Abstract
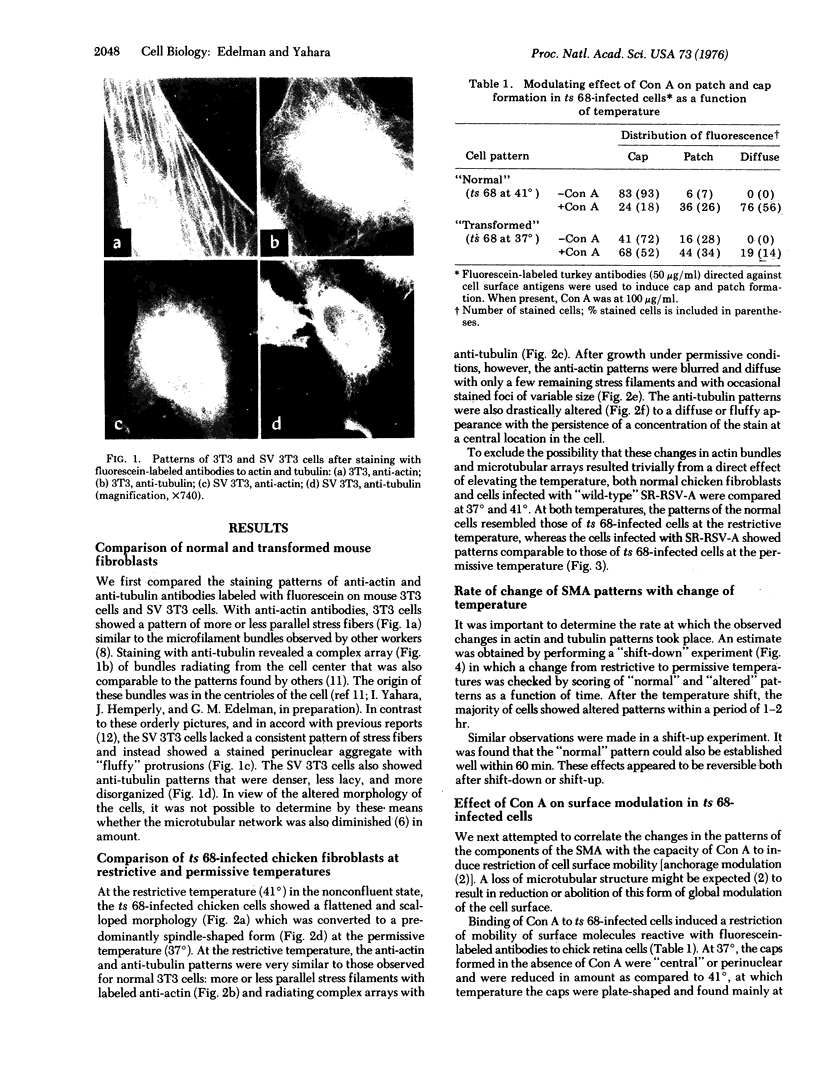
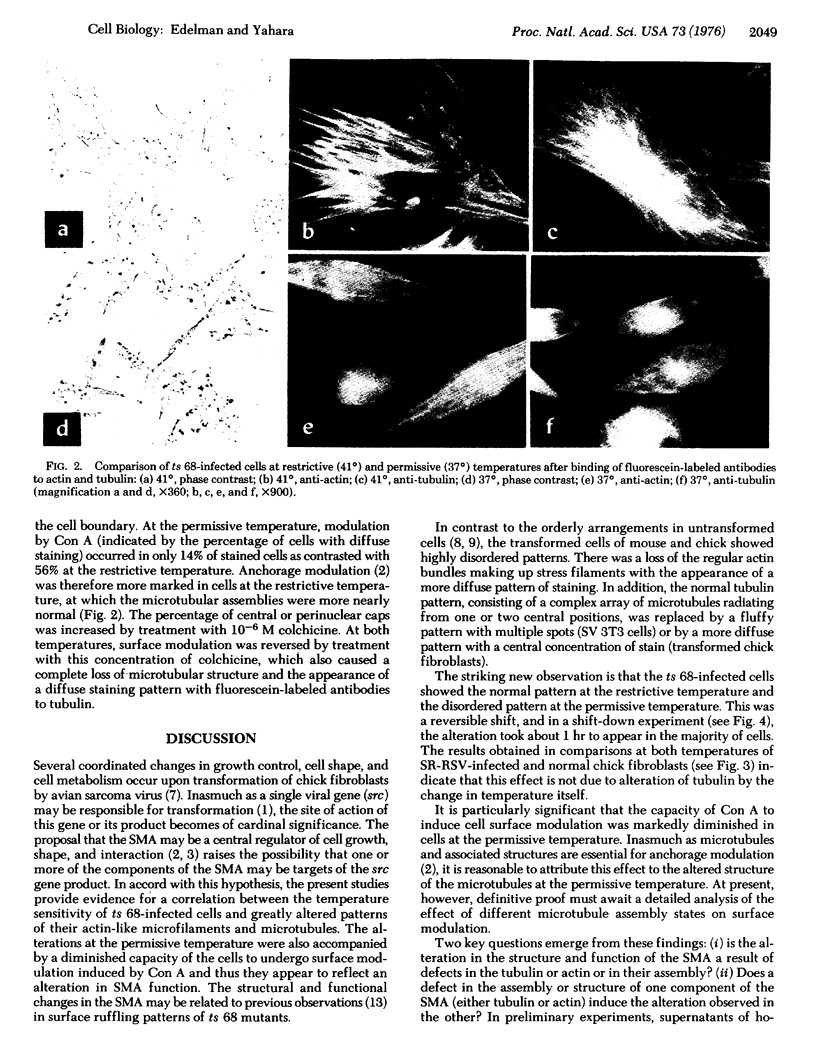
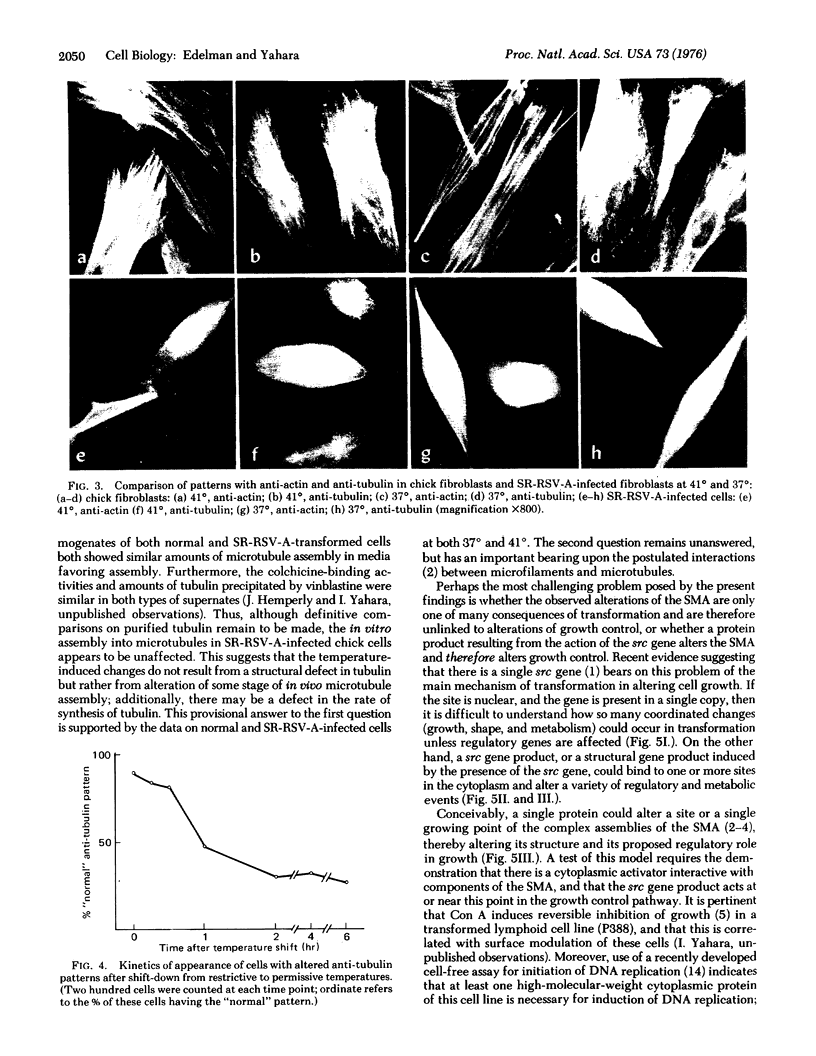
The hypothesis that surface modulating assemblies containing microfilaments and microtubules and altered after cellular transformation was tested on cells infected with temperature-sensitive mutants of avian sarcoma virus. Untransformed cells (mouse 3T3 and chick fibroblasts), cells transformed by simian virus 40 (SV 3T3), and chick fibroblasts infected with Schmidt-Ruppin strain of Rous sarcoma virus (SR-RSV-A-infected cells) were first compared for differences in microfilament and microtubule patterns after treatment with fluorescein-labeled antibodies to actin and tubulin. Transformed cells showed disappearance of ordered stress microfilaments and thickened or diffuse alterations of microtubular arrays. At restrictive temperatures (41 degrees), chick fibroblasts infected with a temperature-sensitive mutant (ts 68) of Rous sarcoma virus showed normal patterns of stress fialments and radial microtubular arrays originating in 1 or 2 centrioles. At permissive temperatures (37 degrees), these patterns were disordered and resembled those of SR-RSV-A-infected cells. After a shift from 41 degrees to 37 degrees, the changes in microtubules were observed in the majority of cells within 1 hr. These changes were reversible and did not result from the inability of tubulin to polymerize. In ts 68-infected cells at permissive temperatures, concanavalin A induced much less surface modulation (inhibition of receptor mobility) than at restrictive temperatures. These results suggest that cellular transformation alters both the structure and function of surface modulating assemblies and prompt the hypothesis that products of viral transforming genes may affect these assemblies with a consequent loss of growth control.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambros V. R., Chen L. B., Buchanan J. M. Surface ruffles as markers for studies of cell transformation by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3144–3148. doi: 10.1073/pnas.72.8.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Edelman G. M. Kinetics of colchicine inhibition of mitogenesis in individual lymphocytes. Exp Cell Res. 1976 Mar 1;98(1):15–22. doi: 10.1016/0014-4827(76)90457-2. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Actin, alpha-actinin, and tropomyosin interaction in the structural organization of actin filaments in nonmuscle cells. J Cell Biol. 1976 Feb;68(2):202–219. doi: 10.1083/jcb.68.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Cytoplasmic microtubules in tissue culture cells appear to grow from an organizing structure towards the plasma membrane. Proc Natl Acad Sci U S A. 1976 Mar;73(3):867–871. doi: 10.1073/pnas.73.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Wang J. L., McClain D. A., Edelman G. M. Modulation of lymphocyte mitogenesis. Proc Natl Acad Sci U S A. 1975 May;72(5):1917–1921. doi: 10.1073/pnas.72.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Bibring T., Osborn M. Specific visualization of tubulin-containing structures in tissue culture cells by immunofluorescence. Cytoplasmic microtubules, vinblastine-induced paracrystals, and mitotic figures. Exp Cell Res. 1975 Oct 1;95(1):111–120. doi: 10.1016/0014-4827(75)90615-1. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Electron microscopic analysis of the modulation of lymphocyte receptor mobility. Exp Cell Res. 1975 Mar 1;91(1):125–142. doi: 10.1016/0014-4827(75)90150-0. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. The effects of concanavalin A on the mobility of lymphocyte surface receptors. Exp Cell Res. 1973 Sep;81(1):143–155. doi: 10.1016/0014-4827(73)90121-3. [DOI] [PubMed] [Google Scholar]