Borrelia burgdorferi induction of indoleamine 2,3-dioxygenase byhuman immune cells correlates with pathogenesis, results in DCactivation, and requires TLR7 signaling and type I and type IIinterferons.
Keywords: Lyme disease, immunosuppression, bacterium, dendritic cells
Abstract
Borrelia burgdorferi, the bacterial agent of Lyme disease, induces the production of type I IFNs by human DCs through TLR7 and TLR9 signaling. This type I IFN response occurs in a genotype-dependent manner, with significantly higher levels of IFN-α elicited by B. burgdorferi strains that have a greater capacity for causing disseminated infection. A B. burgdorferi strain that was previously shown to induce IFN-α was found to elicit significantly higher levels of IDO1 protein and its downstream metabolite, kynurenine, compared with a B. burgdorferi mutant that lacks a single linear plasmid (lp36); this mutant is unable to induce IFN-α and is severely attenuated for infectivity in mice. Production of IDO by mDC and pDC populations, present within human PBMCs, was concomitant with increased expression of the DC maturation markers, CD83 and CCR7. The defects in IDO production and expression of CD83 and CCR7 could be restored by complementation of the mutant with lp36. Maximal IDO production in response to the wild-type strain was dependent on contributions by both type I IFN and IFN-γ, the type II IFN. Induction of IDO was mediated by the same TLR7-dependent recognition of B. burgdorferi RNA that contributes to the production of type I IFNs by human DCs. The ability of IFN-α-inducing B. burgdorferi strains to stimulate production of IDO and kynurenines may be a mechanism that is used by the pathogen to promote localized immunosuppression and facilitate hematogenous dissemination.
Introduction
B. burgdorferi is the causative agent of Lyme disease, the most common arthropod-borne disease in the United States. Acquisition of the spirochete through the bite of an infected tick frequently results in a distinctive skin rash, or EM, which is characterized by an influx of immune cells at the site of inoculation [1, 2]. This inflammatory infiltrate contains cellular components of PBMCs, including T lymphocytes, monocytes, and mDCs and pDCs, which participate in the initial host-pathogen interaction [2]. B. burgdorferi elicits the production of a wide array of cytokines that underlie the inflammation associated with Lyme disease. The development of inflammation is dependent on host recognition of spirochetal PAMPs by PRRs expressed by cells of the innate immune system, especially the TLRs [3–5]. In some patients, disseminated infection occurs when spirochetes migrate from the initial site of infection to distal sites in the body [6]. Sequelae of disseminated Lyme disease are also distinguished by a robust inflammatory response and include carditis, arthritis, and neuroborreliosis [6].
Our group [3, 4] and others [7–9] have shown that this extracellular pathogen induces the production of type I IFNs by human DCs and monocytes, as well as by murine cells. Our previous study [4] used global transcriptional profiling to characterize the response of human PBMCs to a clinical isolate of B. burgdorferi by use of an ex vivo coincubation model. This work demonstrated that B. burgdorferi stimulates the production of high levels of IFN-α protein and downstream type I IFN-associated gene transcripts via TLR7 and TLR9 signaling in human pDC and mDC subsets [4, 10]. In addition, Cervantes et al. [3] has described IFN-β transcriptional activation in human monocytes following stimulation with live B. burgdorferi. Both reports emphasize that type I IFN production is absolutely dependent on phagocytic uptake of spirochetes [3, 4].
DCs, although very low in frequency, are crucial APCs responsible for mounting and initiating a variety of immune processes [11]. DCs undergo rapid maturation upon encountering pathogens and via PAMP recognition by DC-expressed PRRs, produce cytokines and chemokines, up-regulate maturation and migration markers, and prepare to present antigen to T cells for adaptive immune response priming [11–13]. DCs are classified into 2 broad categories, characterized primarily by morphology and expression of surface markers: mDCs and pDCs [12, 14]. mDCs express the cell-surface marker CD11c, at high levels in the case of mDC1s or dimly expressed in mDC2s; pDCs are negative for CD11c [15]. Interestingly, it has been found that CD11c+ mDCs and CD11c− pDCs are enriched in the EM lesions of Lyme disease patients, and there are significantly higher levels of IFN-α found in the serum in patients with evidence of disseminated disease compared with patients with localized disease [2]. A previous study by this laboratory identified pDCs and CD11c+CD14+ mDC precursors to be the predominant producers of the IFN-α observed in human PBMCs in response to B. burgdorferi [4].
Recent reports have given much attention to a new population of tolerogenic DCs [16–18]. These tolerogenic DCs have the ability to express IDO, which can result in an attenuated immune response to a variety of pathogens, including many bacteria [19–21]. IDO is the rate-limiting enzyme in the catabolism of tryptophan, catalyzing the conversion of tryptophan to N-formylkynurenine [22]. It has been proposed that the immunomodulatory mechanisms of IDO are mediated by the generation of cytotoxic kynurenines, as well as via tryptophan depletion [23]. IDO is induced primarily through type I and type II IFN signaling but can be augmented in response to other proinflammatory stimuli [24–26]. These IDO-expressing DCs have been shown to express maturation markers associated with classically activated DCs, such as CD83 and CCR7 [27, 28]. Myeloid-derived suppressor cells, a subtype of tolerogenic DCs, are increased in malignant melanoma patients; these immunosuppressive DCs overexpress CD83 and promote tumorigenesis by suppressing T cell responses [29]. DC-mediated IDO activity is able to mediate localized immunosuppression through the generation of regulatory T cells from naïve T cells and by the induction of effector T cell apoptosis, leading to an overall suppression of T cell immunity [16, 17, 30, 31]. Recent studies of pathogens such as uropathogenic Escherichia coli, Mycobacterium tuberculosis, and Mycobacterium leprae have indicated that IDO expression and activity may facilitate pathogen persistence and in some cases, even promote virulence and pathogenesis by establishing localized immune suppression in epithelial tissues [19, 32].
Significantly higher levels of type I IFN are induced by B. burgdorferi strains with greater pathogenic potential [33]. In addition, these IFN-inducing B. burgdorferi strains associate more avidly with mDCs and pDCs [33]. Phagocytic uptake of B. burgdorferi by DCs initiates signaling through TLR7 and TLR9 that leads to the production of type I IFNs [4], which are potent stimulators of IDO production. Thus, the differential ability of B. burgdorferi strains to induce type I IFN may correlate with the ability to induce IDO by those same DC populations, resulting in regional immune suppression that can be exploited to facilitate hematogenous dissemination of the spirochete. This study was designed to investigate the expression of IDO by human PBMCs and to characterize the potential immunosuppressive phenotypes of human DCs in response to strains of B. burgdorferi with differing pathogenic potential to provide insight into possible mechanisms of dissemination.
MATERIALS AND METHODS
Isolation of human PBMCs
Venous blood was obtained from each of 4 healthy volunteers (2 male, 2 female; 25–65 yr of age) with no prior history of Lyme disease, who had not been vaccinated for Lyme disease and who were seronegative for B. burgdorferi infection. as confirmed by Western immunoblotting. Written, informed consent was obtained from all subjects before blood collection, in accordance with a protocol approved by the Institutional Review Board of New York Medical College. Blood was collected directly into BD Vacutainer CPT tubes (BD Biosciences, San Jose, CA, USA), and PBMCs were obtained by centrifugation, per the manufacturer’s instructions. PBMCs were washed twice with HBSS (Gibco-BRL, Grand Island, NY, USA), without calcium, magnesium, or phenol red (Invitrogen, Grand Island, NY, USA), and resuspended in RPMI 1640 without phenol red (Gibco-BRL), containing 10% (v/v) heat-inactivated and endotoxin-free FBS (HyClone, Waltham, MA, USA). Viability of PBMCs was determined by trypan blue (American Type Culture Collection, Manassas, VA, USA) staining and was found to be >90%. PBMCs were maintained at 37°C in a humidified incubator containing 5% CO2.
B. burgdorferi strains and culture
B. burgdorferi A3-M9 lp36− and A3-M9 lp36−/lp36+ are B31 derivative strains and have been described previously [34]. B. burgdorferi B515, a human clinical isolate, has been described previously [35]. B. burgdorferi were cultivated in modified Barbour-Stoenner-Kelley medium [36] at 37°C until spirochetes reached the mid- to late-log phase of growth [36]. To simulate the temperature shifts that occur during tick-to-mammal transmission and that affect the expression of virulence factors, spirochetes were grown at 25°C, subcultured, and incubated at 37°C until spirochetes reached the mid-log phase of growth (4 × 107–1 × 108/ml). Spirochetes were enumerated and assessed for motility by dark-field microscopy [37]. Before coincubation with PBMCs, spirochetes were centrifuged for 10 min at 8000 g, washed twice with HBSS, and resuspended at a concentration of 5 × 108/ml in RPMI 1640 containing 10% FBS.
Preparation of B. burgdorferi lysate and RNA
Lysate was prepared from 50 ml cultures of B. burgdorferi B515 by use of glass bead-based lysis, as described previously [10]. Spirochete membrane disruption was confirmed by dark-field microscopy [37]. Lysate protein concentration was measured by the Bradford assay and adjusted to 1.0 µg/µl. Total RNA was prepared from a 50 ml culture of B. burgdorferi B515 by use of the ToTally RNA kit (Ambion, Life Technologies, Grand Island, NY, USA), as described previously [10]. Contaminating DNA was removed by treatment with the TURBO DNA-free kit (Ambion, Life Technologies), according to the manufacturer’s instructions. The final concentration of RNA was adjusted to 1.0 μg/μl, 0.5 μl RNase inhibitor (40 U/μl; Promega, Madison, WI, USA) was added, and RNA was stored at −80°C in single-use aliquots. RNA yield for each 50 ml culture was ∼60 μg (4 × 107 B. burgdorferi/μg RNA).
DOTAP methosulfate encapsulation of B. burgdorferi RNA
DOTAP methosulfate (Sigma, St. Louis, MO, USA), a liposomal transfection reagent, was used to deliver B. burgdorferi RNA to PBMCs via the endosomal pathway [38]. B. burgdorferi RNA was mixed with DOTAP at a 1:1 (vol:vol) ratio and incubated at room temperature for 20 min, immediately before stimulation of PBMCs.
Coincubation of human PBMCs with B. burgdorferi
Ex vivo stimulation of freshly isolated human PBMCs was performed in a manner similar to that described previously [4]. Freshly isolated human PBMCs were suspended to a final concentration of 5 × 106 viable cells/ml in RPMI 1640 containing 10% heat-inactivated FBS. Spirochetes were centrifuged at 8000 g for 10 min, washed twice with HBSS, and resuspended in RPMI 1640 without phenol red containing 10% (v/v) heat-inactivated and endotoxin-free FBS. Live B. burgdorferi (5 × 107 MOI = 10:1 viable spirochetes to PBMCs) was added to triplicate wells for a final volume of 1.1 ml. Control wells received 100 µl medium alone. PBMCs were coincubated for 12 or 24 h, as indicated in figure legends. Cell-free culture supernatants were stored at −20°C. PBMC pellets were washed twice with PBS and processed immediately for flow cytometry or aliquoted and frozen at −20°C for Western immunoblot analysis.
TLR ligands and inhibitors
Pam2CSK4 (InvivoGen, San Diego, CA, USA) was added to PBMCs at a final concentration of 1 µg/ml. An ODN inhibitor of TLR7 signaling (IRS661; 5′-GCTTGCAAGCTTGCAAGCA-3′) and a control ODN sequence (5′-TCCTGCAGGTTAAGT-3′) were synthesized by Integrated DNA Technologies (Newark, NJ, USA) and purified by ion-exchange HPLC [39, 40]. Endotoxin levels of all ODNs were <0.1 U/ml, as determined by the LAL assay (Lonza, Allendale, NJ, USA). ODNs were used at a concentration of 5.6 µM, as described previously [4, 39]. Where indicated, ODNs were added to PBMCs, seeded in 24-well culture plates, 1 h before PBMC stimulation with live B. burgdorferi or B. burgdorferi cell components. A synthetic TLR2 agonist (Pam2CSK4) was added to PBMCs at the same time as B. burgdorferi stimuli, at the concentration described above.
Multiparameter flow cytometric analysis of IDO-expressing CD11c+CD14− mDCs
Human PBMCs were coincubated for 12 h with B. burgdorferi, as above. Following coincubation, the contents of each well were transferred to a 5 ml FACS tube and pelleted at 300 g for 10 min at 4°C in a hanging bucket centrifuge. PBMCs were washed twice with 1 ml stain buffer (0.2% BSA in sterile PBS), pelleting as specified above. PBMCs were resuspended in 100 μl antibody cocktail, including Fc blocker. The samples were incubated in the dark for 30 min at 4°C and washed once with stain buffer. To detect intracellular IDO protein, PBMCs were incubated in 250 μl Cytofix/Cytoperm solution (555028; BD Biosciences) for 20 min at 4°C. Cells were pelleted and washed twice with 1 ml 1× BD Perm/Wash buffer (555028; BD Biosciences) and resuspended in 50 μl antibody cocktail [5 μl anti-human IDO-Alexa Fluor 488; IC6030G (R&D Systems, Minneapolis, MN, USA), and 45 μl BD Perm/Wash buffer (BD Biosciences)]. Isotype control samples received 5 μl mouse IgG1-Alexa Fluor 488 (IC002G; R&D Systems). Samples were incubated for 30 min in the dark at 4°C and washed twice with BD Perm/Wash buffer (BD Biosciences). PBMCs were resuspended in 1 ml stain buffer for flow cytometric analysis. Four hundred thousand events were collected on a MACSQuant analyzer and analyzed by use of MACSQuantify software (Miltenyi Biotec, San Diego, CA, USA).
Multiparameter flow cytometric analysis of DC maturation markers
Human PBMCs were coincubated for 12 h with B. burgdorferi and washed with stain buffer as above. PBMCs were resuspended in 100 μl antibody cocktail, including Fc blocker [30 μl anti-human Lineage Cocktail-allophycocyanin and 10 μl anti-human CD11c-V450; 560369 (BD Biosciences)]; 20 μl anti-human CCR7/CD197-PE (560765; BD PharMingen, San Diego, CA, USA); 10 μl anti-human CD83-PE/Cy7 (305326; BioLegend, San Diego, CA, USA); 5 μl human IgG1 κ, purified myeloma protein (5154; Sigma); and 25 μl stain buffer. Mouse IgG2a-PE (555574; BD PharMingen) and mouse IgG1-PE/Cy7 (400126; BioLegend) were used as isotype controls in place of specific antibodies for CD83 or CCR7, respectively. The samples were incubated in the dark for 30 min at 4°C. Staining for intracellular IDO was performed as described above. Five hundred thousand events from samples were collected on a MACSQuant analyzer and analyzed by use of MACSQuantify software (Miltenyi Biotec). PBMCs were phenotyped initially, based on DC markers: mDCs were defined as Lineage-CD11c+, and pDCs were defined as Lineage-CD11c−. Compensation controls for each fluorochrome were prepared by use of OneComp eBeads (01-1111; eBioscience, San Diego, CA, USA).
Detection of IDO1 protein
Western immunoblotting was performed to detect levels of IDO1 in PBMC lysates. In brief, frozen pellets containing 2 × 106 PBMCs were thawed and resuspended in 300 µl of a buffer containing 40 mM Tris-HCl (pH 6.8), 2% SDS, 1.8 mM EDTA, 10% glycerol, 1% 2-ME, and 1× Halt Protease and Phosphatase Inhibitor Cocktail (Pierce, Thermo Fisher Scientific, Rockford, IL, USA). Cell suspensions were vortexed for 30 s and aspirated through 28-gauge insulin syringes to shear DNA. Lysates were then heated at 100°C for 10 min and cooled on ice. Lysates were cleared of cell debris by centrifugation at 18,000 g for 5 min. Aliquots (20 µl) of lysate (corresponding to 1.33 × 105 PBMCs; 40 µg total protein) were resolved by 12.5% SDS-PAGE, run at 40 mA for 2.5 h in SDS Running Buffer (25 mM Tris, 192 mM glycine, 2.5 mM EDTA, 0.1% SDS). Semi-dry electrotransfer of proteins to PVDF membranes was performed at 12 V for 36 min. For detection of IDO1, membranes were blocked for 1 h with 5% nonfat milk in TBST and incubated at 4°C overnight with rabbit IgG anti-human IDO1 antibody (Millipore, Billerica, MA, USA), diluted 1:1000 in 5% nonfat milk in TBST. Membranes were washed 3 times for 10 min with TBST, incubated with HRP-conjugated goat anti-rabbit IgG secondary antibody (Pierce, Thermo Fisher Scientific), diluted 1:20,000 in TBST containing 3% nonfat milk, and developed by use of SuperSignal West Pico ECL substrate (Pierce, Thermo Fisher Scientific). Following detection of protein, PVDF membranes were stripped and reprobed with an antibody to GAPDH. In brief, membranes were incubated in stripping buffer (2% SDS, 62.5 mM Tris HCl, pH 6.8, 1% 2-ME) for 30 min at 55°C and washed 3 times for 10 min in TBST. Membranes were blocked for 1 h in TBST containing 5% nonfat milk, washed 3 times for 10 min with TBST, and incubated overnight at 4°C with primary anti-human GAPDH IgG (Cell Signaling Technology, Danvers, MA, USA), diluted 1:1000 in TBST containing 5% nonfat milk. Membranes were washed, incubated with secondary conjugate, and developed, as described above. Protein levels were quantified by densitometry by use of ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA). The average pixel value was calculated for each protein from boxes of equal size, background was automatically subtracted, and pixel values for IDO1 protein were divided by pixel values for GAPDH. Results from multiple donors were normalized for comparison by designating the smallest and largest densitometry ratios for each donor as baseline values.
Determination of IDO enzymatic activity
To determine whether B. burgdorferi-induced IDO protein was enzymatically active, PBMCs were coincubated with live B. burgdorferi, as described above. At 20 h poststimulation, 15 µM L-tryptophan (Sigma) was added to each well, and the incubation was allowed to proceed for an additional 4 h. PBMCs were pelleted at 300 g for 10 min, and kynurenine concentrations in culture supernatants were assessed by spectrophotometry [21, 31, 41]. In brief, supernatants were mixed 2:1 (90 µl total vol) with 30% TCA (Sigma) to hydrolyze any remaining unconverted N-formylkynurenine to L-kynurenine and incubated at 50°C for 30 min. Samples were then centrifuged at 3000 g for 10 min to pellet precipitated protein. Supernatant (75 µl) was added to an equal volume of Ehrlich’s reagent (2% p-dimethylaminobenzaldehyde in glacial acetic acid; Sigma) in a 96-well plate and incubated for 15 min at room temperature. OD was measured at 492 nm, and unknown kynurenine values were interpolated based on a standard curve of known kynurenine concentrations (0–250 µM).
Identification of IFNs responsible for IDO induction
To investigate which cytokines promote the expression of B. burgdorferi-induced IDO1 in human PBMCs, B. burgdorferi strains were coincubated with human PBMCs as above. Control wells received medium alone. B18R (suspended in PBS, pH 7.2, 150 mM NaCl, 1% BSA), a vaccinia virus-encoded, neutralizing type I IFNR [4, 42–45], was purchased from eBioscience and certified to have an endotoxin level of <0.01 ng/μg. A human α-IFNGR1/CD119 (clone 92101; suspended in PBS at 0.5 mg/ml) [46, 47], was purchased from R&D Systems and certified to have an endotoxin level of <0.1 endotoxin units/μg, as determined by the LAL method. Where indicated, B18R, α-IFNGR1, or both were added before PBMC stimulation at final concentrations of 0.1 μg/ml and 20 μg/ml, respectively [4, 46]. At 20 h poststimulation, 25 µM L-tryptophan (Sigma) was added to each well, and incubation proceeded for an additional 4 h. Following a cumulative 24 h coincubation, PBMC pellets were washed twice with PBS and frozen at −20°C for Western immunoblot analysis.
Statistical analyses
Significant differences between the mean values of experimental groups were determined by one-way ANOVA with Tukey-Kramer’s post-test by use of GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA, USA). For all tests, P < 0.05 was considered statistically significant.
RESULTS
B. burgdorferi induces production of enzymatically active IDO in human PBMCs in a genotype-dependent manner
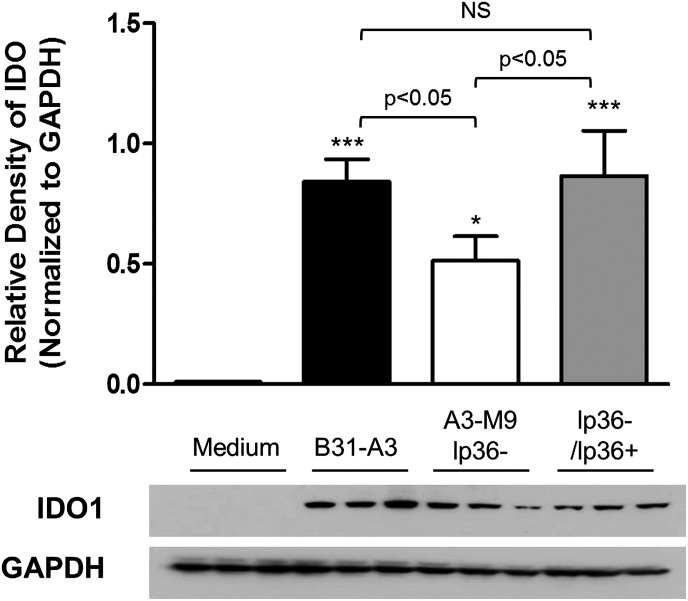
We have previously identified a correlation between pathogenicity of B. burgdorferi and affinity for pDCs and mDCs. Strains of B. burgdorferi that more effectively induce the production of type I IFNs associate more avidly with pDC and mDC subsets; however, these same strains have a greater tendency to disseminate than the strains that are unable to induce type I IFNs [33]. A potential immunoregulatory mechanism used by DCs to modulate the innate immune response is mediated through the production of the enzyme IDO, the rate-limiting enzyme in tryptophan catabolism [26, 41]. There has been considerable attention given to the role of IDO in promoting the pathogenesis of a variety of infectious agents [19, 32, 48]. To investigate whether IDO was playing a role in B. burgdorferi pathogenesis, we performed an ex vivo coincubation by use of PBMCs from healthy donors stimulated with B. burgdorferi strains known to have varying capacities to cause disseminated infection. B31-A3 is a derivative of type strain B31 that induces significant levels of IFN-α by human PBMCs [33] and causes disseminated infection in mice [49]. A3-M9 lp36−, a mutant of B31-A3 that lacks a single linear plasmid (lp36), is unable to induce IFN-α by human PBMCs and is severely attenuated for infectivity [34]. A3-M9 lp36−/lp36+ was generated by reintroduction of lp36 into A3-M9 and is comparable with B31-A3 in its capacity to induce IFN-α [33] and to disseminate in mice [34]. After 12 h of incubation with live spirochetes at an MOI of 10, IDO production was assessed by Western immunoblotting. IDO protein could not be detected in unstimulated PBMCs (Fig. 1). Stimulation of PBMCs with B. burgdorferi strain B31-A3 resulted in significant production of IDO protein (relative density 0.842 IDO:GAPDH; P < 0.001). Interestingly, stimulation of PBMCs with B. burgdorferi A3-M9 lp36−, a strain that is attenuated for infection and is unable to induce type I IFNs, resulted in a more modest production of IDO; this was significantly lower than levels elicited by B31-A3 (relative density 0.514 IDO:GAPDH; P < 0.05 relative to B31-A3; Fig. 1). Furthermore, complementation of A3-M9 lp36− by reintroduction of lp36 (A3-M9 lp36−/lp36+) restored IDO production to that observed after stimulation with B31-A3 (relative density 0.866 IDO:GAPDH; not significantly different from B31-A3).
Figure 1. The presence of lp36 is required for maximal IDO production in human PBMCs.
Human PBMCs were coincubated for 12 h at 37°C with B. burgdorferi strains B31-A3, A3-M9 lp36−, and A3-M9 lp36−/lp36+ at a MOI of 10. PBMC lysates were resolved by 12.5% SDS-PAGE for Western immunoblotting with rabbit anti-human IDO1. Densitometric values of protein expression were normalized to GAPDH and quantitated by use of ImageJ software. Columns show the mean ± sd of PBMCs from 3 donors, assessed in triplicate in independent experiments. Representative Western blot images from 1 donor are shown. One-way ANOVA with Tukey’s post-test was used for statistics. ***P < 0.001; *P < 0.05, relative to PBMCs incubated with medium alone. NS, not significantly different.
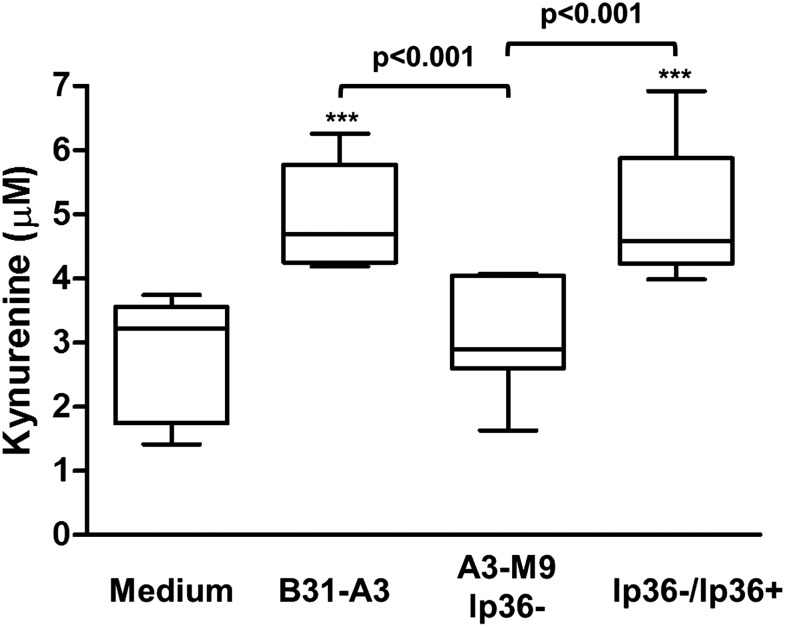
IDO is the rate-limiting enzyme in the catabolism of tryptophan, and the first functional downstream metabolite is L-kynurenine [23, 50]. To investigate whether the induced IDO protein was enzymatically active, we assessed the levels of kynurenine present in culture supernatants following coincubation. PBMCs were stimulated with live B. burgdorferi (MOI = 10) for 20 h. To provide a substrate for IDO, 15 μM L-tryptophan was added to the culture media, and the incubation was allowed to proceed for an additional 4 h before harvesting cell-free culture supernatants. With the use of a modified spectrophotometric assay, significant levels of kynurenine were detected in supernatants of PBMCs stimulated with B31-A3 compared with unstimulated PBMCs (5.03 μM; P < 0.001), whereas levels of kynurenine in response to A3-M9 lp36− were not increased significantly (3.06 μM; P > 0.05; Fig. 2) [21, 31, 41]. Moreover, stimulation of PBMCs with the lp36-complemented strain restored kynurenine production to levels comparable with that induced by B31-A3 (5.00 μM; P < 0.001, relative to unstimulated PBMCs; Fig. 2). Taken together, the data indicate that production of enzymatically active IDO by human PBMCs in response to B. burgdorferi occurs in a manner that correlates with the ability of these strains to induce type I IFN.
Figure 2. IDO produced in response to B. burgdorferi is enzymatically active.
Human PBMCs were coincubated for 24 h at 37°C with B. burgdorferi strains B31-A3, A3-M9 lp36−, and A3-M9 lp36−/lp36+ at a MOI of 10. At 20 h poststimulation, 15 μM L-tryptophan was added to the culture media, and incubation was allowed to proceed for an additional 4 h. Cell-free supernatants were harvested and used for a spectrophotometric assay for secreted kynurenine. Values of kynurenine were interpolated from a standard curve of known kynurenine concentrations. Data are the results from 3 donors assessed in triplicate in independent experiments. The median is represented by the lines inside of the boxes, and whiskers indicate minimum and maximum values. Statistical analysis was performed by use of a one-way ANOVA with Tukey’s post-test. ***P < 0.001, relative to unstimulated PBMCs incubated with medium alone.
Type I IFN-inducing B. burgdorferi promotes maturation and IDO expression within human DC subsets
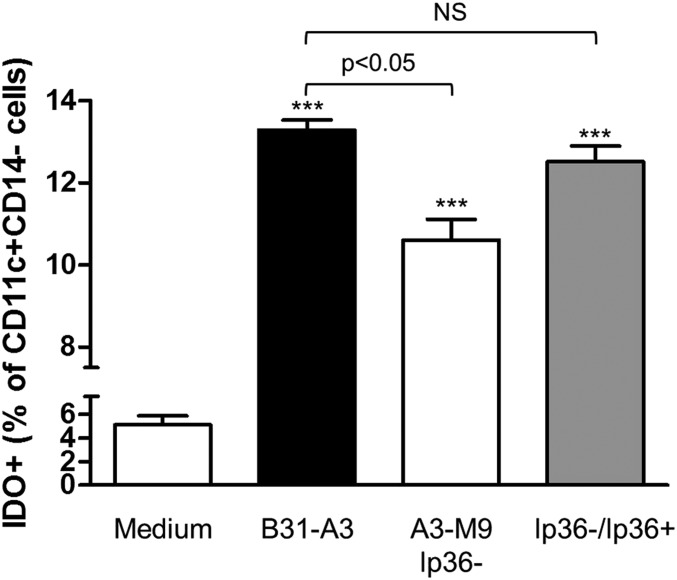
To characterize the predominant IDO-producing cells in the PBMC populations evaluated, multiparameter flow cytometry was performed. PBMCs were stimulated with B. burgdorferi for 12 h, harvested, and stained with fluorophore-conjugated antibodies to the cell-surface markers CD14 and CD11c and to intracellular IDO1. Previous studies had identified an increase in CD11c+CD14− mDCs in PBMCs following stimulation with B31-A3 [33]. There was a significant increase in CD11c+CD14− mDCs in response to B31-A3 (data not shown), consistent with these findings. In addition, B31-A3 elicited a significant increase in the IDO expression in these same mDC populations (13.28% IDO+; P < 0.001; Fig. 3). There was a modest, but significant, increase in IDO-expressing mDCs in response to A3-M9 lp36−, which is unable to induce IFN-α; however, this increase in IDO-expressing mDCs was reduced significantly relative to B31-A3 (10.61% IDO+; P < 0.05; Fig. 3). The proportion of IDO+ mDCs was restored to levels consistent with the parent strain B31-A3 upon reintroduction of lp36 (12.51% IDO+; P < 0.001; Fig. 3). IDO expression by mDCs paralleled the pattern of total IDO protein by the entire PBMC population, with significantly lower levels induced by A3-M9 lp36− (Fig. 1).
Figure 3. The presence of lp36 promotes an increase in IDO-expressing mDCs.
Human PBMCs were coincubated for 12 h at 37°C with B. burgdorferi at a MOI of 10. Cells were immunostained, and FACS was used to assess the percentage of IDO-expressing CD11c+CD14− cells in unstimulated PBMCs (Medium; white bar) or after coincubation with B31-A3 (black bar), A3-M9 lp36− (white bar), and A3-M9 lp36−/lp36+ (gray bar). Statistical analysis was performed by use of a one-way ANOVA with Tukey-Kramer’s post-test. ***P < 0.001, relative to PBMCs incubated with medium alone. Four hundred thousand events/sample were collected.
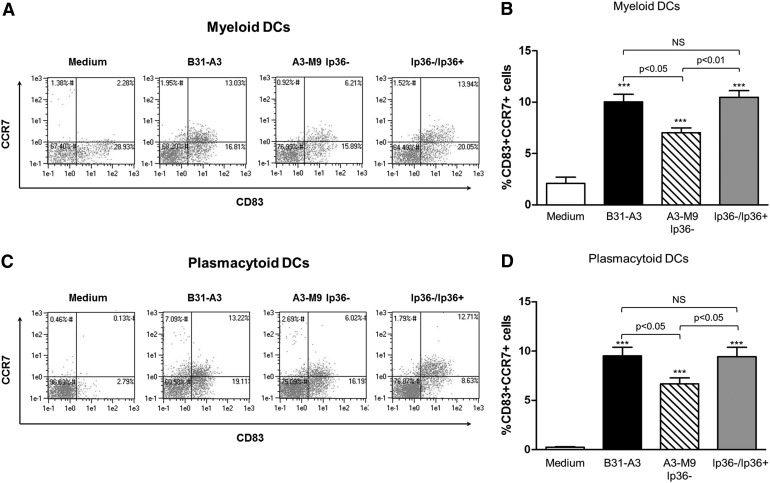
To characterize further changes in DC populations that are induced by B. burgdorferi, PBMCs were stimulated for 12 h, harvested, and stained with fluorophore-conjugated antibodies to the cell-surface markers CCR7, CD83, and CD11c and to intracellular IDO. PBMC populations other than DCs were eliminated from the analysis by staining with an antibody cocktail to lineage markers CD3, CD16, CD19, CD20, CD56, and CD14. Significant increases in mDCs (Lineage−CD11c+) and pDCs (Lineage−CD11c−), expressing the DC maturation markers CD83 and CCR7, were observed in response to B31-A3 (10.02% mDCs, 9.51% pDCs; P < 0.001 for both; Fig. 4). Of these cell populations, nearly 100% was also IDO positive (Fig. 5). In contrast, there was significantly lower expression of CD83 and CCR7 in response to A3-M9 lp36− in mDCs and pDCs (7.03% mDCs, 6.67% pDCs; P < 0.05 for both relative to B31-A3; Fig. 4). This defect in DC maturation marker expression was restored upon reintroduction of lp36 (10.48% mDCs, P < 0.01; 9.44% pDCs, P < 0.05, relative to A3-M9 lp36−; Fig. 4). Taken together, the data suggest that B. burgdorferi-induced IDO expression among mDC and pDC populations in human PBMCs correlates with the induction of a mature DC phenotype.
Figure 4. Type I IFN-inducing strains of B. burgdorferi promote enhanced expression of DC maturation markers.
Human PBMCs were coincubated for 12 h at 37°C with B. burgdorferi strains B31-A3, A3-M9 lp36−, and A3-M9 lp36−/lp36+ at a MOI of 10. Cells were immunostained to assess the expression of CCR7 and CD83 by mDC (A and B) and pDC (C and D) populations following exposure to B. burgdorferi. Columns depict the mean ± sd of results obtained by use of PBMCs from 3 donors, assessed in triplicate in independent experiments. (A and C) Representative dot plots from a single donor are shown. Statistical analysis was performed by use of a one-way ANOVA with Tukey-Kramer’s post-test. ***P < 0.001 relative to unstimulated PBMCs. Five hundred thousand events/sample were collected.
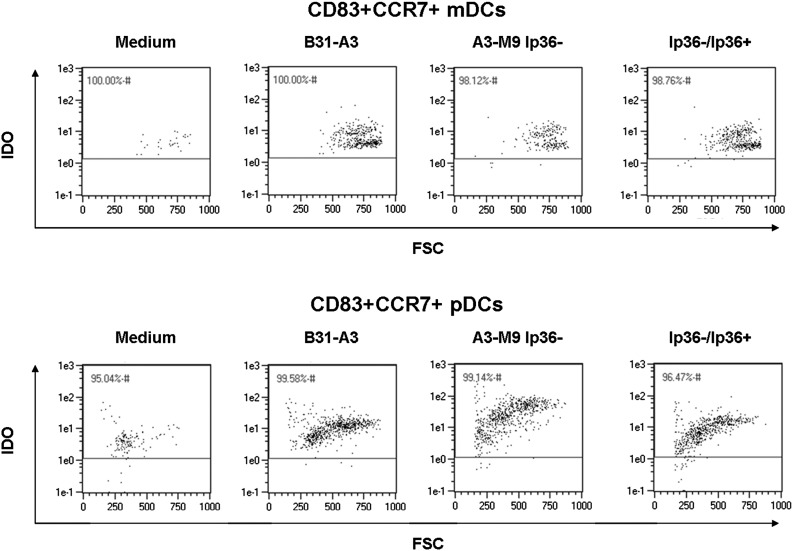
Figure 5. CCR7+CD83+ mature mDC and pDC populations coexpress IDO.
Human PBMCs were coincubated for 12 h at 37°C with B. burgdorferi strains B31-A3, A3-M9 lp36−, and A3-M9 lp36−/lp36+ at a MOI of 10. Cells were immunostained to assess intracellular IDO expression by CD83+CCR7+ mDCs (A) and pDCs (B). Dot plots are representative of 3 donors assessed in triplicate in independent experiments. Five hundred thousand events/sample were collected. FSC, Forward-scatter.
Cooperativity between type I and type II IFNs is required for maximum IDO production in human PBMCs
The human IDO1 gene contains several IFN-responsive regulatory elements within the promoter region [50, 51]. This suggests that the induction of IDO1 is multifactorial; indeed, it can be mediated by host signaling in response to a variety of cytokines [22, 24, 41, 50]. Induction of IDO in many cell types is mediated principally by type II IFNs, with only a small contribution made by the production of type I IFNs [24, 26]. However, in DCs, type I and type II IFNs have equivalent potency with regard to the production of IDO [24]. We have illustrated that B31-A3 and A3-M9 lp36− differ in their ability to induce type I IFN production in human PBMCs, whereas both induce comparable levels of IFN-γ and other cytokines [33]. As both classes of IFN are potentially implicated in IDO production, it was necessary to evaluate the relative contributions of type I and type II IFNs to IDO production in our human PBMC model.
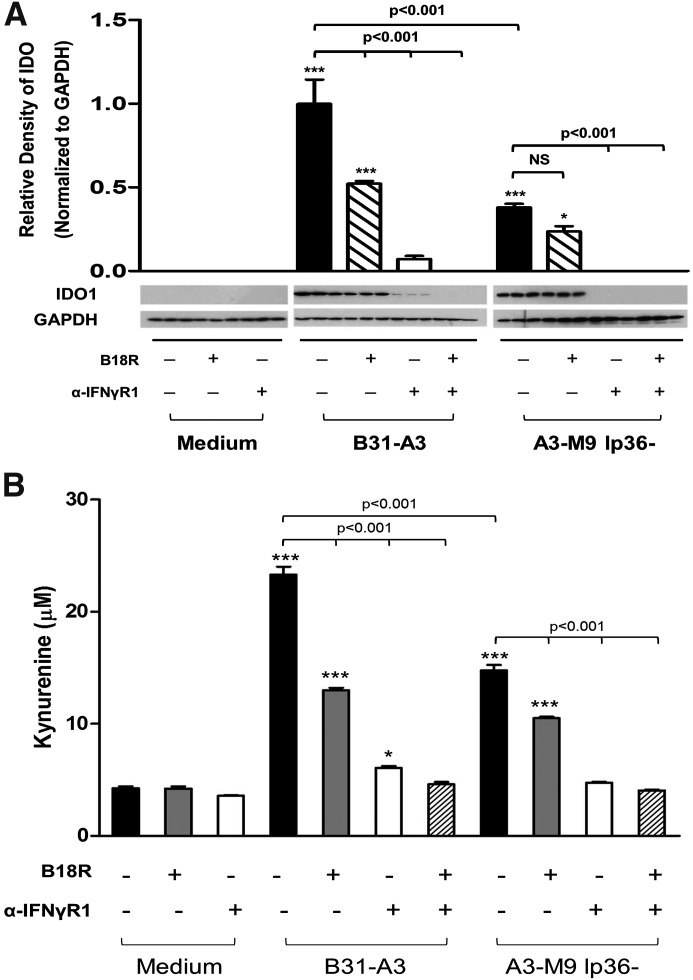
To inhibit type I and type II signaling, a soluble decoy IFN-αR (B18R), or α-IFNGR1, or a combination of both were added to the PBMC culture media, 1 h before stimulation with B. burgdorferi. At 20 h poststimulation, 25 μM l-tryptophan was added to culture media to serve as a substrate for IDO, and the incubation was allowed to proceed for an additional 4 h before harvesting cell-free supernatants and PBMCs pellets. Consistent with previous observations, B31-A3 induced significantly higher levels of IDO than A3-M9 lp36− (relative density = 0.998 IDO:GAPDH, B31-A3; relative density = 0.381 IDO:GAPDH, A3-M9 lp36−; P < 0.001 between groups; Fig. 6A). A significant reduction in IDO was observed when PBMCs were treated with α-IFNGR1 or both α-IFNGR1 and B18R before stimulation with B. burgdorferi B31-A3 or A3-M9 lp36− (Fig. 6A). In contrast, inhibition of type I IFN signaling resulted in a significant reduction in IDO production in response to B31-A3 but not in response to A3-M9 lp36− (Fig. 6A). Moreover, induction of IDO by B31-A3 in the presence of B18R (∼50% of original levels) was comparable with that induced by A3-M9 lp36− in PBMCs that did not receive inhibitors (Fig. 6A). This observation is consistent with previous results demonstrating that both strains induce equivalent levels of IFN-γ, whereas only B31-A3 elicits expression of IFN-α (Krupna-Gaylord et al. [33]). Not unexpectedly, the effects of inhibition of IFN signaling on secreted kynurenine levels paralleled the effects on IDO expression. Treatment of PBMCs with α-IFNGR1 or B18R before stimulation with B31-A3 resulted in significant decreases in levels of secreted kynurenine, but complete abolition of kynurenine production was observed only in the presence of both inhibitors (Fig. 6B). In PBMCs that were stimulated with A3-M9 lp36−, α-IFNGR1 alone was sufficient to ablate kynurenine, whereas B18R resulted in a modest decrease (Fig. 6B). These data indicate that type I and type II IFN signaling are required for B. burgdorferi to elicit maximal production of enzymatically active IDO in human PBMCs.
Figure 6. B. burgdorferi-induced type I and type II IFNs cooperate to drive production of enzymatically active IDO in human PBMCs.
Human PBMCs were coincubated for 24 h at 37°C with B. burgdorferi strains B31-A3 and A3-M9 lp36− at a MOI of 10:1. Where indicated, B18R, a soluble vaccinia virus-encoded IFN-αR, and α-IFNGR1 were added to PBMCs, 1 h before stimulation with B. burgdorferi. (A) PBMC lysates were resolved by 12.5% SDS-PAGE for Western immunoblotting with rabbit anti-human IDO1. Densitometric values of protein expression were normalized to GAPDH and quantitated by use of ImageJ software. (B) Secreted kynurenine concentrations in cell-free culture supernatants were determined by a spectrophotometric assay and interpolated from a standard curve of known kynurenine concentrations. Columns depict the mean ± sd of results from 1 donor assessed in triplicate. Representative Western blot images are shown. Statistical analysis was performed by use of a one-way ANOVA with Tukey-Kramer’s post-test. ***P < 0.001; *P < 0.05, relative to PBMCs incubated with medium alone.
TLR7-mediated recognition of B. burgdorferi RNA contributes to the production of IDO in human PBMCs
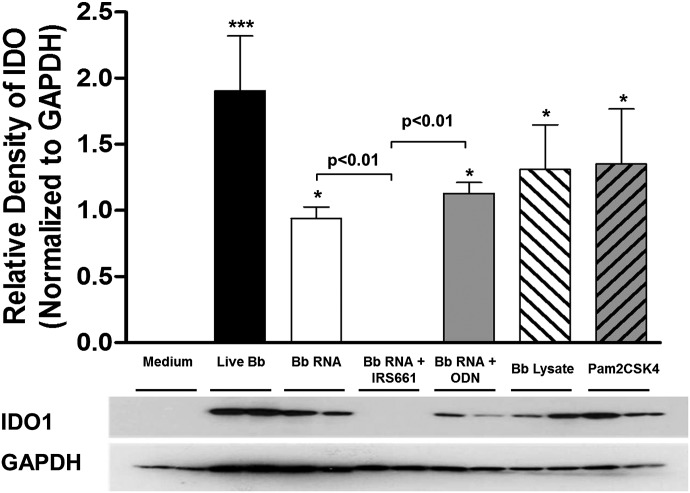
In the human PBMC system used here, B31-A3, a type I IFN-inducing strain of B. burgdorferi, is able to induce the production of significantly higher levels of enzymatically active IDO; A3-M9 lp36−, which is unable to induce IFN-α, exhibits a defect in this ability. We have demonstrated previously that B. burgdorferi is able to induce the observed type I IFN response through recognition of spirochetal RNA by the endosomal TLR7 [10]. Consequently, we sought to determine the contribution of RNA/TLR7-mediated signaling to the production of IDO. Whole-cell lysate and purified RNA were prepared from B. burgdorferi B515, a clinical isolate that like B31-A3, causes disseminated infection in mice [35] and was used previously to demonstrate TLR7 signaling by B. burgdorferi RNA [10]. PBMCs were coincubated for 12 h with live B. burgdorferi B515, isolated B. burgdorferi cellular components, or synthetic TLR agonists. RNA was complexed with DOTAP, a transfection reagent that promotes delivery via the endosomal pathway [38]. Stimulation of human PBMCs with live B. burgdorferi or DOTAP-complexed B. burgdorferi RNA resulted in the expression of significant levels of IDO protein (relative IDO:GAPDH densities: 1.78, live B. burgdorferi; 0.901, B. burgdorferi RNA; P < 0.05 for both compared with unstimulated PBMCs; Fig. 7). Induction of IDO by B. burgdorferi RNA could be ablated by the addition of the TLR7-specific inhibitor IRS661 before PBMC stimulation, whereas a nonspecific control ODN had no effect (Fig. 7). Interestingly, coincubation of PBMCs with B. burgdorferi lysate that had not been complexed with DOTAP and therefore, would be expected to elicit cytokine signaling predominantly through detection of B. burgdorferi lipoproteins by cell surface-localized TLR2 also produced significant levels of IDO, as did Pam2CSK4, a synthetic TLR2 agonist (relative IDO:GAPDH densities: 1.24, B. burgdorferi lysate; 1.85, Pam2CSK4; P < 0.05 for both; Fig. 7). This finding is not unexpected, as we have demonstrated that B. burgdorferi RNA and B. burgdorferi lysate both contributed to the induction of NF-κB-mediated cytokines [10]. These cytokines included IFN-γ, which contributes to the induction of IDO (Fig. 6). Taken together, these data indicate that TLR7-mediated signaling via recognition of B. burgdorferi RNA is a key modulator of IDO production, likely through the contribution to IFN-α and IFN-γ signaling pathways, with an additional contribution to IDO production by TLR2-mediated recognition of B. burgdorferi lipoproteins (Fig. 8).
Figure 7. B. burgdorferi RNA sensing through TLR7 contributes to IDO production.
Human PBMCs were incubated for 12 h with live B. burgdorferi (Bb) B515 at a MOI of 10; DOTAP-complexed B515 RNA (1 µg/ml) and B515 whole-cell lysate (1 µg/ml) added without DOTAP; or a TLR2-specific agonist (Pam2CSK4). Where indicated, a TLR7-specific inhibitor (IRS661) or a control ODN was added 1 h before stimulation. PBMC lysates were resolved by 12.5% SDS-PAGE for Western immunoblotting with rabbit anti-human IDO1. Densitometric values of protein expression were normalized to GAPDH and quantitated by use of ImageJ software. Columns represent the mean ± sd of results from 3 donors assessed in triplicate in independent experiments. Representative Western blot images from a single donor are shown. Statistical analysis was performed by use of a one-way ANOVA with Tukey-Kramer’s post-test. ***P < 0.001; *P < 0.05, relative to PBMCs incubated with medium alone.
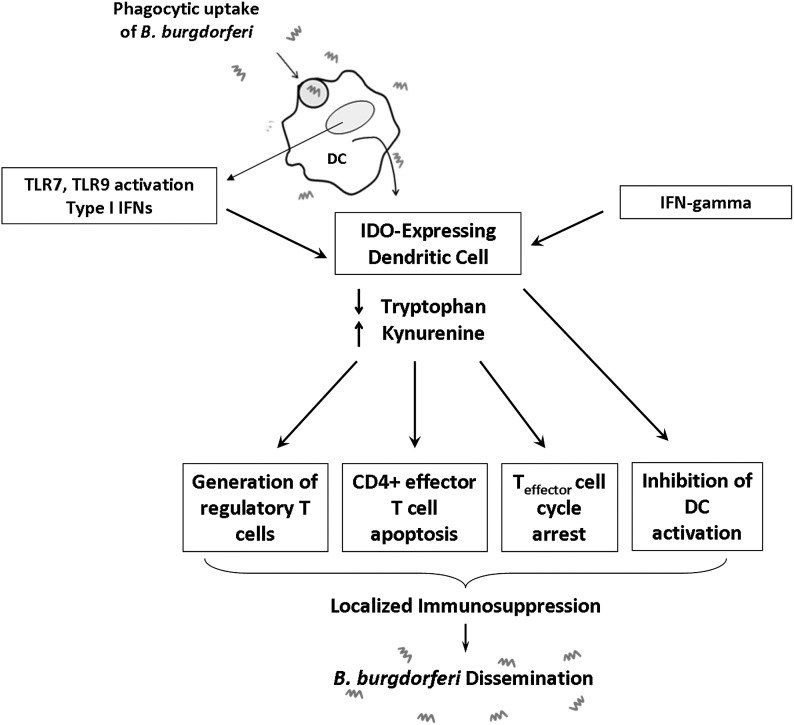
Figure 8. Model depicting potential immunoregulatory mechanisms elicited by the B. burgdorferi-dependent IDO expression observed in human PBMCs.
Following phagocytic uptake of B. burgdorferi, the bacterium is degraded within the endolysosome and cellular contents released. Nucleic acids activate DCs via TLR7- and TLR9-mediated signaling, leading to the generation of type I IFNs [4, 10] and IDO. IDO production, depletion of tryptophan, and generation of kynurenines can exert cytotoxic effects on T cells, inhibit T cell proliferation during G2/M phases of mitosis, generate regulatory T cells, and inhibit activation of adjacent DCs. These effects can work in concert to amplify local immune suppression at the site of infection, which B. burgdorferi may exploit to facilitate hematogenous dissemination.
DISCUSSION
A previous report from our laboratory identified a genotype dependence of type I IFN production in response to B. burgdorferi and a correlation between type I IFN production and pathogenic potential: strains and clinical isolates of B. burgdorferi that induced higher levels of type I IFN were more likely to cause disseminated infection in mice and humans [33]. Furthermore, that study determined that the ability of B. burgdorferi to induce type I IFN and to disseminate correlated with enhanced association of the spirochetes with pDCs and mDC1s. These observations created a paradox, as type I IFNs and uptake by professional APCs characteristically result in microbial killing. However, various pathogens have been shown to use the activation and maturation process of DCs to facilitate spread of infection [52–54].
Recent reports describe a new population of IDO-expressing tolerogenic DCs that can promote an attenuated immune response to a variety of pathogens, including many bacteria [19–21]. Here, we show for the first time that B. burgdorferi induces enzymatically active IDO production by human PBMCs. In this study, a correlation was identified between production of type I IFN and IDO production by mDCs and pDCs: B31-A3, a B. burgdorferi strain that was shown previously to induce IFN-α, was also able to elicit significantly higher levels of enzymatically active IDO protein compared with strain A3-M9 lp36−, a mutant that lacks lp36 and is unable to induce IFN-α (Fig. 3). TLR7 signaling, which was identified as a pathway for IDO production, was previously found to be a mediator of B. burgdorferi-induced IFN-α and the transcription of IFN-responsive genes [4, 10]. Finally, we demonstrated that maximal IDO production in response to B. burgdorferi requires contributions by type I and type II IFN signaling pathways. B31-A3 and A3-M9 lp36− induce comparable levels of IFN-γ but differ in the ability to induce IFN-α [33]. As a result of this crucial difference, the mutant mediates IDO production solely through the action of IFN-γ.
The ability of DC populations to produce IDO is dependent on recognition of PAMPs and the subsequent transcription of IFN-associated genes, which can stimulate the production of type I and type II IFNs by neighboring leukocyte populations [55, 56]. Upon ligand binding by DCs, a rapid maturation process occurs that includes up-regulation of maturation markers (CD83), chemokine receptors (CCR7), and costimulatory molecules [28, 41, 57, 58]. This process enables DCs to capture, process, and present antigen to naïve T cells to initiate immune signaling [11, 59, 60]. We identified significant up-regulation of CD83 and CCR7 in response to the type I IFN-inducing strains of B. burgdorferi compared with the mutant strain (Fig. 4). Moreover, nearly 100% of these populations of CD83+CCR7+ pDCs and mDCs also expressed IDO (Fig. 5). Human DCs produce IFN-α after sensing B. burgdorferi nucleic acids via TLR7 and TLR9 [4, 10]. In the current study, IDO production by DCs was found to be partially dependent on TLR7-mediated signaling. Consistent with our findings, it has been demonstrated that other pathogens, such as HIV, have the ability to activate pDCs by TLR7 signaling, leading to the production of type I IFNs and pronounced up-regulation of CD83 and CCR7 [57]. CD83 is a classic maturation marker that is typically used to characterize activated DCs [61, 62]. Although it was thought previously that only immature DCs could promote tolerance, a newly discovered population of mature DCs that highly express CD83 can induce T cell tolerance through a mechanism similar to that used to induce effector T cells [11, 29, 62, 63]. In addition, the soluble extracellular domain of CD83 can inhibit the maturation of neighboring naïve DCs, thereby preventing immune activation [64].
CCR7, a receptor for CCL19 and CCL21, is expressed predominantly on DCs and T cells [65]. Ligand binding induces production of inflammatory cytokines, increases migratory capacity of DCs, enhances endocytic capability, and increases antigen presentation [65–67]. In addition to aiding in priming the immune system, there has been recent evidence of CCR7 involvement in enhancing disease severity in response to several other human pathogens [52, 68]. Listeria monocytogenes uses the expression of CCR7 by DCs to exploit lymph node homing to facilitate systemic spread [52]. In a recent study, Borrelia garinii, a related spirochete responsible for Lyme borreliosis in Europe, exhibited diminished capacity to induce CCR7 compared with LPS, a potent immune activator, a finding that potentially contradicts our data [69]. However, in that study, CCR7 expression in Borrelia-infected mice was not compared with that in naïve mice. In addition, it has been demonstrated that B. garinii induces significantly lower levels of inflammatory mediators than B. burgdorferi, underscoring the potential differences in the immune response between the 2 closely related spirochetes [70].
IDO production by DCs in response to B. burgdorferi has a variety of potential implications for host-bacterium interactions. IFN-producing pDCs and mDCs have the capability to produce IDO as a tolerogenic mechanism in response to a variety of pathogens [20, 26, 32, 48, 71]. In the presence of IDO, naïve T cells can be directed to adopt a tolerogenic phenotype as opposed to exerting an effector function [16, 17, 48]. Kynurenines, including modified metabolites, such as 3-hydroxylkynurenine and anthranilic acid, can be directly cytotoxic to cells through the generation of free radicals, lipid peroxidation, and oxidative stress, leading to cellular apoptosis [72–74]. Kynurenines and IDO activity have also been linked to inhibition of T cell proliferation, reducing the pool of T cells available to respond to immune stimuli [31, 75, 76]. This can be accomplished through the arrest of the cell cycle, which has been shown to be mediated by tryptophan depletion in G1 and by kynurenines in G2/M phases [31, 75–77]. Interestingly, an additional level of regulation by IDO has been discovered: extensive kynurenine modifications of cellular proteins are linked to cellular dysfunction and ubiquitin tagging of proteins for degradation, inevitably leading to cell-cycle arrest or apoptosis [76]. A limitation of the present study is that potential B. burgdorferi-elicited changes in T cell phenotypes were not assessed. Intriguingly, reduced levels of IDO correlate with disease pathogenesis in patients with rheumatoid arthritis, whereas IDO2, an isotype of IDO1, was recently shown to promote the development of inflammation in a mouse model of autoimmune arthritis [78, 79]. IFNs, which drive the production of IDO, are critical contributors to Lyme arthritis in susceptible mice [9, 80]; it is tempting to speculate regarding a potential contribution of IDO in this model. Taken together, it is likely that the effects of B. burgdorferi-induced IDO are pleiotropic and impact multiple cellular responses (Fig. 8).
These findings demonstrate a positive correlation among the magnitude of the B. burgdorferi-induced type I IFN response, stimulation of host DCs, the ability to induce IDO, and the capacity for dissemination. Production of IDO correlates with expression of DC maturation markers, CD83 and CCR7, and secretion of kynurenine, a metabolite known to be cytotoxic and antiproliferative to T cells and NK cells [76]. We show that the inability of a B. burgdorferi mutant to induce IFN-α significantly impairs IDO and kynurenine production, indicating that the induction of IDO relies, at least in part, on IFN-α signaling. Finally, we demonstrate that IDO production by human PBMCs is mediated by the same TLR7-dependent recognition of B. burgdorferi RNA that contributes to the production of type I IFNs by human DCs [10]. The downstream consequences of enhanced IDO activity may exert a multitude of effects on the ability of the host to mount an effective adaptive immune response. We hypothesize that the ability of certain B. burgdorferi strains to induce IDO may be a mechanism used to evade the immune system and promote dissemination of the spirochete. An understanding of the complex host-pathogen interactions may enable the development of therapeutic approaches to inhibit development of later manifestations of disease pathogenesis, possibly through targeting the activity of B. burgdorferi-induced IDO.
ACKNOWLEDGMENTS
This work was supported by Grant 5U01CK000153 from the U.S. Centers for Disease Control and Prevention (to I.S.) and by a faculty recruitment grant from New York Medical College (to M.M.P.). The authors gratefully acknowledge the staff of the Division of Infectious Diseases for phlebotomy services; Drs. Patricia Rosa (National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health) and Mollie Jewett (University of Central Florida) for B. burgdorferi strains; and Antonella D’Ascanio for technical assistance.
Glossary
- α-IFNGR1
neutralizing antibody against IFN-γR subunit 1
- DC
dendritic cell
- DOTAP
{N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium} methosulfate
- EM
erythema migrans
- IFNGR1
IFN-γR subunit 1
- LAL
Limulus amebocyte lysis
- lp36
linear plasmid 36
- mDC
myeloid dendritic cell
- MOI
multiplicity of infection
- ODN
oligodeoxyribonucleotide
- Pam2CSK4
palmitoyl-2-cysteine-serine-lysine-4
- PAMP
pathogen-associated molecular pattern
- pDC
plasmacytoid dendritic cell
- PRR
pattern recognition receptor
- PVDF
polyvinylidene fluoride
AUTHORSHIP
A.C.L. designed the experiments, performed the experiments, analyzed the data, and wrote the manuscript. I.S. designed the experiments and wrote the manuscript. M.M.P. designed the experiments, analyzed the data, and wrote the manuscript.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Steere A. C., Coburn J., Glickstein L. (2004) The emergence of Lyme disease. J. Clin. Invest. 113, 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salazar J. C., Pope C. D., Sellati T. J., Feder H. M. Jr, Kiely T. G., Dardick K. R., Buckman R. L., Moore M. W., Caimano M. J., Pope J. G., Krause P. J., Radolf J. D.; Lyme Disease Network (2003) Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J. Immunol. 171, 2660–2670. [DOI] [PubMed] [Google Scholar]
- 3.Cervantes J. L., Dunham-Ems S. M., La Vake C. J., Petzke M. M., Sahay B., Sellati T. J., Radolf J. D., Salazar J. C. (2011) Phagosomal signaling by Borrelia burgdorferi in human monocytes involves Toll-like receptor (TLR) 2 and TLR8 cooperativity and TLR8-mediated induction of IFN-beta. Proc. Natl. Acad. Sci. USA 108, 3683–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petzke M. M., Brooks A., Krupna M. A., Mordue D., Schwartz I. (2009) Recognition of Borrelia burgdorferi, the Lyme disease spirochete, by TLR7 and TLR9 induces a type I IFN response by human immune cells. J. Immunol. 183, 5279–5292. [DOI] [PubMed] [Google Scholar]
- 5.Singh S. K., Girschick H. J. (2006) Toll-like receptors in Borrelia burgdorferi-induced inflammation. Clin. Microbiol. Infect. 12, 705–717. [DOI] [PubMed] [Google Scholar]
- 6.Steere A. C., Bartenhagen N. H., Craft J. E., Hutchinson G. J., Newman J. H., Pachner A. R., Rahn D. W., Sigal L. H., Taylor E., Malawista S. E. (1986) Clinical manifestations of Lyme disease. Zentralbl. Bakteriol. Mikrobiol. Hyg. [A] 263, 201–205. [DOI] [PubMed] [Google Scholar]
- 7.Lochhead R. B., Sonderegger F. L., Ma Y., Brewster J. E., Cornwall D., Maylor-Hagen H., Miller J. C., Zachary J. F., Weis J. H., Weis J. J. (2012) Endothelial cells and fibroblasts amplify the arthritogenic type I IFN response in murine Lyme disease and are major sources of chemokines in Borrelia burgdorferi-infected joint tissue. J. Immunol. 189, 2488–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller J. C., Maylor-Hagen H., Ma Y., Weis J. H., Weis J. J. (2010) The Lyme disease spirochete Borrelia burgdorferi utilizes multiple ligands, including RNA, for interferon regulatory factor 3-dependent induction of type I interferon-responsive genes. Infect. Immun. 78, 3144–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller J. C., Ma Y., Bian J., Sheehan K. C., Zachary J. F., Weis J. H., Schreiber R. D., Weis J. J. (2008) A critical role for type I IFN in arthritis development following Borrelia burgdorferi infection of mice. J. Immunol. 181, 8492–8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Love A. C., Schwartz I., Petzke M. M. (2014) Borrelia burgdorferi RNA induces type I and III interferons via Toll-like receptor 7 and contributes to production of NF-κB-dependent cytokines. Infect. Immun. 82, 2405–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banchereau J., Steinman R. M. (1998) Dendritic cells and the control of immunity. Nature 392, 245–252. [DOI] [PubMed] [Google Scholar]
- 12.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y. J., Pulendran B., Palucka K. (2000) Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811. [DOI] [PubMed] [Google Scholar]
- 13.Blander J. M. (2007) Coupling Toll-like receptor signaling with phagocytosis: potentiation of antigen presentation. Trends Immunol. 28, 19–25. [DOI] [PubMed] [Google Scholar]
- 14.Hellman P., Eriksson H. (2007) Early activation markers of human peripheral dendritic cells. Hum. Immunol. 68, 324–333. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D. N., Leenen P. J., Liu Y. J., MacPherson G., Randolph G. J., Scherberich J., Schmitz J., Shortman K., Sozzani S., Strobl H., Zembala M., Austyn J. M., Lutz M. B. (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116, e74–e80. [DOI] [PubMed] [Google Scholar]
- 16.Baban B., Chandler P. R., Sharma M. D., Pihkala J., Koni P. A., Munn D. H., Mellor A. L. (2009) IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J. Immunol. 183, 2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenk M., Scheler M., Koch S., Neumann J., Takikawa O., Häcker G., Bieber T., von Bubnoff D. (2009) Tryptophan deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic cells favoring the induction of human CD4+CD25+ Foxp3+ T regulatory cells. J. Immunol. 183, 145–154. [DOI] [PubMed] [Google Scholar]
- 18.Carvalheiro T., Rodrigues A., Lopes A., Inês L., Velada I., Ribeiro A., Martinho A., Silva J. A., Pais M. L., Paiva A. (2012) Tolerogenic versus inflammatory activity of peripheral blood monocytes and dendritic cells subpopulations in systemic lupus erythematosus. Clin. Dev. Immunol. 2012, 934161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Souza Sales J., Lara F. A., Amadeu T. P., de Oliveira Fulco T., da Costa Nery J. A., Sampaio E. P., Pinheiro R. O., Sarno E. N. (2011) The role of indoleamine 2, 3-dioxygenase in lepromatous leprosy immunosuppression. Clin. Exp. Immunol. 165, 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W., Katz B. P., Spinola S. M. (2011) Haemophilus ducreyi lipooligosaccharides induce expression of the immunosuppressive enzyme indoleamine 2,3-dioxygenase via type I interferons and tumor necrosis factor alpha in human dendritic cells. Infect. Immun. 79, 3338–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popov A., Abdullah Z., Wickenhauser C., Saric T., Driesen J., Hanisch F. G., Domann E., Raven E. L., Dehus O., Hermann C., Eggle D., Debey S., Chakraborty T., Krönke M., Utermöhlen O., Schultze J. L. (2006) Indoleamine 2,3-dioxygenase-expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infection. J. Clin. Invest. 116, 3160–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King N. J., Thomas S. R. (2007) Molecules in focus: indoleamine 2,3-dioxygenase. Int. J. Biochem. Cell Biol. 39, 2167–2172. [DOI] [PubMed] [Google Scholar]
- 23.Thackray S. J., Mowat C. G., Chapman S. K. (2008) Exploring the mechanism of tryptophan 2,3-dioxygenase. Biochem. Soc. Trans. 36, 1120–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puccetti P. (2007) On watching the watchers: IDO and type I/II IFN. Eur. J. Immunol. 37, 876–879. [DOI] [PubMed] [Google Scholar]
- 25.Murakami Y., Hoshi M., Imamura Y., Arioka Y., Yamamoto Y., Saito K. (2013) Remarkable role of indoleamine 2,3-dioxygenase and tryptophan metabolites in infectious diseases: potential role in macrophage-mediated inflammatory diseases. Mediators Inflamm. 2013, 391984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheler M., Wenzel J., Tüting T., Takikawa O., Bieber T., von Bubnoff D. (2007) Indoleamine 2,3-dioxygenase (IDO): the antagonist of type I interferon-driven skin inflammation? Am. J. Pathol. 171, 1936–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munn D. H., Sharma M. D., Lee J. R., Jhaver K. G., Johnson T. S., Keskin D. B., Marshall B., Chandler P., Antonia S. J., Burgess R., Slingluff C. L. Jr, Mellor A. L. (2002) Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 297, 1867–1870. [DOI] [PubMed] [Google Scholar]
- 28.Förster R., Davalos-Misslitz A. C., Rot A. (2008) CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 8, 362–371. [DOI] [PubMed] [Google Scholar]
- 29.Poschke I., Mougiakakos D., Hansson J., Masucci G. V., Kiessling R. (2010) Immature immunosuppressive CD14+HLA−DR−/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 70, 4335–4345. [DOI] [PubMed] [Google Scholar]
- 30.Chung D. J., Rossi M., Romano E., Ghith J., Yuan J., Munn D. H., Young J. W. (2009) Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood 114, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terness P., Bauer T. M., Röse L., Dufter C., Watzlik A., Simon H., Opelz G. (2002) Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J. Exp. Med. 196, 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loughman J. A., Hunstad D. A. (2012) Induction of indoleamine 2,3-dioxygenase by uropathogenic bacteria attenuates innate responses to epithelial infection. J. Infect. Dis. 205, 1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krupna-Gaylord M. A., Liveris D., Love A. C., Wormser G. P., Schwartz I., Petzke M. M. (2014) Induction of type I and type III interferons by Borrelia burgdorferi correlates with pathogenesis and requires linear plasmid 36. PLoS ONE 9, e100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jewett M. W., Lawrence K., Bestor A. C., Tilly K., Grimm D., Shaw P., VanRaden M., Gherardini F., Rosa P. A. (2007) The critical role of the linear plasmid lp36 in the infectious cycle of Borrelia burgdorferi. Mol. Microbiol. 64, 1358–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G., Ojaimi C., Wu H., Saksenberg V., Iyer R., Liveris D., McClain S. A., Wormser G. P., Schwartz I. (2002) Disease severity in a murine model of lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J. Infect. Dis. 186, 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang G., Iyer R., Bittker S., Cooper D., Small J., Wormser G. P., Schwartz I. (2004) Variations in Barbour-Stoenner-Kelly culture medium modulate infectivity and pathogenicity of Borrelia burgdorferi clinical isolates. Infect. Immun. 72, 6702–6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz I., Wormser G. P., Schwartz J. J., Cooper D., Weissensee P., Gazumyan A., Zimmermann E., Goldberg N. S., Bittker S., Campbell G. L., Pavia C. S. (1992) Diagnosis of early Lyme disease by polymerase chain reaction amplification and culture of skin biopsies from erythema migrans lesions. J. Clin. Microbiol. 30, 3082–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mancuso G., Gambuzza M., Midiri A., Biondo C., Papasergi S., Akira S., Teti G., Beninati C. (2009) Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat. Immunol. 10, 587–594. [DOI] [PubMed] [Google Scholar]
- 39.Barrat F. J., Meeker T., Gregorio J., Chan J. H., Uematsu S., Akira S., Chang B., Duramad O., Coffman R. L. (2005) Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 202, 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duramad O., Fearon K. L., Chang B., Chan J. H., Gregorio J., Coffman R. L., Barrat F. J. (2005) Inhibitors of TLR-9 act on multiple cell subsets in mouse and man in vitro and prevent death in vivo from systemic inflammation. J. Immunol. 174, 5193–5200. [DOI] [PubMed] [Google Scholar]
- 41.Braun D., Longman R. S., Albert M. L. (2005) A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood 106, 2375–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colamonici O. R., Domanski P., Sweitzer S. M., Larner A., Buller R. M. (1995) Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon alpha transmembrane signaling. J. Biol. Chem. 270, 15974–15978. [DOI] [PubMed] [Google Scholar]
- 43.Alcamí A., Symons J. A., Smith G. L. (2000) The vaccinia virus soluble alpha/beta interferon (IFN) receptor binds to the cell surface and protects cells from the antiviral effects of IFN. J. Virol. 74, 11230–11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Symons J. A., Alcamí A., Smith G. L. (1995) Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell 81, 551–560. [DOI] [PubMed] [Google Scholar]
- 45.Meyers J. A., Mangini A. J., Nagai T., Roff C. F., Sehy D., van Seventer G. A., van Seventer J. M. (2006) Blockade of TLR9 agonist-induced type I interferons promotes inflammatory cytokine IFN-gamma and IL-17 secretion by activated human PBMC. Cytokine 35, 235–246. [DOI] [PubMed] [Google Scholar]
- 46.Ebert E. C., Mehta V. (2006) Human intestinal intraepithelial lymphocytes keep TNF alpha levels low by cell uptake and feedback inhibition of transcription. Cell. Immunol. 241, 7–13. [DOI] [PubMed] [Google Scholar]
- 47.Nagineni C. N., Cherukuri K. S., Kutty V., Detrick B., Hooks J. J. (2007) Interferon-gamma differentially regulates TGF-beta1 and TGF-beta2 expression in human retinal pigment epithelial cells through JAK-STAT pathway. J. Cell. Physiol. 210, 192–200. [DOI] [PubMed] [Google Scholar]
- 48.Manches O., Munn D., Fallahi A., Lifson J., Chaperot L., Plumas J., Bhardwaj N. (2008) HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J. Clin. Invest. 118, 3431–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elias A. F., Stewart P. E., Grimm D., Caimano M. J., Eggers C. H., Tilly K., Bono J. L., Akins D. R., Radolf J. D., Schwan T. G., Rosa P. (2002) Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70, 2139–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson C. M., Shirey K. A., Carlin J. M. (2003) Synergistic transcriptional activation of indoleamine dioxygenase by IFN-gamma and tumor necrosis factor-alpha. J. Interferon Cytokine Res. 23, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Konan K. V., Taylor M. W. (1996) Importance of the two interferon-stimulated response element (ISRE) sequences in the regulation of the human indoleamine 2,3-dioxygenase gene. J. Biol. Chem. 271, 19140–19145. [DOI] [PubMed] [Google Scholar]
- 52.Pron B., Boumaila C., Jaubert F., Berche P., Milon G., Geissmann F., Gaillard J. L. (2001) Dendritic cells are early cellular targets of Listeria monocytogenes after intestinal delivery and are involved in bacterial spread in the host. Cell. Microbiol. 3, 331–340. [DOI] [PubMed] [Google Scholar]
- 53.Moll H., Flohé S., Röllinghoff M. (1995) Dendritic cells in Leishmania major-immune mice harbor persistent parasites and mediate an antigen-specific T cell immune response. Eur. J. Immunol. 25, 693–699. [DOI] [PubMed] [Google Scholar]
- 54.Masurier C., Salomon B., Guettari N., Pioche C., Lachapelle F., Guigon M., Klatzmann D. (1998) Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice. J. Virol. 72, 7822–7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu X. Y., Kessler D. S., Veals S. A., Levy D. E., Darnell J. E. Jr (1990) ISGF3, the transcriptional activator induced by interferon alpha, consists of multiple interacting polypeptide chains. Proc. Natl. Acad. Sci. USA 87, 8555–8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kessler D. S., Veals S. A., Fu X. Y., Levy D. E. (1990) Interferon-alpha regulates nuclear translocation and DNA-binding affinity of ISGF3, a multimeric transcriptional activator. Genes Dev. 4, 1753–1765. [DOI] [PubMed] [Google Scholar]
- 57.Beignon A. S., McKenna K., Skoberne M., Manches O., DaSilva I., Kavanagh D. G., Larsson M., Gorelick R. J., Lifson J. D., Bhardwaj N. (2005) Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Invest. 115, 3265–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Breloer M., Fleischer B. (2008) CD83 regulates lymphocyte maturation, activation and homeostasis. Trends Immunol. 29, 186–194. [DOI] [PubMed] [Google Scholar]
- 59.Simmons D. P., Wearsch P. A., Canaday D. H., Meyerson H. J., Liu Y. C., Wang Y., Boom W. H., Harding C. V. (2012) Type I IFN drives a distinctive dendritic cell maturation phenotype that allows continued class II MHC synthesis and antigen processing. J. Immunol. 188, 3116–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Austyn J. M. (1996) New insights into the mobilization and phagocytic activity of dendritic cells. J. Exp. Med. 183, 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lechmann M., Zinser E., Golka A., Steinkasserer A. (2002) Role of CD83 in the immunomodulation of dendritic cells. Int. Arch. Allergy Immunol. 129, 113–118. [DOI] [PubMed] [Google Scholar]
- 62.Prechtel A. T., Steinkasserer A. (2007) CD83: an update on functions and prospects of the maturation marker of dendritic cells. Arch. Dermatol. Res. 299, 59–69. [DOI] [PubMed] [Google Scholar]
- 63.Prechtel A. T., Turza N. M., Theodoridis A. A., Steinkasserer A. (2007) CD83 knockdown in monocyte-derived dendritic cells by small interfering RNA leads to a diminished T cell stimulation. J. Immunol. 178, 5454–5464. [DOI] [PubMed] [Google Scholar]
- 64.Bock F., Rössner S., Onderka J., Lechmann M., Pallotta M. T., Fallarino F., Boon L., Nicolette C., DeBenedette M. A., Tcherepanova I. Y., Grohmann U., Steinkasserer A., Cursiefen C., Zinser E. (2013) Topical application of soluble CD83 induces IDO-mediated immune modulation, increases Foxp3+ T cells, and prolongs allogeneic corneal graft survival. J. Immunol. 191, 1965–1975. [DOI] [PubMed] [Google Scholar]
- 65.Parlato S., Santini S. M., Lapenta C., Di Pucchio T., Logozzi M., Spada M., Giammarioli A. M., Malorni W., Fais S., Belardelli F. (2001) Expression of CCR-7, MIP-3beta, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood 98, 3022–3029. [DOI] [PubMed] [Google Scholar]
- 66.Marsland B. J., Bättig P., Bauer M., Ruedl C., Lässing U., Beerli R. R., Dietmeier K., Ivanova L., Pfister T., Vogt L., Nakano H., Nembrini C., Saudan P., Kopf M., Bachmann M. F. (2005) CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity 22, 493–505. [DOI] [PubMed] [Google Scholar]
- 67.Kellermann S. A., Hudak S., Oldham E. R., Liu Y. J., McEvoy L. M. (1999) The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J. Immunol. 162, 3859–3864. [PubMed] [Google Scholar]
- 68.Saleh S., Solomon A., Wightman F., Xhilaga M., Cameron P. U., Lewin S. R. (2007) CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood 110, 4161–4164. [DOI] [PubMed] [Google Scholar]
- 69.Hartiala P., Hytönen J., Pelkonen J., Kimppa K., West A., Penttinen M. A., Suhonen J., Lahesmaa R., Viljanen M. K. (2007) Transcriptional response of human dendritic cells to Borrelia garinii—defective CD38 and CCR7 expression detected. J. Leukoc. Biol. 82, 33–43. [DOI] [PubMed] [Google Scholar]
- 70.Strle K., Drouin E. E., Shen S., El Khoury J., McHugh G., Ruzic-Sabljic E., Strle F., Steere A. C. (2009) Borrelia burgdorferi stimulates macrophages to secrete higher levels of cytokines and chemokines than Borrelia afzelii or Borrelia garinii. J. Infect. Dis. 200, 1936–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Makala L. H., Baban B., Lemos H., El-Awady A. R., Chandler P. R., Hou D. Y., Munn D. H., Mellor A. L. (2011) Leishmania major attenuates host immunity by stimulating local indoleamine 2,3-dioxygenase expression. J. Infect. Dis. 203, 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohib K., Guan Q., Diao H., Du C., Jevnikar A. M. (2007) Proapoptotic activity of indoleamine 2,3-dioxygenase expressed in renal tubular epithelial cells. Am. J. Physiol. Renal Physiol. 293, F801–F812. [DOI] [PubMed] [Google Scholar]
- 73.Németh H., Toldi J., Vécsei L. (2005) Role of kynurenines in the central and peripheral nervous systems. Curr. Neurovasc. Res. 2, 249–260. [DOI] [PubMed] [Google Scholar]
- 74.Lee G. K., Park H. J., Macleod M., Chandler P., Munn D. H., Mellor A. L. (2002) Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 107, 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Munn D. H., Shafizadeh E., Attwood J. T., Bondarev I., Pashine A., Mellor A. L. (1999) Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 189, 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mailankot M., Smith D., Howell S., Wang B., Jacobberger J. W., Stefan T., Nagaraj R. H. (2008) Cell cycle arrest by kynurenine in lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 49, 5466–5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Y., Guillemin G. J. (2009) Kynurenine pathway metabolites in humans: disease and healthy states. Int. J. Tryptophan Res. 2, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Furuzawa-Carballeda J., Lima G., Jakez-Ocampo J., Llorente L. (2011) Indoleamine 2,3-dioxygenase-expressing peripheral cells in rheumatoid arthritis and systemic lupus erythematosus: a cross-sectional study. Eur. J. Clin. Invest. 41, 1037–1046. [DOI] [PubMed] [Google Scholar]
- 79.Merlo L. M., Pigott E., DuHadaway J. B., Grabler S., Metz R., Prendergast G. C., Mandik-Nayak L. (2014) IDO2 is a critical mediator of autoantibody production and inflammatory pathogenesis in a mouse model of autoimmune arthritis. J. Immunol. 192, 2082–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crandall H., Dunn D. M., Ma Y., Wooten R. M., Zachary J. F., Weis J. H., Weiss R. B., Weis J. J. (2006) Gene expression profiling reveals unique pathways associated with differential severity of lyme arthritis. J. Immunol. 177, 7930–7942. [DOI] [PubMed] [Google Scholar]