thus confirming that the Akt function is reduced in these cells (Fig. 6A and 6B and Supplemental Fig. 3A). These reductions in the site-specific phosphorylation of Akt, Erk1/Erk2, and GSK3α/β were correlated with the ∼50% reduction in Pyk2 expression seen in these experiments (Fig. 6A and C and Supplemental Fig. 3). Importantly, no significant decreases in total Akt or Erk1/Erk2 expression were observed at these same time-points (Supplemental Figs. 2, 3, and 3A). Together, these data suggest that Pyk2 controls PI3K function downstream of the TCR.
Figure 6. Pyk2 controls PI3K activation after TCR induction.
(A–C) Control and Pyk2-deficient HuT78 T cells and CD4 hAPBTs were stimulated with anti-TCR antibodies for various times. (A) The phosphorylation (p) of Akt T308, Akt S473, Erk1/Erk2, and GSK3 was analyzed by immunoblotting. Data are representative of at least 3 experiments. (B) The change in the phosphorylation of each of these proteins relative to the maximum Luc sample was calculated. Each dot represents the value calculated for an individual experimental replicate, and the mean of 3 experiments ± sem is also shown. P-values reflect statistical differences between the control and Pyk2-deficient T cells at the indicated time-points. (C) The mean relative expression of Pyk2 across all experimental time-points was also calculated. Each dot represents the value of an individual experiment, and the average of 3 experiments ± sem is graphed.
The kinase activity of Pyk2 controls PI3K activity downstream of the TCR
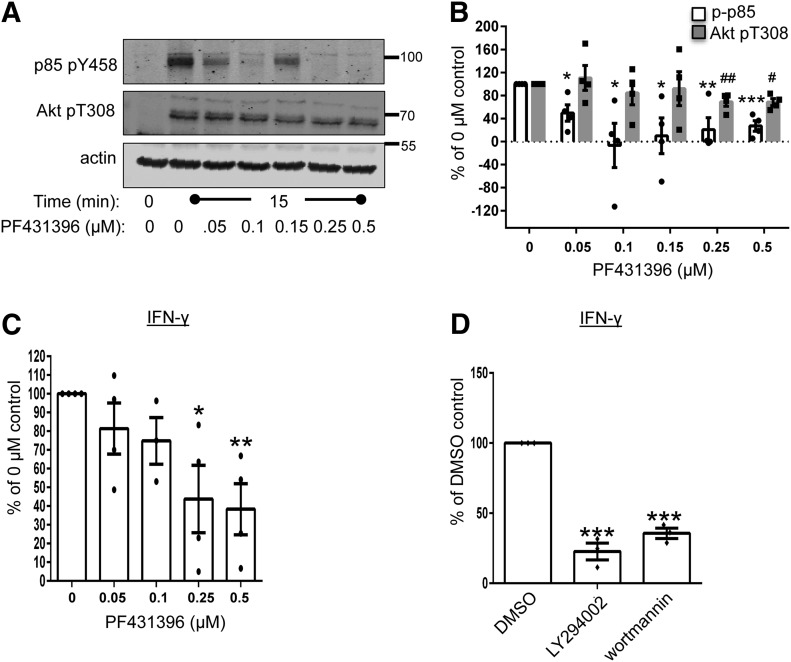
To investigate if the catalytic function of Pyk2 was needed for PI3K function, we pretreated CD4 hAPBTs with PF431396, a compound that suppresses the catalytic functions of FAK and Pyk2 with in vitro IC50 values of 2 nM and 11 nM, respectively [43]. PI3K activity and Jurkat cell proliferation are enhanced when p85-PI3K is tyrosine phosphorylated, and this phosphorylation event and subsequent activation of PI3K require Src family kinase activity [19–21]. The TCR-inducible phosphorylation of Pyk2 is also mediated by the Src family kinases [23, 24]; therefore, Pyk2 may regulate p85-PI3K phosphorylation downstream of the TCR. We first examined if there were differences in p85-PI3K phosphorylation in the absence of Pyk2 activity. There was a significant decrease in the TCR-inducible phosphorylation of p85-PI3K Y458 in CD4 hAPBTs that were treated with PF431396. At doses where PF431396 had the most significant effect on the TCR-inducible phosphorylation of p85-PI3K Y458 (0.25–0.5 μM), Akt T308 phosphorylation was also suppressed by 35–40% (Fig. 7A and B and Supplemental Fig. 3B). The Src family kinases could bind to Pyk2 Y402 to facilitate p85-PI3K phosphorylation [38, 44]. However, it is not likely that the TCR-inducible phosphorylation of p85-PI3K is controlled in this manner, as we did not observe any change in the TCR-mediated phosphorylation of Pyk2 Y402 after PF431396 treatment (unpublished observation). Instead, these results suggest that Pyk2 directly phosphorylates p85-PI3K to augment PI3K function upon TCR activation.
Figure 7. The catalytic activity of Pyk2 controls PI3K function downstream of the TCR.
CD4 hAPBTs were pretreated with various doses of P431396 for 1 h at 37°C. The cells were then stimulated with anti-TCR antibodies for 0 or 15 min. The phosphorylation of p85 Y458 and Akt T308 was detected by immunoblotting. A representative of 4 independent experiments is shown. (B) The immunoblot data from 4 independent experiments were normalized to the 0 μM PF431396 sample. The normalized values for each experiment replicate are shown in the dots, and the mean ± sem is also graphed. (C) CD4 hAPBTs were pretreated with the indicated doses of PF431396 for 1 h at 37°C and then stimulated with immobilized anti-CD3 antibody in the presence of the inhibitor for 24 h. The production of IFN-γ was detected by ELISA. Data were normalized to the 0 μM sample. The dots represent normalized values for individual donor, and the mean ± sem of the 4 independent experiments is also graphed. (D) CD4 hAPBTs were pretreated with the LY294002 or wortmannin for 15 min and stimulated as in C. The data were normalized to the DMSO sample. Each dot represents normalized data from an individual donor, and the mean ± sem from 3 independent experiments was calculated and graphed. *P < 0.05, **P < 0.01, and ***P < 0.001 denote statistical values for p85 Y458 phosphorylation (B) or IFN-γ production (C and D) relative to the control sample; #P < 0.05, and ##P < 0.01 denote statistical values for Akt T308 phosphorylation relative to the control sample.
We next wanted to determine if the catalytic function of Pyk2 was required for TCR-mediated IFN-γ production. As shown in Fig. 7C, CD4 hAPBTs that were treated with PF431396 and stimulated with immobilized anti-CD3 displayed a dose-dependent decrease in IFN-γ production. There was a dose-dependent decrease in TCR-inducible IFN-γ production following PF431396 treatment, and maximum inhibition in IFN-γ secretion was observed after treating cells with 0.25–0.5 μM PF431396, the same doses that significantly suppressed p85 and Akt phosphorylation (Fig. 7A and B and Supplemental Fig. 3B). Interestingly, the TCR-mediated production of IFN-γ was suppressed in a similar fashion whether the enzymatic activity of Pyk2 or PI3K was inhibited (compare Fig. 7C with D). Collectively, these data suggest that the catalytic function of Pyk2 controls PI3K-dependent signaling that regulates IFN-γ production after TCR induction.
DISCUSSION
In this manuscript, we found that Pyk2 controls specific TCR-induced functions in human T cells. Pyk2-deficient T cells had proliferation defects and produced less IFN-γ after TCR stimulation. We also demonstrated that Pyk2 is recruited to the activated LAT complex, where it associates with the regulatory subunit of PI3K. This association appears to be important for PI3K function, as loss of Pyk2 expression or catalytic function led to impaired p85-PI3K, Akt, GSK3α/β, and Erk1/Erk2 phosphorylation, events that are downstream of PI3K activation (Supplemental Fig. 4). These data reveal a novel function for Pyk2 as a positive regulator of TCR-induced PI3K function in human T cells. Thus, these studies significantly enhance our understanding of the pathways that control PI3K activation downstream of the TCR.
We demonstrated that long- and short-term suppression of Pyk2 differentially altered the phenotype of human T cells. Transient inhibition of Pyk2 expression repressed T cell proliferation and TCR-induced PI3K activation, whereas stable Pyk2-deficient HuT78 T cells retained their proliferative ability and had normal-to-elevated TCR-inducible Akt T308 and Akt S473 phosphorylation (Supplemental Fig. 1 and unpublished observation). Furthermore, in contrast to our published work [25], TCR-driven adhesion was also normal to enhanced in the stable Pyk2-deficient T cells (unpublished observation). These phenotypic discrepancies can likely be explained by differences in protein expression, as the HuT78 T cells that stably expressed the Pyk2-specific miRNA had increased Akt, LAT, SLP-76, and PI3K regulatory subunit expression compared with the cells that expressed the Luc-specific miRNA. Moreover, these cells expressed ∼50% less FAK protein, which our lab has recently demonstrated is a negative regulator of TCR function [31]. Therefore, long-term Pyk2 suppression leads to compensatory changes in positive and negative signaling pathways. These results may explain why Pyk2-deficient mice have no T cell developmental defects and why only minimal functional impairments are observed in murine Pyk2-deficient T cells [26, 39, 45].
We and others [26, 27] have demonstrated that T cell proliferation is impaired in the absence of Pyk2, whereas IL-2 production is not substantially altered. Our study mechanistically links Pyk2 to PI3K activation downstream of the TCR. Consistent with our results, the inhibition of PI3K function dramatically reduces TCR-induced proliferation and IFN-γ production in human T cells, whereas IL-2 production and CD69 up-regulation are not altered substantially [10–12]. However, it should be noted that the inhibition of PI3K function by use of the PI3K inhibitor, LY294002, or the deletion of specific p110 catalytic isoforms impairs IL-2 production under certain conditions [10–16]. Thus, although Pyk2 appears to regulate PI3K function to promote specific TCR-induced functions in CD4 hAPBTs, future studies could investigate if Pyk2 regulates the activity of specific PI3K isoforms to modulate T cell responses.
In contrast to our study, Wiemer and coworkers [46] suggested that the Pyk2-related kinase FAK controls T cell proliferation. When human CD4 T cells are treated with PF562271, a pharmacological inhibitor that suppresses the catalytic function of FAK and Pyk2, anti-CD3 and anti-CD28 antibody-induced proliferation is impaired. These cells also have reductions in ZAP-70 and Erk1/Erk2 phosphorylation, which we also observed in FAK-deficient T cells [31]. As Wiemer et al. [46] treated the cells with doses of PF562271 that likely suppress the catalytic activity of Pyk2 [47], our data suggest that the differences in proliferation observed following PF562271 treatment were attributed to loss of Pyk2 and not FAK function. It is interesting to note that both and Pyk2 appear to modulate Erk1/Erk2 activity positively downstream of the TCR [31, 46].
PI3K signaling is activated upon recruitment of p85-PI3K to the LAT/SLP-76 complex [3]. The data presented in this manuscript suggest that Pyk2 is also a critical component of this complex. Although our results suggest that Pyk2 phosphorylates p85-PI3K directly after TCR induction, it is unclear if Pyk2 has additional scaffolding functions that facilitate PI3K activation downstream of the TCR. Pyk2 may recruit different effector proteins to the LAT/SLP-76 complex to promote PI3K activation. For instance, Pyk2 constitutively associates with Vav1 in Jurkat T cells. Importantly, Vav1 regulates PI3K function in T cells and binds SLP-76 Y113 and/or Y128, the phosphotyrosine residues that control PI3K activation downstream of the TCR [3, 28, 48]. The scaffolding function of Pyk2 may also stabilize the interaction of p85-PI3K with LAT or SLP-76, as neither of these adaptor proteins contains the consensus pYXXM motif that binds to the SH2 domain(s) of p85-PI3K [18]. These possibilities are under active investigation by our laboratory. Future experiments will also elucidate how Pyk2 is recruited to the LAT complex after TCR activation.
After receiving TCR signals, costimulatory and adhesive receptors, including CD28 and LFA-1, drive T cell activation. Coengagement of the TCR and CD28 induces more sustained PI3K activity than TCR engagement alone [49]. Although CD28 binds to p85-PI3K, mutagenesis of the pYXXM motif in the cytoplasmic tail of CD28 does not impair Akt recruitment to the IS, IL-2 production, or proliferation in primary T cells [49–52]. Therefore, CD28 costimulatory signals enhance the magnitude and duration of TCR-mediated PI3K activation in a manner largely independent of PI3K binding to the CD28. As CD28 costimulation sustains Lck activity at the IS [53], CD28 costimulation may prolong PI3K activation by enhancing Pyk2, LAT, and/or SLP-76 activation [23, 24, 51]. In this regard, Pyk2-deficient murine CD4+ T cells have markedly decreased proliferative responses following anti-CD3 and anti-CD28 antibody stimulation [27]. PI3K activation also promotes LFA-1 affinity maturation, which enhances T cell adhesion to potentiate T cell activation [54]. Although the precise molecular mechanisms that couple TCR/CD28-induced PI3K function to LFA-1 activation are unknown, our data that show a link between Pyk2 and PI3K activation may explain why Pyk2-deficient CD8+ T cells have reduced TCR-induced adhesion to the LFA-1 ligand, ICAM-1, and reduced antigen-specific effector responses [26]. How Pyk2 coordinates signaling among the TCR, CD28, and LFA-1 remains unknown.
We demonstrated recently that TCR activation leads to 2 separate phases of T cell adhesion. The 1st phase occurs within 5–15 min after TCR activation and is driven by the enzymatic activities of Lck/Fyn and to a lesser extent, the catalytic functions of FAK and/or Pyk2. By contrast, the 2nd phase is observed 30–60 min after TCR stimulation, and it is regulated by the nonenzymatic functions of Fyn and Pyk2. This latter observation was demonstrated by the fact that adhesion was resistant to treatment with the Src family kinase inhibitor PP2 and the FAK/Pyk2 kinase inhibitor PF431396. Furthermore, PI3K inhibitors did not ablate this 2nd phase of TCR-driven adhesion [25]. Therefore, Pyk2 has a kinase-dependent function, which induces PI3K activation and adhesion after TCR stimulation. Moreover, Pyk2 has a kinase-independent function to stabilize TCR-driven adhesion at later times after stimulation.
Pyk2 is localized to 2 subcellular locations upon T cell activation. In this study, we showed that a fraction of membrane-associated Pyk2 colocalizes with phosphorylated LAT, whereas another fraction does not. These 2 pools may be consistent with the 2 fractions of Pyk2 identified by St-Pierre and colleagues [37]. They observed that 1 fraction of Pyk2 is constitutively bound to paxillin and localized to the MTOC after TCR activation, whereas another fraction is phosphorylated more strongly on serine/threonine and tyrosine residues and localized to the T cell-APC interface. We propose that the latter pool of Pyk2 is the enzymatically active form that associates with the LAT complex and controls TCR-induced PI3K activation and early actin polymerization events that regulate T cell adhesion [25]. Furthermore, we predict that the hypophosphorylated, MTOC-associated fraction and/or the pool of Pyk2 that does not colocalize with phosphorylated LAT control late-stage adhesion. This function is independent of its role in controlling PI3K function, as we found that neither the catalytic function of Pyk2 nor PI3K activity was needed for this process [25]. Thus, Pyk2 exists in 2 separate pools in activated T cells, where it controls 2 separate functions, ultimately to shape how T cells respond to pathogens (Supplemental Fig. 4).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by an American Heart Association Pre-doctoral fellowship (11PRE7390070; to N.M.C.), the U.S. National Institutes of Health (NIH) Predoctoral Training Grant in Immunology (T32 AI007485; to N.M.C., M.Y.B., and S.F.C.), an American Heart Association undergraduate fellowship (12UFEL12060651; to A.N.Y.), an Iowa Center for Research by Undergraduate fellowship (to K.M.B.), a Scientist Development Grant (0830244N) from the American Heart Association, and NIH National Cancer Institute Grant R01-CA136729 (to J.C.D.H.). The authors thank Dr. Anton McCaffrey for useful discussions and assistance in developing the miRNA vectors and Dr. Bruce Hostager for the vectors used to generate the retroviruses. The authors also thank Jonathan Light, Mikaela Tremblay, and Aldo Vacaflores for their critical discussions and critique of this manuscript.
Glossary
- Akt
protein kinase B
- FAK
focal adhesion kinase
- GSK
glycogen synthetase kinase
- hAPBT
human activated peripheral blood T cell
- IS
immunologic synapse
- LAT
linker for activation of T cells
- Lck
lymphocyte-specific protein tyrosine kinase
- LFA-1
leukocyte function-associated antigen 1
- MFI
mean fluorescence intensity
- miRNA
microRNA
- MTOC
microtubule-organizing complex
- p85-PI3K
regulatory p85 subunit of PI3K
- Pyk2
proline-rich tyrosine kinase 2
- SH2
Src homology 2
- SLP-76
Src homology 2 domain-containing leukocyte protein of 76 kDa
- TIRF
total internal reflection fluorescence microscopy
- US
unstimulated
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
AUTHORSHIP
N.M.C. and J.C.D.H. wrote the manuscript. N.M.C., M.Y.B., S.F.C., and J.C.D.H. conceived of, designed, and performed experiments and analyzed the data. A.N.Y. and K.M.B. performed experiments, analyzed data, and edited the manuscript.
DISCLOSURES
The authors declare no competing financial or commercial interests.
REFERENCES
- 1.Smith-Garvin J. E., Koretzky G. A., Jordan M. S. (2009) T cell activation. Annu. Rev. Immunol. 27, 591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartelt R. R., Houtman J. C. (2013) The adaptor protein LAT serves as an integration node for signaling pathways that drive T cell activation. Wiley Interdiscip. Rev. Syst. Biol. Med. 5, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shim E. K., Jung S. H., Lee J. R. (2011) Role of two adaptor molecules SLP-76 and LAT in the PI3K signaling pathway in activated T cells. J. Immunol. 186, 2926–2935. [DOI] [PubMed] [Google Scholar]
- 4.Shim E. K., Moon C. S., Lee G. Y., Ha Y. J., Chae S. K., Lee J. R. (2004) Association of the Src homology 2 domain-containing leukocyte phosphoprotein of 76 kD (SLP-76) with the p85 subunit of phosphoinositide 3-kinase. FEBS Lett. 575, 35–40. [DOI] [PubMed] [Google Scholar]
- 5.Fukazawa T., Reedquist K. A., Panchamoorthy G., Soltoff S., Trub T., Druker B., Cantley L., Shoelson S. E., Band H. (1995) T cell activation-dependent association between the p85 subunit of the phosphatidylinositol 3-kinase and Grb2/phospholipase C-gamma 1-binding phosphotyrosyl protein pp36/38. J. Biol. Chem. 270, 20177–20182. [DOI] [PubMed] [Google Scholar]
- 6.Lahesmaa R., Allsup A., Soderberg C., Jackman J., Findell P., Peltz G. (1995) Modulation of the Grb2-associated protein complex in human CD4+ T cells by receptor activation. J. Immunol. 155, 3815–3822. [PubMed] [Google Scholar]
- 7.Paz P. E., Wang S., Clarke H., Lu X., Stokoe D., Abo A. (2001) Mapping the Zap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. Biochem. J. 356, 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Orcutt N., Houtman J. C. (2009) PI3 kinase function is vital for the function but not formation of LAT-mediated signaling complexes. Mol. Immunol. 46, 2274–2283. [DOI] [PubMed] [Google Scholar]
- 9.Robertson L. K., Mireau L. R., Ostergaard H. L. (2005) A role for phosphatidylinositol 3-kinase in TCR-stimulated ERK activation leading to paxillin phosphorylation and CTL degranulation. J. Immunol. 175, 8138–8145. [DOI] [PubMed] [Google Scholar]
- 10.Okkenhaug K., Bilancio A., Farjot G., Priddle H., Sancho S., Peskett E., Pearce W., Meek S. E., Salpekar A., Waterfield M. D., Smith A. J., Vanhaesebroeck B. (2002) Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science 297, 1031–1034. [DOI] [PubMed] [Google Scholar]
- 11.Okkenhaug K., Patton D. T., Bilancio A., Garçon F., Rowan W. C., Vanhaesebroeck B. (2006) The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J. Immunol. 177, 5122–5128. [DOI] [PubMed] [Google Scholar]
- 12.Soond D. R., Bjørgo E., Moltu K., Dale V. Q., Patton D. T., Torgersen K. M., Galleway F., Twomey B., Clark J., Gaston J. S., Taskén K., Bunyard P., Okkenhaug K. (2010) PI3K p110delta regulates T-cell cytokine production during primary and secondary immune responses in mice and humans. Blood 115, 2203–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alcázar I., Marqués M., Kumar A., Hirsch E., Wymann M., Carrera A. C., Barber D. F. (2007) Phosphoinositide 3-kinase gamma participates in T cell receptor-induced T cell activation. J. Exp. Med. 204, 2977–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji H., Rintelen F., Waltzinger C., Bertschy Meier D., Bilancio A., Pearce W., Hirsch E., Wymann M. P., Rückle T., Camps M., Vanhaesebroeck B., Okkenhaug K. Rommel C. (2007) Inactivation of PI3Kgamma and PI3Kdelta distorts T-cell development and causes multiple organ inflammation. Blood 110, 2940–2947. [DOI] [PubMed] [Google Scholar]
- 15.Kane L. P., Andres P. G., Howland K. C., Abbas A. K., Weiss A. (2001) Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat. Immunol. 2, 37–44. [DOI] [PubMed] [Google Scholar]
- 16.Ladygina N., Gottipati S., Ngo K., Castro G., Ma J. Y., Banie H., Rao T. S., Fung-Leung W. P. (2013) PI3Kγ kinase activity is required for optimal T-cell activation and differentiation. Eur. J. Immunol. 43, 3183–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanhaesebroeck B., Guillermet-Guibert J., Graupera M., Bilanges B. (2010) The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 11, 329–341. [DOI] [PubMed] [Google Scholar]
- 18.Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J., Neel B. G., Birge R. B., Fajardo J. E., Chou M. M., Hanafusa H., Schaffhausen B., Cantley L. C. (1993) SH2 domains recognize specific phosphopeptide sequences. Cell 72, 767–778. [DOI] [PubMed] [Google Scholar]
- 19.Cuevas B. D., Lu Y., Mao M., Zhang J., LaPushin R., Siminovitch K., Mills G. B. (2001) Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphatidylinositol 3-kinase. J. Biol. Chem. 276, 27455–27461. [DOI] [PubMed] [Google Scholar]
- 20.Martínez-Lorenzo M. J., Anel A., Monleón I., Sierra J. J., Piñeiro A., Naval J., Alava M. A. (2000) Tyrosine phosphorylation of the p85 subunit of phosphatidylinositol 3-kinase correlates with high proliferation rates in sublines derived from the Jurkat leukemia. Int. J. Biochem. Cell Biol. 32, 435–445. [DOI] [PubMed] [Google Scholar]
- 21.Carrera A. C., Rodriguez-Borlado L., Martinez-Alonso C., Merida I. (1994) T cell receptor-associated alpha-phosphatidylinositol 3-kinase becomes activated by T cell receptor cross-linking and requires pp56lck. J. Biol. Chem. 269, 19435–19440. [PubMed] [Google Scholar]
- 22.Von Willebrand M., Baier G., Couture C., Burn P., Mustelin T. (1994) Activation of phosphatidylinositol-3-kinase in Jurkat T cells depends on the presence of the p56lck tyrosine kinase. Eur. J. Immunol. 24, 234–238. [DOI] [PubMed] [Google Scholar]
- 23.Collins M., Bartelt R. R., Houtman J. C. (2010) T cell receptor activation leads to two distinct phases of Pyk2 activation and actin cytoskeletal rearrangement in human T cells. Mol. Immunol. 47, 1665–1674. [DOI] [PubMed] [Google Scholar]
- 24.Collins M., Tremblay M., Chapman N., Curtiss M., Rothman P. B., Houtman J. C. (2010) The T cell receptor-mediated phosphorylation of Pyk2 tyrosines 402 and 580 occurs via a distinct mechanism than other receptor systems. J. Leukoc. Biol. 87, 691–701. [DOI] [PubMed] [Google Scholar]
- 25.Chapman N. M., Yoder A. N., Houtman J. C. (2012) Non-catalytic functions of Pyk2 and Fyn regulate late stage adhesion in human T cells. PLoS ONE 7, e53011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beinke S., Phee H., Clingan J. M., Schlessinger J., Matloubian M., Weiss A. (2010) Proline-rich tyrosine kinase-2 is critical for CD8 T-cell short-lived effector fate. Proc. Natl. Acad. Sci. USA 107, 16234–16239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson D., Shi X., Zhong M. C., Rhee I., Veillette A. (2010) The phosphatase PTP-PEST promotes secondary T cell responses by dephosphorylating the protein tyrosine kinase Pyk2. Immunity 33, 167–180. [DOI] [PubMed] [Google Scholar]
- 28.Katagiri T., Takahashi T., Sasaki T., Nakamura S., Hattori S. (2000) Protein-tyrosine kinase Pyk2 is involved in interleukin-2 production by Jurkat T cells via its tyrosine 402. J. Biol. Chem. 275, 19645–19652. [DOI] [PubMed] [Google Scholar]
- 29.Houtman J. C., Houghtling R. A., Barda-Saad M., Toda Y., Samelson L. E. (2005) Early phosphorylation kinetics of proteins involved in proximal TCR-mediated signaling pathways. J. Immunol. 175, 2449–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keck K., Volper E. M., Spengler R. M., Long D. D., Chan C. Y., Ding Y., McCaffrey A. P. (2009) Rational design leads to more potent RNA interference against hepatitis B virus: factors effecting silencing efficiency. Mol. Ther. 17, 538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapman N. M., Connolly S. F., Reinl E. L., Houtman J. C. (2013) Focal adhesion kinase negatively regulates Lck function downstream of the T cell antigen receptor. J. Immunol. 191, 6208–6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartelt R. R., Cruz-Orcutt N., Collins M., Houtman J. C. (2009) Comparison of T cell receptor-induced proximal signaling and downstream functions in immortalized and primary T cells. PLoS ONE 4, e5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunnell S. C., Barr V. A., Fuller C. L., Samelson L. E. (2003) High-resolution multicolor imaging of dynamic signaling complexes in T cells stimulated by planar substrates. Sci. STKE 2003, PL8. [DOI] [PubMed] [Google Scholar]
- 34.Chapman N. M., Bilal M. Y., Cruz-Orcutt N., Knudson C., Madinaveitia S., Light J., Houtman J. C. (2013) Distinct signaling pathways regulate TLR2 co-stimulatory function in human T cells. Cell. Signal. 25, 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finkelstein L. D., Shimizu Y., Schwartzberg P. L. (2005) Tec kinases regulate TCR-mediated recruitment of signaling molecules and integrin-dependent cell adhesion. J. Immunol. 175, 5923–5930. [DOI] [PubMed] [Google Scholar]
- 36.Sancho D., Nieto M., Llano M., Rodríguez-Fernández J. L., Tejedor R., Avraham S., Cabañas C., López-Botet M., Sánchez-Madrid F. (2000) The tyrosine kinase PYK-2/RAFTK regulates natural killer (NK) cell cytotoxic response, and is translocated and activated upon specific target cell recognition and killing. J. Cell Biol. 149, 1249–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.St-Pierre J., Lysechko T. L., Ostergaard H. L. (2011) Hypophosphorylated and inactive Pyk2 associates with paxillin at the microtubule organizing center in hematopoietic cells. Cell. Signal. 23, 718–730. [DOI] [PubMed] [Google Scholar]
- 38.Berg N. N., Ostergaard H. L. (1997) T cell receptor engagement induces tyrosine phosphorylation of FAK and Pyk2 and their association with Lck. J. Immunol. 159, 1753–1757. [PubMed] [Google Scholar]
- 39.Okigaki M., Davis C., Falasca M., Harroch S., Felsenfeld D. P., Sheetz M. P., Schlessinger J. (2003) Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc. Natl. Acad. Sci. USA 100, 10740–10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi C. S., Kehrl J. H. (2001) PYK2 links G(q)alpha and G(13)alpha signaling to NF-kappa B activation. J. Biol. Chem. 276, 31845–31850. [DOI] [PubMed] [Google Scholar]
- 41.Melikova S., Dylla S. J., Verfaillie C. M. (2004) Phosphatidylinositol-3-kinase activation mediates proline-rich tyrosine kinase 2 phosphorylation and recruitment to beta1-integrins in human CD34+ cells. Exp. Hematol. 32, 1051–1056. [DOI] [PubMed] [Google Scholar]
- 42.Rumsey L. M., Teague R. M., Benedict S. H., Chan M. A. (2001) MIP-1alpha induces activation of phosphatidylinositol-3 kinase that associates with Pyk-2 and is necessary for B-cell migration. Exp. Cell Res. 268, 77–83. [DOI] [PubMed] [Google Scholar]
- 43.Tse K. W., Dang-Lawson M., Lee R. L., Vong D., Bulic A., Buckbinder L., Gold M. R. (2009) B cell receptor-induced phosphorylation of Pyk2 and focal adhesion kinase involves integrins and the Rap GTPases and is required for B cell spreading. J. Biol. Chem. 284, 22865–22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ostergaard H. L., Lysechko T. L. (2005) Focal adhesion kinase-related protein tyrosine kinase Pyk2 in T-cell activation and function. Immunol. Res. 31, 267–282. [DOI] [PubMed] [Google Scholar]
- 45.Guinamard R., Okigaki M., Schlessinger J., Ravetch J. V. (2000) Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1, 31–36. [DOI] [PubMed] [Google Scholar]
- 46.Wiemer A. J., Wernimont S. A., Cung T. D., Bennin D. A., Beggs H. E., Huttenlocher A. (2013) The focal adhesion kinase inhibitor PF-562,271 impairs primary CD4+ T cell activation. Biochem. Pharmacol. 86, 770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts W. G., Ung E., Whalen P., Cooper B., Hulford C., Autry C., Richter D., Emerson E., Lin J., Kath J., Coleman K., Yao L., Martinez-Alsina L., Lorenzen M., Berliner M., Luzzio M., Patel N., Schmitt E., LaGreca S., Jani J., Wessel M., Marr E., Griffor M., Vajdos F. (2008) Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 68, 1935–1944. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds L. F., Smyth L. A., Norton T., Freshney N., Downward J., Kioussis D., Tybulewicz V. L. (2002) Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and -independent pathways. J. Exp. Med. 195, 1103–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garçon F., Patton D. T., Emery J. L., Hirsch E., Rottapel R., Sasaki T., Okkenhaug K. (2008) CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood 111, 1464–1471. [DOI] [PubMed] [Google Scholar]
- 50.Gogishvili T., Elias F., Emery J. L., McPherson K., Okkenhaug K., Hünig T., Dennehy K. M. (2008) Proliferative signals mediated by CD28 superagonists require the exchange factor Vav1 but not phosphoinositide 3-kinase in primary peripheral T cells. Eur. J. Immunol. 38, 2528–2533. [DOI] [PubMed] [Google Scholar]
- 51.Boomer J. S., Green J. M. (2010) An enigmatic tail of CD28 signaling. Cold Spring Harb. Perspect. Biol. 2, a002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dodson L. F., Boomer J. S., Deppong C. M., Shah D. D., Sim J., Bricker T. L., Russell J. H., Green J. M. (2009) Targeted knock-in mice expressing mutations of CD28 reveal an essential pathway for costimulation. Mol. Cell. Biol. 29, 3710–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holdorf A. D., Lee K. H., Burack W. R., Allen P. M., Shaw A. S. (2002) Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nat. Immunol. 3, 259–264. [DOI] [PubMed] [Google Scholar]
- 54.Mueller K. L., Daniels M. A., Felthauser A., Kao C., Jameson S. C., Shimizu Y. (2004) Cutting edge: LFA-1 integrin-dependent T cell adhesion is regulated by both ag specificity and sensitivity. J. Immunol. 173, 2222–2226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.