The relationships between CD4 and CD8 T cell phenotype, function, and anatomic distribution in the context of acuteversus persistent LCMV infections.
Keywords: CD8 T cells, homing
Abstract
Vaccines are desired that maintain abundant memory T cells at nonlymphoid sites of microbial exposure, where they may be anatomically positioned for immediate pathogen interception. Here, we test the impact of antigen persistence on mouse CD8 and CD4 T cell distribution and differentiation by comparing responses to infections with different strains of LCMV that cause either acute or chronic infections. We used in vivo labeling techniques that discriminate between T cells present within tissues and abundant populations that fail to be removed from vascular compartments, despite perfusion. LCMV persistence caused up to ∼30-fold more virus-specific CD8 T cells to distribute to the lung compared with acute infection. Persistent infection also maintained mucosal-homing α4β7 integrin expression, higher granzyme B expression, alterations in the expression of the TRM markers CD69 and CD103, and greater accumulation of virus-specific CD8 T cells in the large intestine, liver, kidney, and female reproductive tract. Persistent infection also increased LCMV-specific CD4 T cell quantity in mucosal tissues and induced maintenance of CXCR4, an HIV coreceptor. This study clarifies the relationship between viral persistence and CD4 and CD8 T cell distribution and mucosal phenotype, indicating that chronic LCMV infection magnifies T cell migration to nonlymphoid tissues.
Introduction
The establishment of memory T cells may be important for optimizing vaccine-elicited protection against several recalcitrant infectious diseases, including AIDS, malaria, tuberculosis, and hepatitis C. To favor rapidity of T cell-mediated control, it is thought that pathogen-specific memory T cells should be positioned at the initial site of infection, where they are poised for immediate pathogen interception [1–4]. In support of this concept, mice that contain effector-like CD8 TRM within the skin exhibit much more rapid control against local rechallenges [5, 6]. Likewise, CD4 TRM within the lung have been shown to hasten control of respiratory infections [7, 8]. These data provide a compelling rationale for defining the rules that regulate the distribution and maintenance of antigen-experienced T cells.
Analyses of T cell responses specific for various acute and chronic infections provide empirical evidence that antigen persistence favors the differentiation of TEM, which may promote recirculating or possibly even TRM within nonlymphoid tissues, including the mucosae [9–11]. Such an outcome might be highly desirable for rapid defense against peripheral infections. Indeed, concomitant immunity has been noted in the context of several parasitic infections [12]. Here, prevention of a secondary infection is highly dependent on the persistence of the primary infection. This could be related to the maintenance of antigen-specific T cell quantity, distribution in nonlymphoid tissues, or maintenance of effector functions. In addition, exploitation of chronic CMV as a vaccine vector has resulted in very promising, protective immunity against mucosal challenge of rhesus macaques with a stringent SIV challenge, a phenomenon that likely depends on CD8 T cells [13]. This striking observation supports the hypothesis that vaccines against HIV that rely on T cells, which may need to intercept the infection very rapidly at mucosal sites of infection, would benefit by use of persisting viral vectors. These data and concepts demand further investigation into the role of antigen persistence on T cell distribution and function.
However, it is difficult to attribute differences in T cell distribution specifically to antigen persistence rather than other aspects of pathogenesis when responses are compared between phylogenetically unrelated acute and chronic infectious agents. In this regard, infection of mice with different strains of LCMV does offer a reductionist approach for investigating the role of antigen persistence on T cell distribution. LCMV Armstrong induces an acute viscerotropic infection that is cleared within ∼8 days after viral challenge of adult immunocompetent C57BL/6J mice [14, 15]. LCMV Cl-13 is a variant of LCMV Armstrong that differs by only 2 aa. These mutations affect the viral polymerase and glycoprotein yet do not alter immunodominant T cell epitopes [16–18]. Nevertheless, LCMV Cl-13 infection persists for >100 days in kidney, brain, salivary gland, and possibly many additional tissues [15, 19–23].
The distribution of T cells after acute and persistent LCMV infections has been compared previously with the lung, liver, and kidney serving as representative nonlymphoid tissues [15, 24]. These studies revealed relatively little difference or a mild augmentation of antigen-specific CD8 T cells within these tissues as a result of chronic infection. We have shown recently that T cells isolated from these particular tissues after LCMV Armstrong infection are mostly present within blood-borne compartments of these organs [25, 26]. Indeed, up to 97% of isolated LCMV Armstrong-specific CD8 T cells, regardless of perfusion, are not actually present within the tissue parenchyma [25]. It is unknown how this issue applies to LCMV-specific CD4 T cells or in the context of LCMV Cl-13 infection. Intravascular staining via i.v. injection of antibodies distinguishes between tissue and blood-borne populations [26]. Here, we exploit this technique to redress the influence of LCMV persistence on CD8 T cell distribution to lung and kidney, and we extend this analysis to the red and white pulp of spleen, numerous mucosal tissues, CD4 T cells, and the expression of molecules of relevance for homing, function, and differentiation state and revise the perceived distribution of T cells following LCMV.
MATERIALS AND METHODS
Mice and infections
Six- to 7-week-old C57BL/6J mice, purchased from The Jackson Laboratory (The Jackson Laboratory, Bar Harbor, ME, USA), were used for virus infections. These mice were infected with 2 × 105 PFU LCMV Armstrong i.p. or 2 × 106 PFU LCMV Cl-13 i.v. to establish acute and chronic LCMV infections, respectively, as described before [27]. All mice were used in accordance with the Institutional Animal Care and Use Committee guidelines at the University of Minnesota.
Intravascular staining and lymphocyte isolation
The intravascular staining protocol to label cells in the vasculature has been described earlier [32]. In brief, at the indicated time postinfection, animals were injected i.v. with anti-CD8α-eFluor 450 (53-6.7) or anti-CD45.2-FITC (104) through the tail vein. Three minutes postinjection, animals were euthanized, and tissues were harvested.
Lymphocyte isolation from secondary lymphoid organs and nonlymphoid tissue was performed as described [27]. Spleen, lymph nodes, and livers were homogenized through a 70 μm filter in RPMI 1640 containing 5% FBS. Lung was removed and dissected into small pieces, and the pieces were incubated with 1.3 mM EDTA in HBSS (30 min at 37°C, 450 rpm), followed by treatment with 100 U/ml type I collagenase in 5% RPMI-1640 medium/2 mM MgCl2/2 mM CaCl2 (45 min/37°C, 450 rpm). For isolation of IELs from small and large intestine, the organs were isolated, fecal contents removed, and Peyer’s patches excised (from small intestine only) and cut longitudinally and then into 1 cm pieces. Intestine pieces were incubated in 10% 1× HBSS/HEPES bicarbonate containing 15.4 mg/100 ml dithioerythritol (30 min at 37°C, 450 rpm) to extract IEL. After separating IELs, gut pieces were treated further with 100 U/ml type I collagenase (Worthington Biochemical, Lakewood, NJ, USA) for lamina propria lymphocyte isolation. Kidneys and the female reproductive tract (containing uterine horns, cervix, and vaginal tissue) were removed and cut into small pieces, followed by treatment with 100 U/ml type I (Worthington Biochemical) and type IV (Sigma, St. Louis, MO, USA) collagenase in 5% RPMI 1640/2 mM MgCl2/2 mM CaCl2 (45 min at 37°C, 400 rpm) respectively. All tissue pieces were washed several times before enzymatic digestion with 5% RPMI-1640 medium to remove excess of injected antibody so as to prevent staining extravascular cells. Lymphocytes from liver, lung, gut, kidney, and female reproductive tract were purified on a 44%/67% Percoll gradient (800 g at 23°C for 20 min).
In vitro stimulation assays
Isolated lymphocytes were incubated in RPMI 1640, supplemented with 10% FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, and 50 mM 2-ME, with or without gp33 peptide (0.2 μg/ml) at 37°C for 3 h. PE-Cy7 conjugated IFN-γ (XMG 1.2; Affymetrix eBioscience, San Diego, CA, USA) intracellular staining was performed using the Cytofix/Cytoperm kit (BD PharMingen. San Diego, CA, USA), according to the manufacturer’s instructions.
Phenotyping of T cells
Isolated cells were surface stained with anti-CD8α (53-6.7; for e.v. staining), CD45.2 (104), CD4 (RM4-5), CD62L (MEL-14), CD44 (IM7), CD69 (H1.2F3), CD103 (M290), Ly6C (AL21), CD27 (LG.3A10), PD-1 (RMP1-30), KLRG1 (2F1), α4β7 (DATK32), and CXCR4 (2B11; all purchased from BD PharMingen or Affymetrix eBioscience, directly conjugated to different fluorochromes). PE-conjugated granzyme B (Invitrogen, Carlsbad, CA, USA) intracellular staining was performed using the Cytofix/Cytoperm kit (BD PharMingen), according to the manufacturer’s instructions. Cells were stained with H-2Db/GP33 (MHC class I tetramer), conjugated to APC to detect LCMV-specific CD8 T cells. LCMV-specific CD4 T cells were detected by staining with the GP66–77:IAb tetramer, conjugated to APC using a protocol described previously [28]. The samples were acquired using LSR II or LSRFortessa flow cytometers (BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
Tissue freezing, immunofluorescence, and microscopy
At the indicated time postinfection, harvested tissues were embedded in tissue-freezing medium OCT and snap frozen in isopentane liquid bath. Frozen blocks were cut to prepare 7 μm-thick sections using a Leica cryostat. Slides were fixed in acetone. Before staining, sections were rehydrated using PBS and blocked with 5% BSA solution. To detect LCMV antigen, purified rat anti-LCMV nucleoprotein antibody VL-4 (BioXCell, West Lebanon, NH, USA) and donkey anti-rat Alexa Fluor 488 (Invitrogen) were used. Cells were counterstained with DAPI to detect nuclei. Immunofluorescence microscopy was performed using a Leica DM5500 B microscope. Separate images, taken with a 20× objective, were collected for each channel and overlaid to obtain a multicolor image. Image processing was done using Adobe Photoshop software.
RESULTS
Persistence of LCMV in murine mucosal tissues
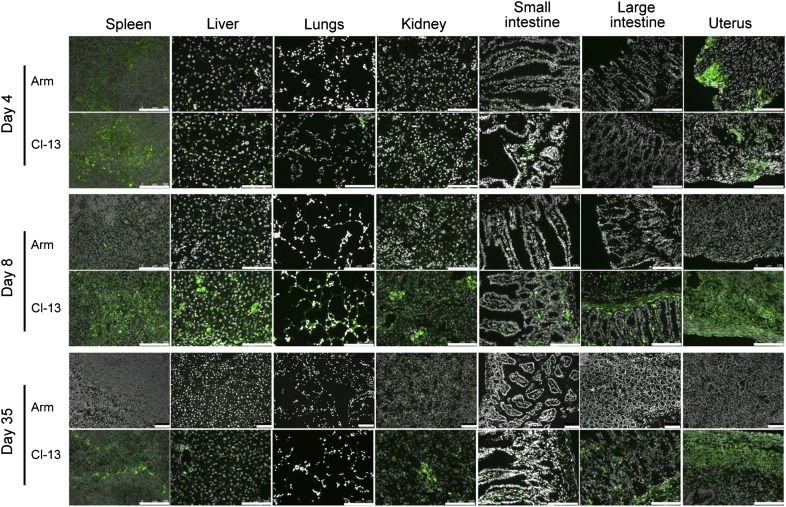
We sought a model that would allow one to measure the impact of local antigen persistence, particularly within mucosal tissues, on T lymphocyte distribution. LCMV Armstrong is undetectable in most tissues within ∼1 week of exposure, whereas prolonged Cl-13 replication has been well documented in peripheral tissues, including liver, kidney, and brain [15, 19–23].To interrogate whether this strain-dependent dichotomy in antigen persistence extended to frontline mucosal tissues, C57BL/6J mice were infected with LCMV Armstrong or LCMV Cl-13. At various times postinfection, organs were assessed for viral persistence by staining frozen sections with immunofluorescence microscopy using a mAb against the highly expressed nucleoprotein of LCMV [29]. Kidney and spleen, tissues in which viral persistence has been extensively characterized previously, served as positive controls. As shown in Fig. 1, LCMV Armstrong was detectable in spleen and liver, 4 days after infection, as described previously. Here, we show that the female reproductive tract was also a major site of viral replication. Nevertheless, per expectations, infection was largely absent by 8 days postinfection and was undetectable at Day 35. In contrast, infection with LCMV Cl-13 resulted in readily detectable virus staining throughout mucosal tissues for at least 35 days after infection, including lungs, small and large intestines, and the uterus (Fig. 1), as well as salivary gland, stomach, and tongue (data not shown). Thus, comparisons between LCMV Armstrong and Cl-13 infection provide a model to assess T cell differentiation, homing, and distribution in a situation whereby antigen is cleared rapidly (LCMV Armstrong) versus a situation in which antigen persists in frontline mucosal tissues (LCMV Cl-13).
Figure 1. Comparison of LCMV Armstrong and Cl-13 persistence in mouse tissues.
C57BL/6J mice were infected with LCMV Armstrong (Arm) or Cl-13, and on the days indicated, tissue sections were stained with DAPI (gray) and LCMV nucleoprotein-specific mAb (green) to assess viral replication. Images are representative of three independent experiments, with ≥3 mice/infection condition. Original scale bars = 100 μm.
LCMV persistence affects distribution of virus-specific CD8 T cells
We next wished to compare the anatomic distribution of virus-specific CD8 T cells responding to acute and chronic strains of LCMV. Whereas similar analyses have been performed previously in the lung and liver [15, 24], recent data demonstrate that interpretation of these studies may have been conflated by contamination with T cells present within the vasculature, despite perfusion. Indeed, up to 100% of naïve lymphocytes and >95% of virus-specific CD8 T cells isolated from lung actually represent cells in capillaries rather than tissue parenchyma, which confound interpretations of pulmonary T cell-homing requirements, phenotype, and function [25]. Similar issues are associated with cells isolated from liver and kidney [26]. However, vascular cells can be readily distinguished from tissue lymphocytes by their accessibility to in vivo intravascular antibody labeling [25]. This method also provides an opportunity to distinguish between lymphocytes present in the splenic red pulp (which become labeled) from those that are protected from labeling in white pulp [25, 30]. Although T cells in each compartment are rarely compared, red and white pulp is distinct with respect to immunologic function, viral tropism, and antigen presentation.
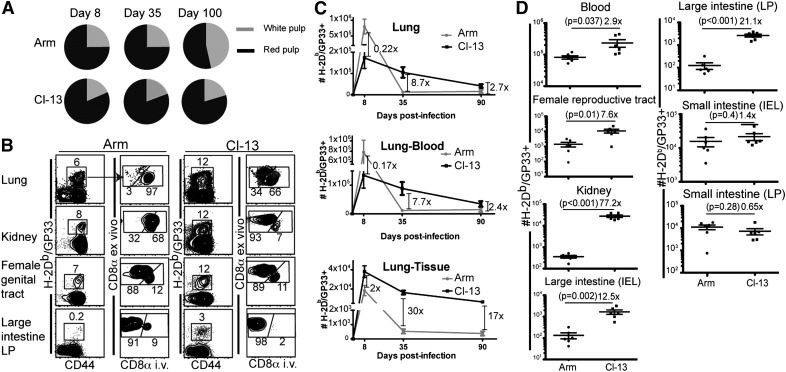
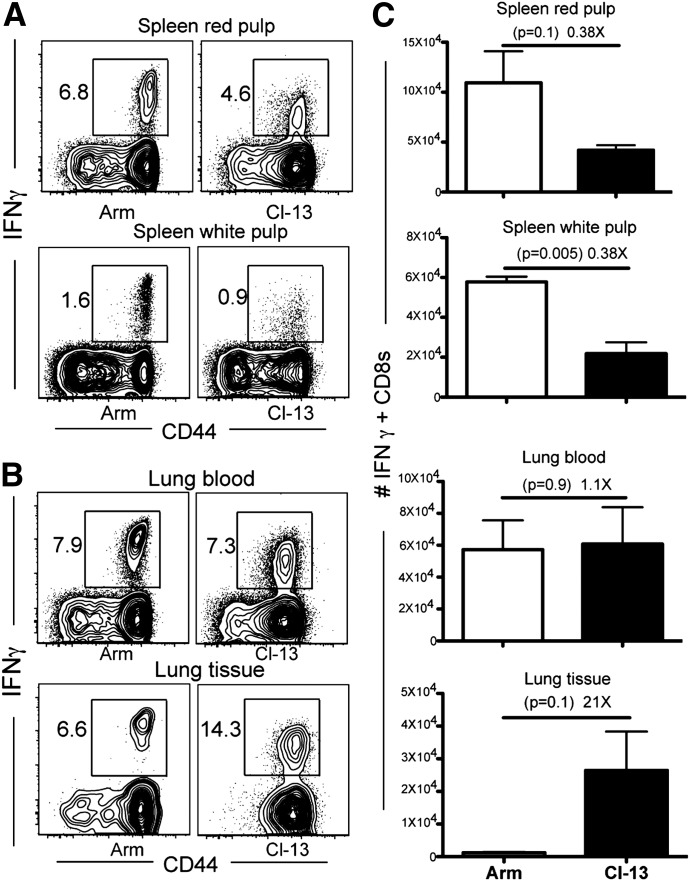
To redress the anatomic distribution of LCMV-specific CD8 T cells, we infected mice with LCMV Armstrong or Cl-13. At various times after infection, anti-CD8α mAb was injected i.v., mice were euthanized 3 min later, and lymphocytes were isolated from the indicated tissues. LCMV-specific CD8 T cells were identified via e.v. staining with H-2Db/GP33 MHC I tetramers. Figure 2A depicts the distribution of H-2Db/GP33-specific CD8 T cells between splenic red and white pulp, as distinguished by i.v. mAb labeling. After resolution of LCMV Armstrong infection, H-2Db/GP33 tetramer+ CD8 T cells gradually redistributed from the red pulp to the white pulp. In contrast, persistent LCMV Cl-13 infection caused virus-specific CD8 T cells to patrol splenic red pulp preferentially and significantly biased CD8 T cells toward nonlymphoid tissues (Fig. 2A and B). Remarkably, there were 17- to 30-fold more cells established in lung tissue by LCMV Cl-13 infection compared with Armstrong infection (Fig. 2C). Without distinguishing blood and parenchymal T cell populations via i.v. mAb staining, this difference would have been largely obscured (particularly by 90 days after infection; Fig. 2C), which may explain the novelty of this finding. In addition to the lung, persistent infection biased the distribution of virus-specific CD8 T cells to many nonlymphoid tissues, such as the large intestine epithelium (13-fold increase) and lamina propria (21-fold increase), the female reproductive tract (8-fold increase), and the kidney (77-fold increase; Fig. 2D). However, LCMV Cl-13 did not promote accumulation of specific CD8 T cells in the small intestinal mucosa, suggesting that there may be tissue-specific regulation.
Figure 2. LCMV persistence affects distribution of virus-specific CD8 T cells.
CD8 T cells were isolated from tissues on various days after LCMV Armstrong or Cl-13 infection. (A) H-2Db/GP33 MHC class I tetramer+ CD8 T cells were enumerated independently in the red and white pulp of the spleen via intravascular staining (see Materials and Methods). (B) The frequency and intravascular staining pattern of H-2Db/GP33-specific CD8 T cells in various tissues, 35 days after infection. Plots gated on CD8+ lymphocytes, representative of 10 mice from 3 independent experiments. LP, Lamina propria. (C) The kinetics of the H-2Db/GP33-specific CD8 T cell response in the entire lung and the compartments that were stained with (lung blood) or protected from (lung tissue) intravascular-injected antibody. (D) The number of H-2Db/GP33-specific CD8 T cells protected from intravascular CD8α staining in various tissues (including peripheral blood, where all CD8 T cells are stained with i.v.-injected antibody) was compared, 35 days after each infection. Error bars indicate sem.
Sublocalization within spleen and LCMV persistence delineates CD8 T cell phenotype
T cells occupy 2 anatomically and functionally distinct compartments within the spleen: lymphocyte-rich secondary lymphoid organ-inductive sites (white pulp) and a dense network of extralymphoid reticular fibers associated with numerous red and white blood cells (red pulp). Unfortunately, multiparameter flow cytometric methods of phenotyping splenocytes are typically performed on mixed populations of cells isolated from red and white pulp, as there is no easy way to separate these compartments physically. Intravascular staining affords an opportunity to examine T cells within each compartment independently [25]. As LCMV persistence affected the distribution of H-2Db/GP33-specific CD8 T cells in spleen, we interrogated whether persistence correlated with distinct phenotypes within each compartment.
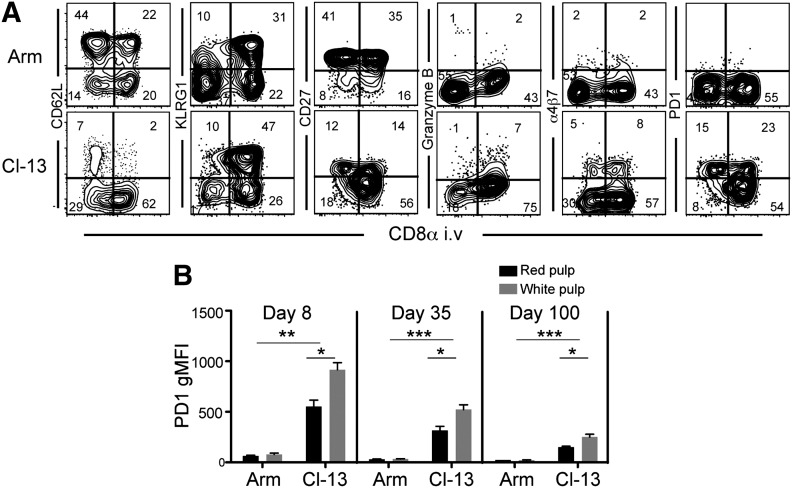
We found that 100 days after LCMV Armstrong infection, CD8 TCM (defined by CD62L expression and the absence of granzyme B expression) were enriched within the white pulp, and CD8 TEM (defined by KLRG1 expression and the absence of CD62L and CD27) were preferentially distributed within red pulp (Fig. 3A). These data highlight that heterogeneity among memory CD8 T cell phenotype in spleen is, in fact, correlated with the distribution of cells into 2 anatomically and functionally distinct compartments.
Figure 3. Sublocalization within spleen and LCMV persistence delineates CD8 T cell phenotype.
C57BL/6J mice were infected with LCMV Armstrong or Cl-13. One hundred days later, mice were injected with anti-CD8α i.v., and splenocytes were isolated. (A) CD8α staining versus the indicated markers, gated on H-2Db/GP33-specific CD8 T cells. (B) Geometric mean fluorescence intensity (gMFI) of PD-1 expression on H-2Db/GP33-specific CD8 T cells isolated from red or white pulp. Data are representative of 9 mice from 3 independent experiments. Error bars indicate sem; ***P < 0.001; **P < 0.01; *P < 0.05.
We also asked how infection with LCMV Cl-13 would influence the phenotype of T cells within each compartment, as compared with LCMV Armstrong, this infection establishes higher viral load in the spleen, persists within the reticular fibers of the white pulp, and maintains a biased distribution of specific CD8 T cells to red rather than white pulp (Fig. 2A) [31]. CD62L and CD27 expression was down-regulated on T cells in the context of antigen persistence, and this was even true among those cells that localized to the white pulp. However, markers associated with terminal differentiation (KLRG1) and cytolytic function (granzyme B) were still excluded from white pulp (Fig. 3A). Strikingly, Cl-13 infection resulted in the maintenance of the mucosal-homing integrin, α4β7, in stark contrast to acute viral infection, particularly in red pulp. This suggests that the homing program from CD8 T cells specific for chronic infections might differ from those specific for infections that have been cleared.
Interestingly, PD-1, an inhibitory receptor associated with antigenic stimulation and T cell dysfunction, was expressed more highly by cells in the white pulp (Fig. 3A and B). This expression pattern may reflect the distribution of antigen and may contribute to persistence of LCMV Cl-13 within the white pulp. From a therapeutic perspective, this reminds us that the blockage of PD-1 antibodies should target sites of antigen presentation and that this may be more difficult when that includes tissues that are less efficiently accessible to injected mAb. In summary, antigen persistence and anatomic compartmentalization were predictive of the T cell phenotype within the spleen.
LCMV persistence regulates effector differentiation and mucosal-homing molecule expression in nonlymphoid organs
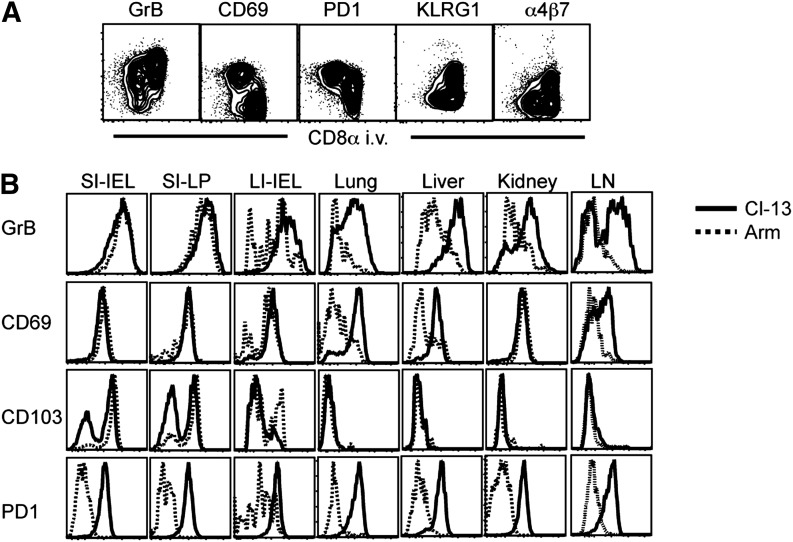
As persistent infection promoted abundant T cell distribution to nonlymphoid tissues, we tested the hypothesis of whether persistent infection would also maintain heightened expression of effector molecules and mucosal-homing molecules. To address this issue, we compared nonlymphoid CD8 T cell populations, 35 days after LCMV Armstrong and Cl-13 infection. It should be noted that this analysis exploited intravascular staining, and analysis was restricted to cells protected from intravascular antibody and thus were actually present within tissues. In other words, the tissue populations under investigation were not conflated with blood-borne subsets that would otherwise confound interpretation. To illustrate this principle, LCMV Cl-13-specific CD8 T cells, isolated from the lung tissue (protected from CD8α i.v. labeling), expressed higher levels of CD69 and PD-1 and lower levels of KLRG1, granzyme B, and α4β7 when compared with cells within the lung vasculature (permissive to CD8α i.v. labeling; see Fig. 4A).
Figure 4. LCMV persistence regulates effector differentiation and mucosal-homing molecule expression in nonlymphoid organs.
H-2Db/gp33 CD8 T cells were phenotyped by flow cytometry, 35 days after LCMV Armstrong or Cl-13 infection. Anatomic distribution was determined by permissiveness to intravascular anti-CD8α staining. (A) Antigen-specific CD8 T cells isolated from lung after Cl-13 infection. (B) H-2Db/GP33 MHC I tetramer+ cells that were protected from intravascular staining after LCMV Armstrong (dashed black histogram) or Cl-13 (solid black histogram) infection isolated from indicated tissues. Data are representative of 9 mice from 3 independent experiments. GrB, Granzyme B; SI, small intestine; LI, large intestine; LN, lymph node.
Previous reports demonstrated that CD8 TRM that are retained within nonlymphoid tissues acquire a unique phenotype that is influenced by the tissue microenvironment. This resident memory phenotype has been associated with the induction of CD103 and/or CD69 on CD8 T cells subsequent to nonlymphoid tissue migration [27, 32–36]. How persistent infection might affect the phenotype of CD8 T cells within nonlymphoid tissues is unclear. We now wished to test how persistent LCMV Cl-13 infection would regulate the expression of effector molecules and mucosal-homing molecules within the intestine and to extend these observations to other nonlymphoid tissues.
Figure 4B demonstrates that an effector phenotype was maintained in most peripheral tissues following acute LCMV Armstrong infection, suggesting that this CD8 T cell differentiation program is a general feature of nonlymphoid distribution. These data demonstrate that the maintenance of effector properties in most organs is not necessarily indicative of stimulation by chronic infections, consistent with previous work [27, 37]. In support of this interpretation, H-2Db/GP33-specific CD8 T cells in Armstrong infection did not express PD-1, a marker of recent TCR stimulation. In some tissues, most notably in lung, liver, and kidney, the majority of LCMV Armstrong-specific CD8 T cells did not maintain an effector-like phenotype (Fig. 4B), but this was promoted by infection with persistent LCMV Cl-13. The uniform expression of PD-1 in the context of LCMV Cl-13 infection was consistent with the hypothesis that this phenotype was associated with chronic local infection. Curiously, it was in these tissues, lung, liver, and kidney, where a CD69+ TRM phenotype was enhanced by LCMV Cl-13 infection, and it was these tissues that experienced the greatest increase in virus-specific CD8 T cells (Fig. 2D). Taken together, these observations indicate that there are separate pathways for the maintenance of an effector-like differentiation state among CD8 T cells and that persistent infection maintains this phenotype in locations where that is not an axiomatic feature of the tissue microenvironment.
There are more IFN-γ-competent CD8 T cells in lung tissue after persistent LCMV infection
LCMV Cl-13 infection is associated with functional exhaustion of CD8 T cells compared with LCMV Armstrong infection. Consequently, reports demonstrate that LCMV Cl-13 infection results in fewer gp33-specific IFN-γ-producing CD8 T cells after brief in vitro restimulation with peptide [15]. We confirmed these results in spleen (Fig. 5). However, this issue has not been addressed properly for mucosal tissues, such as the lung, which we have now shown exhibits significant inclusion of cells from outside of the actual lung parenchyma and stroma. Ergo, we redressed this issue while incorporating intravascular staining to segregate vascular cells from those isolated from bona fide pulmonary mucosa. In contrast to expectations, LCMV Cl-13 infection was associated with significantly greater numbers of IFN-γ-positive cells after peptide restimulation and intracellular cytokine staining (Fig. 5B and C). These results were largely a result of the fact that LCMV Cl-13 infection induced greater CD8 T cell distribution to the lung, as shown in Fig. 2. However, it should be noted that the intensity of IFN-γ staining was lower on gp33-specific CD8 T cells in the context of LCMV Cl-13 compared with LCMV Armstrong infections.
Figure 5. There are more IFN-γ-competent CD8 T cells in lung after persistent LCMV infection.
Forty-five days after LCMV Armstrong or Cl-13 infection, lymphocytes were isolated, stimulated with gp33 for 3 h in vitro, and then stained with an IFN-γ antibody. Representative flow cytometry plots showing the frequency of IFN-γ-positive cells on CD8+ lymphocytes isolated from (A) spleen and (B) lung blood and tissue. (C) As in A and B, however, IFN-γ+ cells in each location were enumerated. Error bars indicate sem.
Persistent LCMV drives enhanced expression of nonlymphoid-homing markers on CD4 T cells and preferential distribution to nonlymphoid tissues
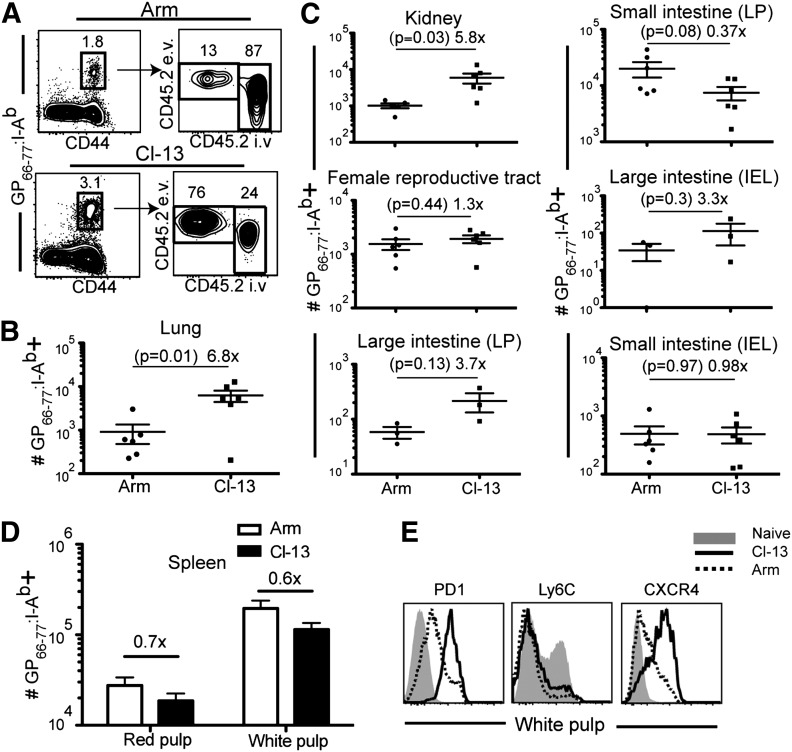
CD4 T cells positioned within mucosal tissues contribute to local antimicrobial immunity and also serve as a major target of SIV and HIV-1 infection and pathogenesis [9]. To address whether LCMV persistence drives increased antigen-specific CD4 T cell homing to nonlymphoid organs, we infected mice with LCMV Armstrong or Cl-13 (as in Fig. 1) and constructed I-Ab MHC class II tetramers containing the immunodominant peptide GP66–77, which we used to detect LCMV-specific CD4 T cells. Like CD8 T cell responses, chronic infection maintained a significantly higher frequency of LCMV-specific CD4 T cells within the pulmonary mucosa. In fact, >70% of antigen-specific CD4 T cells were localized in the lung parenchyma rather than the vascular compartment (Fig. 6A), and chronic infection resulted in ∼7-fold more antigen-specific CD4 T cells isolated from lung tissue than after LCMV Armstrong infection (Fig. 6B). Chronic LCMV infection also biased CD4 T cell distribution to kidney but in contrast to CD8 T cells, had little impact on CD4 T cell maintenance in the female reproductive tract and lamina propria and epithelial compartments of the large intestine (Fig. 6C). Consistent with previous reports [38], spleen contained fewer antigen-specific cells after Cl-13 infection compared with acute Armstrong infection (Fig. 6D), but chronic infection did elevate the expression of PD-1 as well as CXCR4 (Fig. 6E). However, expression of Ly6C, which has been linked to CD4 T cell longevity and proliferation potential remains unchanged in chronically infected mice [39]. These data illustrate that persistent infections may augment the number of mucosal CD4 T cells and also induce maintenance of the murine homolog of human CXCR4, an important peripheral-homing molecule and a major HIV-1 coreceptor in humans.
Figure 6. Persistent LCMV drives enhanced expression of nonlymphoid-homing markers on CD4 T cells and preferential distribution to nonlymphoid tissues.
The phenotype of GP66–77:I-Ab MHC II tetramer+ LCMV-specific CD4 T cells from various tissues was analyzed, 35 days after LCMV Armstrong or Cl-13 infection. (A) Representative intravascular staining on MHC II tetramer+ CD4 T cells in lung. (B) Enumeration of isolated GP66–77:I-Ab MHC II tetramer+ CD4 T cells that were protected from intravascular staining in the lung, (C) indicated tissues, and (D) spleen white pulp (intravascular staining + red pulp cells are also shown). Error bars represent sem. (E) Comparison of PD-1, Ly6C, and CXCR4 expression on spleen white pulp-derived GP66–77:I-Ab MHC II tetramer+ CD4 T cells after LCMV Armstrong (dashed black line) or Cl-13 (solid black line) infection. Phenotype of naïve CD44lo splenocytes (solid gray histogram) is shown for comparison. Data are representative of two independent experiments totaling ≥6 mice/group.
DISCUSSION
We exploited a reductionist acute versus chronic infection model using two related strains of LCMV to examine the consequences of persistent infection on the distribution and differentiation of antigen-specific CD8 and CD4 T cells. For CD8 and CD4 T cells, persistent infection favored the development of subsets (TRM and TEM) that patrol nonlymphoid organs rather than TCM that patrol secondary lymphoid organs. Chronic infection also sustained the expression of effector and peripheral-homing molecules on antigen-specific T cells (Fig. 4). The latter finding was consistent with the observation that LCMV persistence was associated with greater accumulation of virus-specific CD8 and CD4 T cells within nonlymphoid tissues, including mucosae (Figs. 2 and 6), which include tissues that harbor TRM [27]. Moroever, there were actually greater numbers of functional IFN-γ-competent CD8 T cells within the lung after LCMV Cl-13 infection compared with LCMV Armstrong infection. These findings were predicated on discriminating tissue-bound cells from those merely populating the vasculature. Taken together, these results refine and revise our understanding of the distribution of T cells following this persistent viral infection.
In the context of acute infections, CD8 T cells migrate into the intestinal mucosa and skin only during the effector stage of differentiation and then differentiate into TRM in situ [32, 36, 40]. Local tissue signals induce CD69 and CD103 expression, which regulates retention within the tissue and also programs the maintenance of an effector-like phenotype (including constitutive granzyme B expression) that does not depend on persistent antigen [27, 37, 41]. Thus, TRM adapt to the tissue environments in which they reside and remain poised to exert effector functions more rapidly in the event of pathogen re-exposure. However, TRM differentiation varies among tissues. For instance, lung CD8+ TRM often does not maintain granzyme B after many primary infections and undergoes attrition after antigen clearance [35, 42]. Here, we demonstrate that chronic LCMV infection sustained CD69 and granzyme B expression among CD8 T cells within the lung (and also the liver) and resulted in a 30-fold increase in the number of antigen-specific cells, 35 days after priming when compared with an acute LCMV infection. Local accumulation within the lung may have resulted from increased retention as a result of enhanced CD69 expression (as suggested during influenza virus infection; ref. [35]), local proliferation of T cells within the lung, or sustained entry into the tissue. With regards to the latter hypothesis, it should be noted that chronic infection resulted in the sustained expression of peripheral-homing molecules on T cells, even 100 days after LCMV Cl-13 exposure. This raises the possibility that the temporal window of opportunity for T cell migration into nonlymphoid tissues after priming may depend on the persistence of antigen. Our findings raise the possibility that HIV-1 exploits this phenomenon, as chronic viral infection sustained the expression of CXCR4 and HIV-1 coreceptor on antigen-specific CD4 T cells. These results also indicate that further studies should interrogate the recirculation properties of antigen-experienced T cells in the contexts of persistent as well as acute infections.
ACKNOWLEDGMENTS
This study was supported by U.S. National Institutes of Health Grants R01AI084913 and R01AI111671 (to D.M.).
Glossary
- APC
allophycocyanin
- CD62L
cluster of differentiation 62 ligand
- Cl-13
clone 13
- e.v.
ex vivo
- IEL
intraepithelial lymphocyte
- KLRG1
killer cell lectin-like receptor subfamily G member 1
- LCMV
lymphocytic choriomeningitis virus
- PD-1
programmed death 1
- TCM
central memory T cell(s)
- TEM
effector memory T cell(s)
- TRM
resident memory T cell(s)
Footnotes
SEE CORRESPONDING EDITORIAL ON PAGE 211
AUTHORSHIP
L.K.B., V.V., and D.M. designed the experiments. L.K.B., K.G.A., J.M.S., J.J.L., and K.A.F. performed the experiments and analyzed data. M.P. contributed critical reagent. L.K.B. and D.M. wrote the manuscript.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Cauley L. S., Lefrançois L. (2013) Guarding the perimeter: protection of the mucosa by tissue-resident memory T cells. Mucosal Immunol. 6, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller S. N., Gebhardt T., Carbone F. R., Heath W. R. (2013) Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 31, 137–161. [DOI] [PubMed] [Google Scholar]
- 3.Kohlmeier J. E., Woodland D. L. (2009) Immunity to respiratory viruses. Annu. Rev. Immunol. 27, 61–82. [DOI] [PubMed] [Google Scholar]
- 4.Masopust D., Picker L. J. (2012) Hidden memories: frontline memory T cells and early pathogen interception. J. Immunol. 188, 5811–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gebhardt T., Wakim L. M., Eidsmo L., Reading P. C., Heath W. R., Carbone F. R. (2009) Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530. [DOI] [PubMed] [Google Scholar]
- 6.Jiang X., Clark R. A., Liu L., Wagers A. J., Fuhlbrigge R. C., Kupper T. S. (2012) Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 483, 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teijaro J. R., Turner D., Pham Q., Wherry E. J., Lefrançois L., Farber D. L. (2011) Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 187, 5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogan R. J., Zhong W., Usherwood E. J., Cookenham T., Roberts A. D., Woodland D. L. (2001) Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J. Exp. Med. 193, 981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haase A. T. (2005) Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5, 783–792. [DOI] [PubMed] [Google Scholar]
- 10.Appay V., Dunbar P. R., Callan M., Klenerman P., Gillespie G. M. A., Papagno L., Ogg G. S., King A., Lechner F., Spina C. A., Little S., Havlir D. V., Richman D. D., Gruener N., Pape G., Waters A., Easterbrook P., Salio M., Cerundolo V., McMichael A. J., Rowland-Jones S. L. (2002) Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8, 379–385. [DOI] [PubMed] [Google Scholar]
- 11.Picker L. J., Hansen S. G., Lifson J. D. (2012) New paradigms for HIV/AIDS vaccine development. Annu. Rev. Med. 63, 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown S. P., Grenfell B. T. (2001) An unlikely partnership: parasites, concomitant immunity and host defence. Proc. Biol. Sci. 268, 2543–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen S. G., Ford J. C., Lewis M. S., Ventura A. B., Hughes C. M., Coyne-Johnson L., Whizin N., Oswald K., Shoemaker R., Swanson T., Legasse A. W., Chiuchiolo M. J., Parks C. L., Axthelm M. K., Nelson J. A., Jarvis M. A., Piatak M. Jr., Lifson J. D., Picker L. J. (2011) Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed R., Oldstone M. B. (1988) Organ-specific selection of viral variants during chronic infection. J. Exp. Med. 167, 1719–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wherry E. J., Blattman J. N., Murali-Krishna K., van der Most R., Ahmed R. (2003) Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matloubian M., Somasundaram T., Kolhekar S. R., Selvakumar R., Ahmed R. (1990) Genetic basis of viral persistence: single amino acid change in the viral glycoprotein affects ability of lymphocytic choriomeningitis virus to persist in adult mice. J. Exp. Med. 172, 1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matloubian M., Kolhekar S. R., Somasundaram T., Ahmed R. (1993) Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J. Virol. 67, 7340–7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvato M., Borrow P., Shimomaye E., Oldstone M. B. (1991) Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J. Virol. 65, 1863–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Southern P. J., Blount P., Oldstone M. B. (1984) Analysis of persistent virus infections by in situ hybridization to whole-mouse sections. Nature 312, 555–558. [DOI] [PubMed] [Google Scholar]
- 20.Mims C. A. (1966) Immunofluorescence study of the carrier state and mechanism of vertical transmission in lymphocytic choriomeningitis virus infection in mice. J. Pathol. Bacteriol. 91, 395–402. [DOI] [PubMed] [Google Scholar]
- 21.Sydora B. C., Jamieson B. D., Ahmed R., Kronenberg M. (1996) Intestinal intraepithelial lymphocytes respond to systemic lymphocytic choriomeningitis virus infection. Cell. Immunol. 167, 161–169. [DOI] [PubMed] [Google Scholar]
- 22.Wilsnack R. E., Rowe W. P. (1964) Immunofluorescent studies of the histopathogenesis of lymphocytic choriomeningitis virus infection. J. Exp. Med. 120, 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rai S. K., Micales B. K., Wu M. S., Cheung D. S., Pugh T. D., Lyons G. E., Salvato M. S. (1997) Timed appearance of lymphocytic choriomeningitis virus after gastric inoculation of mice. Am. J. Pathol. 151, 633–639. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou S., Ou R., Huang L., Price G. E., Moskophidis D. (2004) Differential tissue-specific regulation of antiviral CD8+ T-cell immune responses during chronic viral infection. J. Virol. 78, 3578–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson K. G., Sung H., Skon C. N., Lefrançois L., Deisinger A., Vezys V., Masopust D. (2012) Cutting edge: intravascular staining redefines lung CD8 T cell responses. J. Immunol. 189, 2702–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson K. G., Mayer-Barber K., Sung H., Beura L., James B. R., Taylor J. J., Qunaj L., Griffith T. S., Vezys V., Barber D. L., Masopust D. (2014) Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc. 9, 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casey K. A., Fraser K. A., Schenkel J. M., Moran A., Abt M. C., Beura L. K., Lucas P. J., Artis D., Wherry E. J., Hogquist K., Vezys V., Masopust D. (2012) Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 188, 4866–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepper M., Pagán A. J., Igyártó B. Z., Taylor J. J., Jenkins M. K. (2011) Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 35, 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Battegay M., Cooper S., Althage A., Bänziger J., Hengartner H., Zinkernagel R. M. (1991) Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods 33, 191–198. [DOI] [PubMed] [Google Scholar]
- 30.Arnon T. I., Horton R. M., Grigorova I. L., Cyster J. G. (2013) Visualization of splenic marginal zone B-cell shuttling and follicular B-cell egress. Nature 493, 684–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller S. N., Matloubian M. (2007) Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection.Proc. Natl. Acad. Sci. USA 104, 15430–15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masopust D., Vezys V., Wherry E. J., Barber D. L., Ahmed R. (2006) Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol. 176, 2079–2083. [DOI] [PubMed] [Google Scholar]
- 33.Gebhardt T., Whitney P. G., Zaid A., Mackay L. K., Brooks A. G., Heath W. R., Carbone F. R., Mueller S. N. (2011) Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219. [DOI] [PubMed] [Google Scholar]
- 34.Wakim L. M., Woodward-Davis A., Liu R., Hu Y., Villadangos J., Smyth G., Bevan M. J. (2012) The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J. Immunol. 189, 3462–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y.-T., Suarez-Ramirez J. E., Wu T., Redman J. M., Bouchard K., Hadley G. A., Cauley L. S. (2011) Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J. Virol. 85, 4085–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackay L. K., Rahimpour A., Ma J. Z., Collins N., Stock A. T., Hafon M.-L., Vega-Ramos J., Lauzurica P., Mueller S. N., Stefanovic T., Tscharke D. C., Heath W. R., Inouye M., Carbone F. R., Gebhardt T. (2013) The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 14, 1294–1301. [DOI] [PubMed] [Google Scholar]
- 37.Mackay L. K., Stock A. T., Ma J. Z., Jones C. M., Kent S. J., Mueller S. N., Heath W. R., Carbone F. R., Gebhardt T. (2012) Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl. Acad. Sci. USA 109, 7037–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brooks D. G., Teyton L., Oldstone M. B. A., McGavern D. B. (2005) Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J. Virol. 79, 10514–10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall H. D., Chandele A., Jung Y. W., Meng H., Poholek A. C., Parish I. A., Rutishauser R., Cui W., Kleinstein S. H., Craft J., Kaech S. M. (2011) Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity 35, 633–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masopust D., Choo D., Vezys V., Wherry E. J., Duraiswamy J., Akondy R., Wang J., Casey K. A., Barber D. L., Kawamura K. S., Fraser K. A., Webby R. J., Brinkmann V., Butcher E. C., Newell K. A., Ahmed R. (2010) Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207, 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohlmeier J. E., Miller S. C., Woodland D. L. (2007) Cutting edge: antigen is not required for the activation and maintenance of virus-specific memory CD8+ T cells in the lung airways. J. Immunol. 178, 4721–4725. [DOI] [PubMed] [Google Scholar]
- 42.Wu T., Hu Y., Lee Y.-T., Bouchard K. R., Benechet A., Khanna K., Cauley L. S. (2014) Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 95, 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]