Elucidation of the molecular mechanisms behind differentialGlut1 expression in human conventional and regulatory T cells.
Keywords: signal transduction, metabolism, lymphocyte, autoimmunity
Abstract
CD4+CD25+Foxp3+ Tregs have a diminished capacity to activate the PI3K/Akt pathway. Although blunted Akt activity is necessary to maintain Treg function, the consequences of this altered signaling are unclear. Glut1 is a cell-surface receptor responsible for facilitating glucose transport across plasma membranes, whose expression is tightly coupled to costimulatory signals and Akt phosphorylation. Freshly isolated human Tregs were unable to up-regulate Glut1 in response to TCR and costimulatory signals compared with Tconv. Consequently, the ability of Tregs to use glucose was also reduced. Introduction of Foxp3 into Tconv inhibited Akt activation and Glut1 expression, indicating that Foxp3 can regulate Glut1. Finally, pharmacologic activation of Akt in Tregs can induce Glut1, overcoming the effects of Foxp3. Together, these results illustrate the molecular basis behind differential glucose metabolism in Tregs.
Introduction
The PI3K/Akt pathway is essential for Tconv growth, survival, cell-cycle progression, and function [1, 2]. In contrast to Tconv, Foxp3+ Tregs are unable to activate this pathway in response to TCR, costimulatory, and IL-2 signals [3–5]. Moreover, minimal or absent Akt phosphorylation is a requirement for Treg function, phenotype, and development [3, 6]. Although much work has been done examining the mechanisms responsible for blunted Akt activation in Tregs [5, 7, 8], the consequences of this differential signaling remain unclear.
Glut1 is the primary glucose transporter of CD4+ T cells [2, 9]. Glut1 is not expressed on resting T cells, but its expression is induced following TCR and CD28 ligation, sufficient to activate Akt [9, 10]. Failure to activate Akt appropriately, through insufficient TCR and CD28 ligation, coinhibitory receptor ligation, or direct inhibition of the PI3K/Akt pathway, will not result in Glut1 induction [9, 10]. Furthermore, Glut1 expression and adequate glucose uptake are necessary for maintaining effector T cell function, growth, and survival [2]. The expression of Glut 1 has also been studied during the differentiation of murine Th1, Th2, Th17, and TGF-β iTreg. Th1, Th2, and Th17 cells all had high surface expression of Glut 1, whereas iTreg expressed low levels and used lipid oxidation [11, 12]. In contrast to studies in mice, there is a lack of consensus in the literature as to whether human iTregs can be generated [13], and expression of Glut 1 had not been analyzed on human Foxp3+ Treg.
In this study, we demonstrate that Glut1 is regulated in a different way in human Foxp3+ Tregs. Foxp3, the master regulator of Tregs, inhibits Akt phosphorylation. Blunted Akt activation, in turn, results in an inability to express Glut1 on the cell surface. Lastly, with the use of a small molecule activator of Akt, SC79 [14], we were able to overcome the effects of Foxp3-mediated Akt repression, resulting in Glut1 induction.
MATERIALS AND METHODS
Cell isolation, cell expansion, cell stimulation, and glucose determination
Peripheral blood was obtained from healthy adult donors by the Department of Transfusion Medicine at the U.S. National Institutes of Health. The acquisition of blood products was approved according to the Institutional Review Board and in accordance with the Declaration of Helsinki. Primary human CD4+ T cells from healthy donors were purified by positive selection as described previously [15]. CD4+ T cells were sorted on a FACSAria into CD4+CD127+CD25− (Tconv) and CD4+CD127−CD25+ (Treg) populations. Tconv and Tregs were stimulated with anti-CD3/anti-CD28 mAb-coated beads (Invitrogen, Carlsbad, CA, USA) and human rIL-2 (300 U/ml; Chiron, Emeryville, CA, USA) for 15 min or 20 h as described previously [16]. T cells were cultured with SC79 (0.5 μg/ml; Sigma-Aldrich, St. Louis, MO, USA) or DMSO control (1 μl/ml; Sigma-Aldrich) for 15 min or 20 h where indicated. PMA (50 ng; Sigma-Aldrich) and ionomycin (500 ng; Sigma-Aldrich) were added to the cells before performing intracellular cytokine staining. For glucose-consumption studies, following stimulation, media were removed carefully from the cells and analyzed by an ELISA kit for glucose (Abcam, Cambridge, MA, USA), per the manufacturer’s recommendation.
Flow cytometric analysis
Surface staining for CD4, CD25, and CD127 (BD PharMingen, San Diego, CA, USA) was performed according to the manufacturer’s recommendations. Intracellular staining was performed by use of the FOXP3 Fix/Perm kit (BioLegend, San Diego, CA, USA) for Foxp3 (BioLegend), IL-2 (BD PharMingen), and phospho-Ser473 Akt (Cell Signaling Technology, Danvers, MA, USA) and the Caltag Fix & Perm kit (Invitrogen) for Glut1 (R&D Systems, Minneapolis, MN, USA), per each manufacturer’s recommendation. Data were collected by use of a FACSCalibur (BD Biosciences, San Jose, CA, USA). Events (12,000–15,000) were collected for each sample, and data were analyzed by use of FlowJo software (Tree Star, Ashland, OR, USA).
Production of lentiviral vectors and transduction of CD4+CD127+CD25− T cells
GFP and YFP 2A Foxp3 were cloned upstream of the elongation factor-1α promoter in a lentiviral vector described previously [17] so that all transduced cell populations could be detected by FL1. The 2A sequence used to allow coexpression of YFP and Foxp3 was GSGEGRGSLLTCGDVEENPGP. High titer vector was used to transduce T cells as described previously [17].
Statistical analysis
Data were analyzed by use of the paired Student’s t-test via Prism 6.0 software (GraphPad Software, La Jolla, CA, USA), and P < 0.01 was considered significant.
RESULTS AND DISCUSSION
Foxp3-expressing Tregs are unable to phosphorylate Akt and up-regulate Glut1
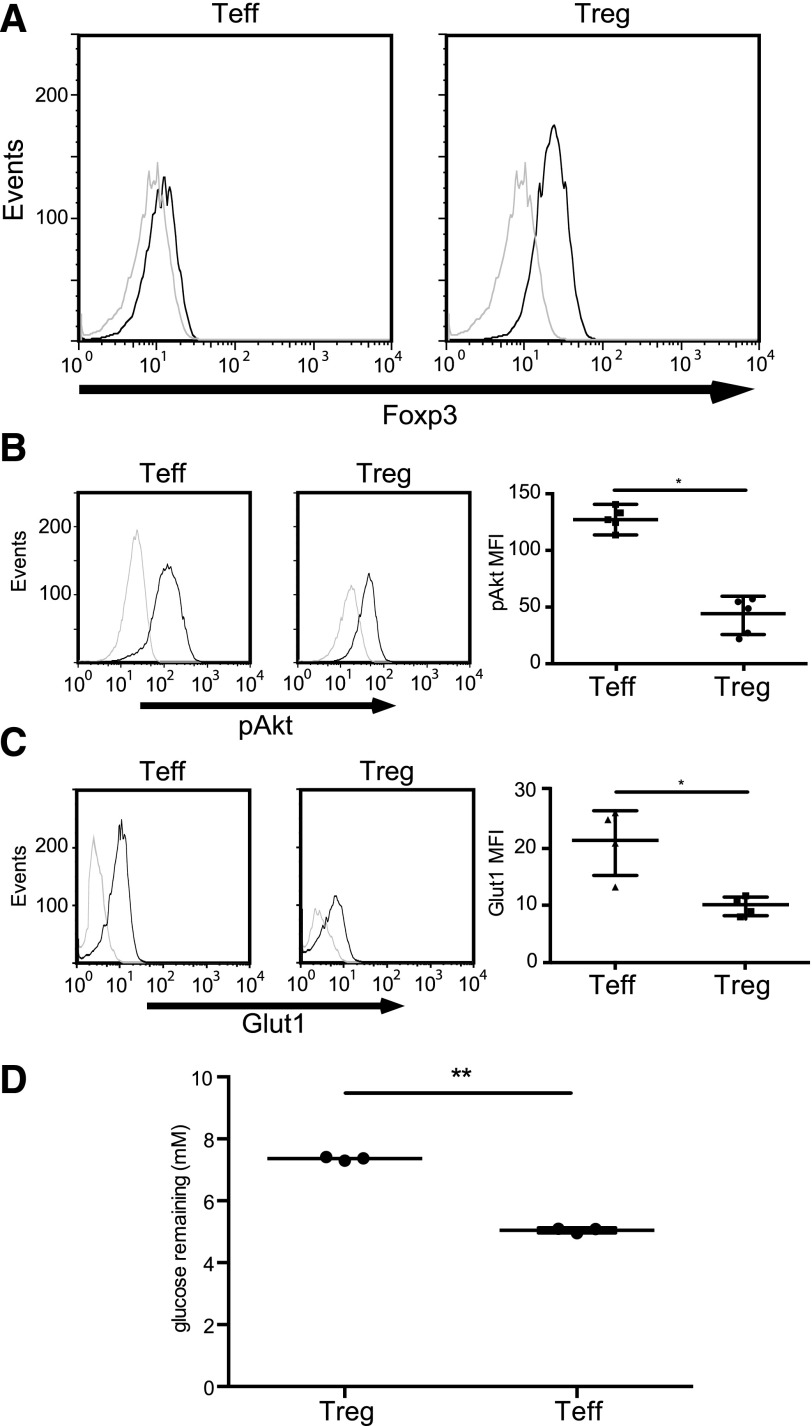
Given the dependency of Glut1 expression on Akt signaling, it would be expected that Tregs have a reduced capacity to induce Glut1. We purified Tregs and Tconv from isolated leukapheresis products and examined each population’s ability to phosphorylate Akt and express Glut1 upon activation. Measurement of Foxp3 expression by flow cytometry established that we were able to enrich successfully for Tregs (Fig. 1A). Stimulation of freshly sorted CD4+CD127+CD25− and CD4+CD127−CD25+ T cells with a combination of anti-CD3/anti-CD28 mAb-coated beads and IL-2 resulted in substantial phosphorylation of Akt at serine residue 473 in Tconv and in minimal phosphorylation in Tregs (Fig. 1B), as reported previously [3, 4]. Furthermore, activated Tregs and Tconv do not express Glut1 equally (Fig. 1C). The differential expression of Glut 1 correlated with differential glucose consumption (Fig. 1D). Taken together, these results suggest that human Tregs may be less dependent than Tconv on use of glucose as an energy source. Indeed, 1 study has shown that murine Tregs rely on nonglucose sources, such as short-chain fatty acids [18]. It is somewhat surprising that enhanced expression of Glut1 by Treg has not been noted in microarray studies of gene expression by Tregs and Tconv [19, 20]. Some of the differences in Glut1 expression may therefore occur at a post-translational level.
Figure 1. Foxp3-expressing Tregs are unable to phosphorylate Akt and up-regulate Glut1.
(A) Freshly isolated Tconv [effector T cells (Teff; left)] and Tregs (right) were stained with anti-Foxp3 (black histograms) or an isotype control (gray histograms) and analyzed by flow cytometry. (B, left 2 panels) Freshly isolated Tconv and Tregs (as shown in A) were stimulated with CD3/CD28 mAb-coated beads and IL-2 (300 U/ml) for 15 min (black histograms) or left unstimulated for 15 min (gray histograms). Cells were then stained with phospho-Ser473 Akt antibody (pAkt). (Right) Summary of 5 independent experiments (*P < 0.01). The data were plotted as the mean ± sd. MFI, Mean fluorescence intensity. (C, left 2 panels) Freshly isolated Tconv and Tregs (as shown in A) were stimulated with CD3/CD28 mAb-coated beads and IL-2 (300 U/ml) for 20 h (black histograms) or left unstimulated (gray histograms). Cells were then stained with anti-Glut1 mAb. (Right) Summary of 4 independent experiments (*P < 0.01). (D) Freshly isolated Tregs and Tconv (as shown in A) were stimulated with CD3/CD28 antibody-coated beads and IL-2 (300 U/ml) for 20 h. Following stimulation, media were removed carefully and then analyzed by ELISA for glucose. Data are summary of 3 independent experiments (**P < 0.001). The data were plotted as the mean ± sd.
Foxp3 expression inhibits Akt activation and Glut1 expression
Foxp3 expression is necessary for Treg function, and its ectopic expression in CD4+ Tconv results in a suppressive phenotype [16]. Thus, any investigation to determine the relationship between Akt and Glut1 in Tregs would begin with Foxp3. We activated CD4+CD127+CD25− Tconv with anti-CD3/anti-CD28 mAb-coated beads and transduced them with GFP or YFP-2A-Foxp3 expression vectors (Fig. 2A). To confirm that transduction of Foxp3 resulted in a bona fide Treg phenotype [21], we determined IL-2 production by Foxp3-expressing cells (Fig. 2A). Upon stimulation with PMA and ionomycin, 40% of GFP-transduced cells and 48% of untransduced cells produced IL-2. By comparison, only 7.4% of Foxp3-transduced cells were able to secrete IL-2. These results demonstrate that our Foxp3 expression vector is functional, as the level of IL-2 production by the transduced cells was comparable with that seen with freshly explanted Tregs (Fig. 2A).
Figure 2. Foxp3 expression inhibits Akt activation and Glut1 expression.
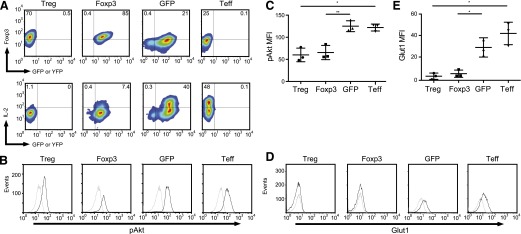
(A, upper) Tregs and Tconv were activated with CD3/CD28 mAb-coated beads and were transduced with lentiviral vectors expressing YFP-2A-Foxp3, GFP, or left untransduced. The correlation between YFP or GFP and Foxp3 was determined by flow cytometry. (Lower) Untransduced Treg, YFP-2A-Foxp3, GFP, and untransduced effector T cells were restimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 3 h, and intracellular IL-2 expression was determined by flow cytometry. (B) Untransduced Treg, YFP-2A-Foxp3, GFP, and untransduced Tconv (as shown in A) were restimulated with CD3/CD28 mAb-coated beads and IL-2 (300 U/ml) for 15 min (black histograms) or left unstimulated for 15 min (gray histograms). Cells were then stained with phospho-Ser473 Akt antibody. (C) Summary of 3 independent experiments as shown in B (*P < 0.01; **P < 0.001). The data were plotted as the mean ± sd. (D) Untransduced Treg, YFP-2A-Foxp3, GFP, and untransduced Tconv (as shown in A) were restimulated with CD3/CD28 mAb-coated beads and IL-2 (300 U/ml) for 20 h (black histograms) or left unstimulated (gray histograms). Cells were then stained with anti-Glut1 antibody. (E) Summary of 3 independent experiments as shown in D (*P < 0.01). The data were plotted as the mean ± sd.
Next, we examined the ability of Foxp3 to inhibit Akt activation and subsequently, Glut1 expression in human CD4+ T cells. We observed that transduction of Foxp3 blunted Akt phosphorylation (Fig. 2B and C). Moreover, the level of Akt phosphorylation in the transduced cells was similar to that seen in freshly explanted Tregs. To demonstrate that decreased Akt activity in T cells causes a concomitant decrease in Glut1, we transduced primary human CD4+CD127+CD25− T cells with oxp3 or control GFP expression vectors as described above. Following transduction, we stimulated cells with anti-CD3/anti-CD28 mAb-coated beads and IL-2 and measured Glut1 expression (Fig. 2D and E). Both Tregs and Foxp3-transduced cells were unable to induce Glut1 when compared with GFP-transduced and nontransduced controls. It is likely that the differences in the absolute levels of background staining and Glut1 staining in Figs. 1 and 2 reflect the differences in cell size of the populations being assayed (resting versus in vitro stimulated and expanded).
Although the PI3K/Akt pathway is differentially regulated in Tregs, the molecular mechanisms underlying these differences remain unclear [3, 5, 6, 8]. Contributing to the complexity of Akt signaling in Tregs are reports that many known antagonists of Akt activity are not differentially expressed in Tregs. Several studies have shown that phosphatases PTEN and SHIP are equally expressed in Tconv and Tregs [7, 8]. Confounding matters further, induced murine Tregs can be generated through attenuation of Akt phosphorylation via increased PTEN activity by way of programmed death 1 ligation [22]. However, recent data addressing the differential effects of PHLPP expression in Tregs and Tconv may provide some clues [5]. These studies illustrate that PHLPP, a phosphatase known to dephosphorylate Akt at serine residue 473, is up-regulated in human Tregs. In murine Tregs, PHLPP is also known to be responsible for Akt dephosphorylation. Furthermore, knockdown of PHLPP activity in human Tregs and murine Tregs results in restoration of Akt phophorylation comparable with levels found in Tconv [5]. Finally, it has been demonstrated that in human Tregs, Foxp3 binds PHLPP near its transcriptional start site [23]. Taken together, Foxp3 may exert its effects on Akt and Glut1 through transcriptional up-regulation of PHLPP. Increased PHLPP activity is then responsible for diminished Akt activity and Glut1 expression.
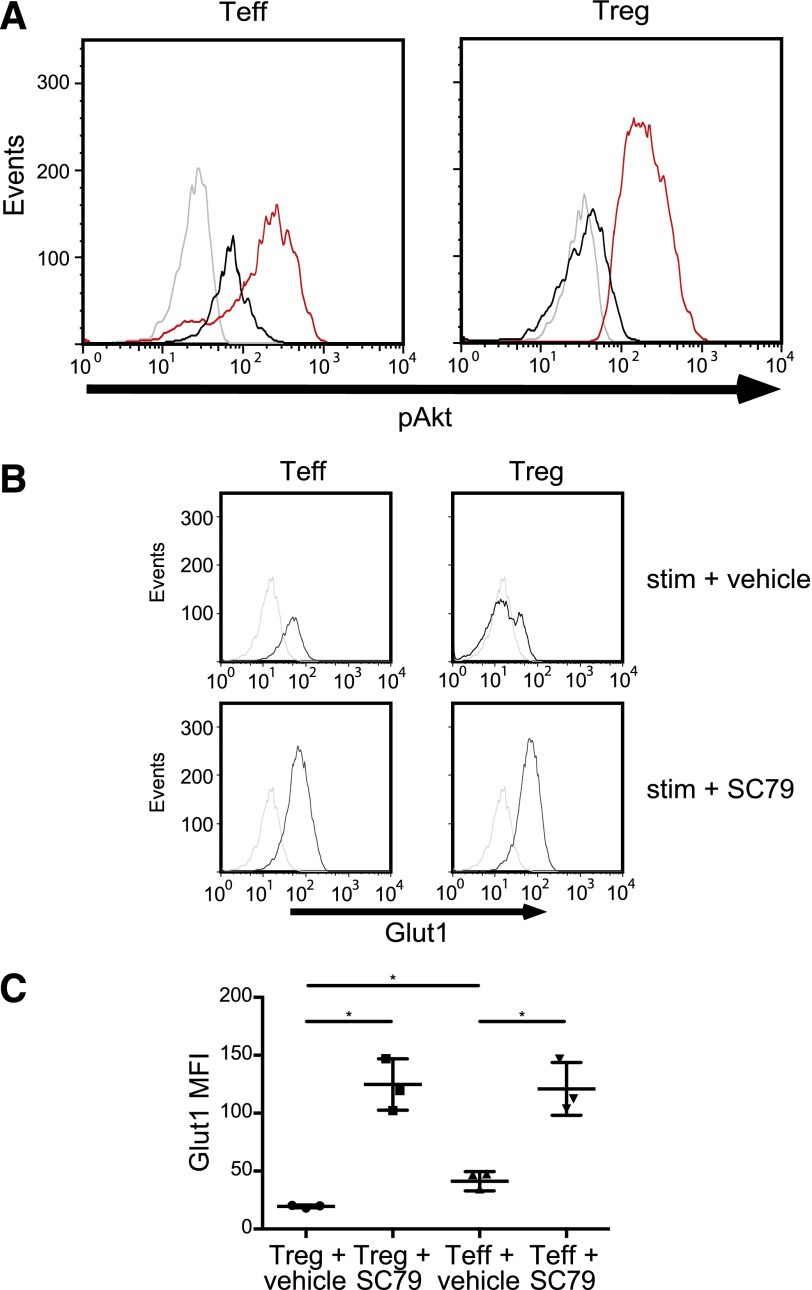
Pharmacologic Akt activation induces Glut1 in Tregs
Our data until this point not only confirm previous work showing that Tregs have a reduced ability to activate Akt [3, 4, 6] but also that they fail to express Glut1 as a consequence. To determine if Glut1 is targeted directly by Foxp3 or indirectly via Akt, we sought to overactivate Akt in Tregs. SC79 is a small molecule activator of Akt described recently [14]. SC79 has been shown to activate Akt in neurons and in Hela cells. We first determined if SC79 could induce Akt activation in primary Tregs and Tconv. Indeed, following a brief treatment of freshly isolated Tregs and Tconv with SC79, considerable Akt activation was observed (Fig. 3A). Surprisingly, the level of Akt activation in both cell types following SC79 treatment was nearly identical. Next, we examined the ability of SC79 to induce Glut1 in human CD4+ T cells. We observed that pharmacologic activation of Akt in freshly isolated Tconv or Tregs resulted in a profound up-regulation of Glut1 (Fig. 3B and C). The nearly equal expression of Glut1 in Tregs and Tconv corresponds to the nearly identical levels of phospho-Ser473 Akt following SC79 treatment in each population. Taken together, our results indicate that this reduced capacity to induce Glut1 is a function of diminished Akt phosphorylation, which in turn, is Foxp3 dependent. In summary, Akt activation is down-regulated in human Tregs in a Foxp3-dependent manner, and this down-regulation prevents Glut1 cell-surface expression. These data demonstrate further a molecular basis behind recent studies [18] that Tregs may not rely on glucose or glycolysis and may use lipid oxidation and short-chain fatty acids. In light of these results, media used for the ex vivo expansion of human Tregs may not benefit from glucose supplementation.
Figure 3. Pharmacologic Akt activation induces Glut1 in Tregs.
(A) Freshly isolated Tconv and Tregs (as shown in Fig. 1A) were stimulated with the Akt agonist SC79 (red histograms), CD3/CD28 mAb-coated beads and 300 U/ml IL-2 (black histograms) or left unstimulated (gray histograms) for 15 min. Cells were then stained with phospho-Ser473 Akt mAb. Data are representative of 3 independent experiments. (B, upper) Freshly isolated Tconv and Tregs (as shown in Fig. 1A) were stimulated with CD3/CD28 mAb-coated beads, IL-2 (300 U/ml), and a vehicle control (black histograms) or left unstimulated (gray histograms) for 20 h. (Lower) Freshly isolated Tconv and Tregs were stimulated with CD3/CD28 mAb-coated beads, IL-2 (300 U/ml), and SC79 (0.5 μg/ml) or left unstimulated for 20 h. Cells were then stained with anti-Glut1 mAb. (C) Summary of 3 independent experiments as shown in B (*P < 0.01). The data were plotted as the mean ± sd.
ACKNOWLEDGMENTS
This research was supported by the Intramural Program of National Institute of Allergy and Infectious Diseases (NIAID), U.S. National Institutes of Health, and by a Cooperative Research and Development Agreement (CRADA) with Boerhinger-Ingelheim. The authors thank the NIAID Flow Cytometry Section, particularly Elina Stregvensky and Bishop Hague for all of their help in sorting our cells. The authors also thank the Department of Transfusion Medicine for providing the blood donors.
Glossary
- Foxp3
forkhead box P3 transcription factor
- Glut1
glucose transporter 1
- iTreg
induced regulatory T cell
- NIAID
National Institute of Allergy and Infectious Diseases
- PHLPP
pH domain and leucine-rich repeat protein phosphatase
- PTEN
phosphatase and tensin homolog
- Tconv
conventional T cell
- Treg
regulatory T cell, YFP = yellow fluorescent protein
AUTHORSHIP
S.B. designed experiments, performed research and analyses, and wrote the manuscript. B.H. isolated and purified cells and performed cytokine staining. E.M.S. supervised research, designed experiments, edited the manuscript, and provided research funding.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Smith-Garvin J. E., Koretzky G. A., Jordan M. S. (2009) T cell activation. Annu. Rev. Immunol. 27, 591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox C. J., Hammerman P. S., Thompson C. B. (2005) Fuel feeds function: energy metabolism and the T-cell response. Nat. Rev. Immunol. 5, 844–852. [DOI] [PubMed] [Google Scholar]
- 3.Crellin N. K., Garcia R. V., Levings M. K. (2007) Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood 109, 2014–2022. [DOI] [PubMed] [Google Scholar]
- 4.Crellin N. K., Garcia R. V., Levings M. K. (2007) Flow cytometry-based methods for studying signaling in human CD4+CD25+FOXP3+ T regulatory cells. J. Immunol. Methods 324, 92–104. [DOI] [PubMed] [Google Scholar]
- 5.Patterson S. J., Han J. M., Garcia R., Assi K., Gao T., O’Neill A., Newton A. C., Levings M. K. (2011) Cutting edge: PHLPP regulates the development, function, and molecular signaling pathways of regulatory T cells. J. Immunol. 186, 5533–5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sauer S., Bruno L., Hertweck A., Finlay D., Leleu M., Spivakov M., Knight Z. A., Cobb B. S., Cantrell D., O’Connor E., Shokat K. M., Fisher A. G., Merkenschlager M. (2008) T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. USA 105, 7797–7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bensinger S. J., Walsh P. T., Zhang J., Carroll M., Parsons R., Rathmell J. C., Thompson C. B., Burchill M. A., Farrar M. A., Turka L. A. (2004) Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J. Immunol. 172, 5287–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh P. T., Buckler J. L., Zhang J., Gelman A. E., Dalton N. M., Taylor D. K., Bensinger S. J., Hancock W. W., Turka L. A. (2006) PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J. Clin. Invest. 116, 2521–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frauwirth K. A., Riley J. L., Harris M. H., Parry R. V., Rathmell J. C., Plas D. R., Elstrom R. L., June C. H., Thompson C. B. (2002) The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs S. R., Herman C. E., Maciver N. J., Wofford J. A., Wieman H. L., Hammen J. J., Rathmell J. C. (2008) Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 180, 4476–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michalek R. D., Gerriets V. A., Jacobs S. R., Macintyre A. N., MacIver N. J., Mason E. F., Sullivan S. A., Nichols A. G., Rathmell J. C. (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macintyre A. N., Gerriets V. A., Nichols A. G., Michalek R. D., Rudolph M. C., Deoliveira D., Anderson S. M., Abel E. D., Chen B. J., Hale L. P., Rathmell J. C. (2014) The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 20, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shevach E. M., Thornton A. M. (2014) tTregs, pTregs, and iTregs: similarities and differences. Immunol. Rev. 259, 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jo H., Mondal S., Tan D., Nagata E., Takizawa S., Sharma A. K., Hou Q., Shanmugasundaram K., Prasad A., Tung J. K., Tejeda A. O., Man H., Rigby A. C., Luo H. R. (2012) Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc. Natl. Acad. Sci. USA 109, 10581–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran D. Q., Andersson J., Wang R., Ramsey H., Unutmaz D., Shevach E. M. (2009) GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 106, 13445–13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu S., Golovina T., Mikheeva T., June C. H., Riley J. L. (2008) Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin. J. Immunol. 180, 5794–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chemnitz J. M., Lanfranco A. R., Braunstein I., Riley J. L. (2006) B and T lymphocyte attenuator-mediated signal transduction provides a potent inhibitory signal to primary human CD4 T cells that can be initiated by multiple phosphotyrosine motifs. J. Immunol. 176, 6603–6614. [DOI] [PubMed] [Google Scholar]
- 18.Smith P. M., Howitt M. R., Panikov N., Michaud M., Gallini C. A., Bohlooly-Y M., Glickman J. N., Garrett W. S. (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugimoto N., Oida T., Hirota K., Nakamura K., Nomura T., Uchiyama T., Sakaguchi S. (2006) Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int. Immunol. 18, 1197–1209. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y., Josefowicz S. Z., Kas A., Chu T. T., Gavin M. A., Rudensky A. Y. (2007) Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature 445, 936–940. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler S. F. (2006) FOXP3: of mice and men. Annu. Rev. Immunol. 24, 209–226. [DOI] [PubMed] [Google Scholar]
- 22.Francisco L. M., Sage P. T., Sharpe A. H. (2010) The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 236, 219–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadlon T. J., Wilkinson B. G., Pederson S., Brown C. Y., Bresatz S., Gargett T., Melville E. L., Peng K., D’Andrea R. J., Glonek G. G., Goodall G. J., Zola H., Shannon M. F., Barry S. C. (2010) Genome-wide identification of human FOXP3 target genes in natural regulatory T cells. J. Immunol. 185, 1071–1081. [DOI] [PubMed] [Google Scholar]