Abstract
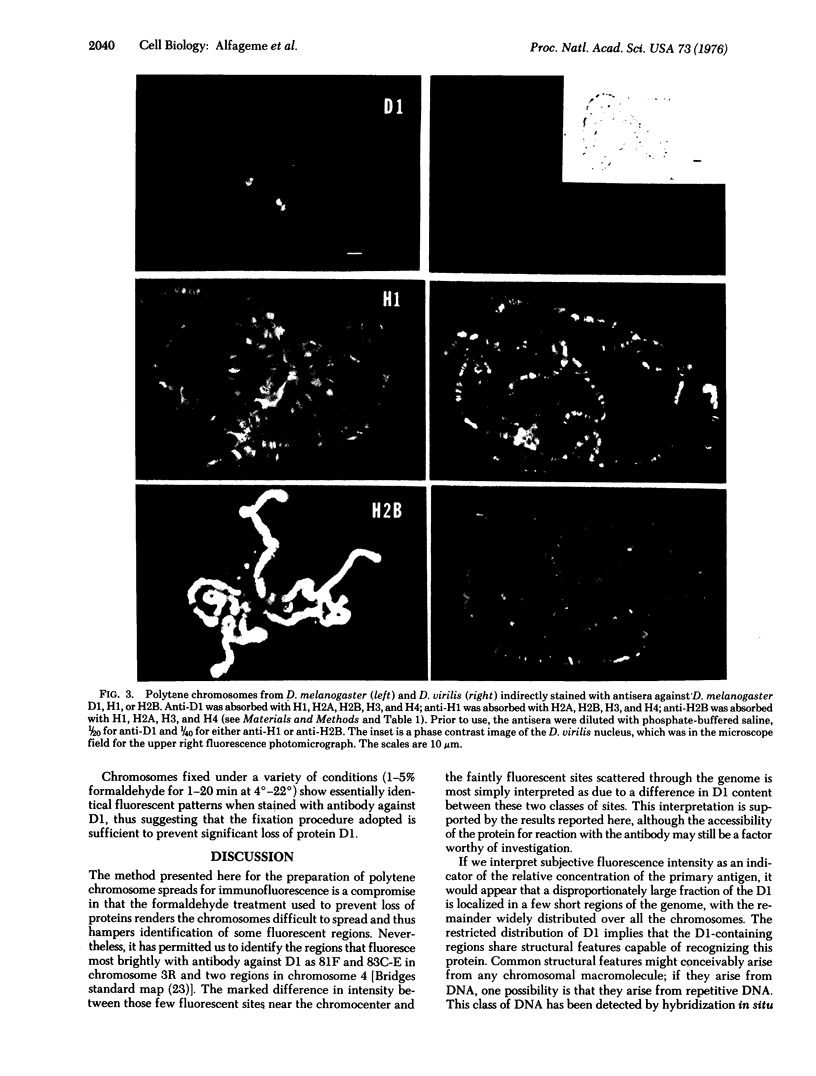
D1, a nonhistone chromosomal protein rich in both basic and acidic amino acids, has been localized at a limited number of specific loci in polytene chromosomes of Drosophila melanogaster. H2B, a nucleosomal histone, and H1, a nonnucleosomal histone, are both found throughout most chromosomal regions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkisson K. P., Perreault W. J., Gay H. Differential fluorescent staining of Drosophila chromosomes with quinacrine mustard. Chromosoma. 1971;34(2):190–205. doi: 10.1007/BF00285186. [DOI] [PubMed] [Google Scholar]
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Barr H. J., Ellison J. R. Ectopic pairing of chromosome regions containing chemically similar DNA. Chromosoma. 1972;39(1):53–61. doi: 10.1007/BF00320590. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Brody T. Histones in cytological preparations. Exp Cell Res. 1974 Apr;85(2):255–263. doi: 10.1016/0014-4827(74)90125-6. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Schlehuber C., Bonner J. Properties of formaldehyde-treated nucleohistone. Biochemistry. 1969 Aug;8(8):3214–3218. doi: 10.1021/bi00836a013. [DOI] [PubMed] [Google Scholar]
- COONS A. H., LEDUC E. H., CONNOLLY J. M. Studies on antibody production. I. A method for the histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J Exp Med. 1955 Jul 1;102(1):49–60. doi: 10.1084/jem.102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWLE A. J. Four modifications of the micro agar diffusion precipitin test. J Lab Clin Med. 1960 Apr;55:593–604. [PubMed] [Google Scholar]
- Chalkley R., Hunter C. Histone-histone propinquity by aldehyde fixation of chromatin. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1304–1308. doi: 10.1073/pnas.72.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C., Bonner J. Partial fractionation and chemical characterization of the major nonhistone chromosomal proteins. Biochemistry. 1972 Feb 29;11(5):772–781. doi: 10.1021/bi00755a015. [DOI] [PubMed] [Google Scholar]
- Ellison J. R., Barr H. J. Differences in the quinacrine staining of the chromosomes of a pair of sibling species: Drosophila melanogaster and Drosophila simulans. Chromosoma. 1971;34(4):424–435. doi: 10.1007/BF00326314. [DOI] [PubMed] [Google Scholar]
- Ellison J. R., Barr H. J. Quinacrine fluorescence of specific chromosome regions. Late replication and high A: T content in Samoaia leonensis. Chromosoma. 1972;36(4):375–390. doi: 10.1007/BF00336794. [DOI] [PubMed] [Google Scholar]
- Gall J. G., Cohen E. H., Polan M. L. Reptitive DNA sequences in drosophila. Chromosoma. 1971;33(3):319–344. doi: 10.1007/BF00284948. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P., Ananieva L. N., Kozlov J. V. Stepwise removal of protein from a deoxyribonucleoprotein complex and de-repression of the genome. J Mol Biol. 1966 Dec 28;22(2):365–371. doi: 10.1016/0022-2836(66)90140-9. [DOI] [PubMed] [Google Scholar]
- Goldblatt D., Bustin M. Exposure of histone antigenic determinants in chromatin. Biochemistry. 1975 Apr 22;14(8):1689–1695. doi: 10.1021/bi00679a022. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Johns E. W. Isolation and characterisation of two calf-thymus chromatin non-histone proteins with high contents of acidic and basic amino acids. Eur J Biochem. 1973 Dec 3;40(1):215–219. doi: 10.1111/j.1432-1033.1973.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Holmquist G. Hoechst 33258 fluorescent staining of Drosophila chromosomes. Chromosoma. 1975;49(4):333–356. doi: 10.1007/BF00285127. [DOI] [PubMed] [Google Scholar]
- Kinkade J. M., Jr, Cole R. D. The resolution of four lysine-rich histones derived from calf thymus. J Biol Chem. 1966 Dec 25;241(24):5790–5797. [PubMed] [Google Scholar]
- Kornberg R. D. Chromatin structure: a repeating unit of histones and DNA. Science. 1974 May 24;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Thomas J. O. Chromatin structure; oligomers of the histones. Science. 1974 May 24;184(4139):865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- Manning J. E., Schmid C. W., Davidson N. Interspersion of repetitive and nonrepetitive DNA sequences in the Drosophila melanogaster genome. Cell. 1975 Feb;4(2):141–155. doi: 10.1016/0092-8674(75)90121-x. [DOI] [PubMed] [Google Scholar]
- Marushige K., Dixon G. H. Transformation of trout testis chromatin. J Biol Chem. 1971 Sep 25;246(18):5799–5805. [PubMed] [Google Scholar]
- Mayfield J. E., Ellison J. R. The organization of interphase chromatin in drosophilidae: the self adhesion of chromatin containing the same DNA sequences. Chromosoma. 1975 Sep 15;52(1):37–48. doi: 10.1007/BF00285787. [DOI] [PubMed] [Google Scholar]
- Murray K. Stepwise removal of histones from native deoxyribonucleoprotein by titration with acid at low temperature and some properties of the resulting partial nucleoproteins. J Mol Biol. 1969 Jan 14;39(1):125–144. doi: 10.1016/0022-2836(69)90338-6. [DOI] [PubMed] [Google Scholar]
- Ohlenbusch H. H., Olivera B. M., Tuan D., Davidson N. Selective dissociation of histones from calf thymus nucleoprotein. J Mol Biol. 1967 Apr 28;25(2):299–315. doi: 10.1016/0022-2836(67)90143-x. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E. Spheroid chromatin units (v bodies). Science. 1974 Jan 25;183(4122):330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- Peacock W. J., Brutlag D., Goldring E., Appels R., Hinton C. W., Lindsley D. L. The organization of highly repeated DNA sequences in Drosophila melanogaster chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:405–416. doi: 10.1101/sqb.1974.038.01.043. [DOI] [PubMed] [Google Scholar]
- Rae P. M. Chromosomal distribution of rapidly reannealing DNA in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1970 Oct;67(2):1018–1025. doi: 10.1073/pnas.67.2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudkin G. T., Tartof K. D. Repetitive DNA in polytene chromosomes of Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1974;38:397–403. doi: 10.1101/sqb.1974.038.01.042. [DOI] [PubMed] [Google Scholar]
- Sederoff R., Lowenstein L. Polypyrimidine segments in Drosophila melanogaster DNA: II. Chromosome location and nucleotide sequence. Cell. 1975 Jun;5(2):183–194. doi: 10.1016/0092-8674(75)90026-4. [DOI] [PubMed] [Google Scholar]
- Silver L. M., Elgin S. C. A method for determination of the in situ distribution of chromosomal proteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):423–427. doi: 10.1073/pnas.73.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Stocken L. A. The characterization of a non-histone protein isolated from histone F1 preparations. Biochem J. 1973 Apr;131(4):859–861. doi: 10.1042/bj1310859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar B. D., Ward M. Rabbit antibodies to histone fractions as specific reagents for preparative and comparative studies. J Biol Chem. 1970 Mar 25;245(6):1261–1266. [PubMed] [Google Scholar]
- Vosa C. G. The discriminating fluorescence patterns of the chromosomes of Drosophila melanogaster. Chromosoma. 1970;31(4):446–451. doi: 10.1007/BF00285835. [DOI] [PubMed] [Google Scholar]
- Weisblum B., De Haseth P. L. Quinacrine, a chromosome stain specific for deoxyadenylate-deoxythymidylaterich regions in DNA. Proc Natl Acad Sci U S A. 1972 Mar;69(3):629–632. doi: 10.1073/pnas.69.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B. Fluorescent probes of chromosomal DNA structure: three classes of acridines. Cold Spring Harb Symp Quant Biol. 1974;38:441–449. doi: 10.1101/sqb.1974.038.01.048. [DOI] [PubMed] [Google Scholar]
- Wilhelm X., Champagne M. Dissociation de la nucléoprotéine d'érythrocytes de poulets par les sels. Eur J Biochem. 1969 Aug;10(1):102–109. [PubMed] [Google Scholar]






