Abstract
Background
With a prevalence of about 1:500 to 1:1,000, autosomal dominant polycystic kidney disease (ADPKD) often causes renal failure, with many serious complications. However, there is no Food and Drug Administration (FDA) approved therapy available.
Material/Methods
MiR-199a-5p level in ADPKD patient samples, rat model, and cell lines were determined with Realtime PCR assay. After miR-199a-5p inhibitor was transfected, we detected the cell proliferation and apoptosis using an MTT assay and an Annexin V-FITC staining kit, respectively. Finally, TargetScan version 5.1 was used to predict the miRNA target and the target gene of miR-199a-5p was proved by a Luciferase assay.
Results
We identified a dramatically up-regulated microRNA, miR-199a-5p, in ADPKD tissues and cell lines. Our data show that inhibition of miR-199a-5p suppressed cyst cells proliferation and induced cell apoptosis. We found that miR-199a-5p might exert this effect through targeting CDKN1C/p57.
Conclusions
Up-regulation of miR-199a-5p in ADPKD tissues might promote cell proliferation through suppressing CDKN1C, suggesting miR-199a-5p as a novel target for ADPKD treatment.
MeSH Keywords: Cell Proliferation; Cyclin-Dependent Kinase Inhibitor p57; MicroRNAs; Polycystic Kidney, Autosomal Dominant
Background
Polycystic kidney disease (PKD) is a serious human kidney disease characterized by many fluid-filled cysts in the renal parenchyma. It is a monogenetic renal disease and can be classified into autosomal dominant PKD (ADPKD) and autosomal recessive PKD (ARPKD) [1–4]. With a prevalence of about 1:500 to 1:1000, ADPKD is quite common and is attributed to mutations of PKD1 and PKD2, with severe clinical manifestations that include abdominal mass, chronic flank, or back pain, gross hematuria, urinary tract infection, and urolithiasis [5–7]. In addition, cysts can cause renal failure, with many serious complications. However, no FDA approved drugs or therapy are available for ADPKD patients and even the underlying mechanism of ADPKD is still unclear.
MicroRNAs (miRNAs, miRs) are a class of endogenous non-coding RNAs with a length of around 22nt RNAs, and can regulate the expressions of the target gene in a post-transcriptional manner [8,9]. MiRNA has been reported to participate in many biological processes, including cell proliferation, cell apoptosis, and carcinogenesis [10–13]. The most important features in ADPKD are the high proliferation of cyst epithelial cells and the secretion of fluid, which lead to cyst expansion [14]. Recently, Patel et al. reported that miRNA 17–92 cluster plays a role in several models of PKD [13]. In the present study, we demonstrate that miR-199a-5p is up-regulated in ADPKD tissues and plays a critical role in the proliferation of cyst epithelial cells, suggesting miR-199a-5p as a potential target for treatment.
Material and Methods
Ethics statement
The entire study was approved by the Ethics Committee of Second Military Medical University, Shanghai, China. After signing informed consent, human cystic kidney tissues were obtained from ADPKD patients who receive nephrectomies. Normal renal cortical tissues were obtained from kidneys that were removed for circumscribed tumors. Histological examination of these kidney samples did not show any renal pathology.
Animal and treatment
An established animal model of ADPKD heterozygous (Cy/+) and normal littermate control (+/+) Han: SPRD rats were used as described in our previous study [15]. All the experiments were approved by the Second Military Medical University, China in accordance with the Guide for Care and Use of Laboratory Animals published by the US NIH (publication No. 96-01). Rats had free access to tap water and standard rat diet [15].
Cell culture and transfection
Human ADPKD cystic lining epithelial cells OX161 (a kind gift from Sheffield Kidney Institute, Division of Clinical Sciences (North), University of Sheffield, UK), human ADPKD cell lines WT9–12 (a gift given by prof. Jing Zhou of Harvard University), human renal tubular epithelial UCL93, RCTEC and HK2 cells, human mesangial cell HMCL, and mouse mesangial cell RCM cell lines, maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (PAA, Austria) at 37°C. After being used for checking the level of miR-199a-5p, OX161 cells were used for transfection. Firstly, cells were seeded in 24-well plates at a 6×104 cells per well and transfected with miR-199a-5p inhibitor and normal control inhibitor (Genema Technology, Shanghai, China) by using Lipofectamine 2000 (Invitrogen, Canada) transfection reagent according to the manufacturer’s instructions. Cells were used for further experiments 48 h later.
RNA extraction and real time PCR
Paired ADPKD tissues and adjacent normal tissues of renal cancer resected surgically used for qRT-PCR were collected from 5 ADPKD patients during surgery at Changzheng Hospital (Shanghai, China) [16]. RNA from human renal tissues, rat renal tissues, and cells were homogenized in Trizol (Invitrogen) and isolated according to the manufacturer’s instructions. The miR-199a-5p were determined with Roche technology, as described in a previous study [17].
Cell proliferation assay
After being seeded into 96-well plates at 4000 cells per well, cells were transfected with miR-199a-5p inhibitor/normal control inhibitor. Cell viability was determined by an MTT assay at Days 1–5 after transfection, as described in previous studies [18–20].
Apoptosis
Cell apoptosis were detected by using the Apoptosis Detecting Kit (Invitrogen) and analyzed by flow cytometry [17]. Briefly, after different treatments, cells were labeled with annexin V-FITC and propidium iodide (PI) following the manufacturer’s instructions. Samples were determined by flow cytometry and the results were analyzed using CellQuest software (Becton Dickinson, San Jose, CA) as described previously.
Plasmids
The luciferase-3′ UTR reporter constructs were generated by introducing the wild type and mutated CDKN1C/p57 3′-UTR into a pGL3 promoter vector (Promega) by the Xba1 site (Generay, Shanghai, China) in a method described previously [17,21,22]. All PCR products were verified by DNA sequencing.
Western blot assay
Proteins from normal and ADPKD tissues were obtained using ProteoJET™ Mammalian Cell Lysis Reagent (Fermentas) according to manufacturer’s protocol, and then analyzed by Western blotting to detect CDKN1C (Cell Signaling Technology, 1:1000) and β-actin (Cell Signaling Technology, 1:1000). The secondary antibody was purchased from Sigma.
miRNA-target prediction and luciferase assays
TargetScan version 5.1 (http://www.targetscan.org/index.html) was used to find potential targets for miR-199a-5p. Luciferase assays were performed by using a luciferase assay kit (E1910, Promega) according to the manufacturer’s instructions. TK Renilla was used for normalization in Dual Luciferase assays.
Statistical analysis
Data are expressed as means ±SEM for each experiment. For single comparisons, we performed an unpaired 2-tailed t-test; for multiple comparisons, we used an analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc test. We performed experiments for quantification in a blinded fashion. P<0.05 was considered to be significantly different.
Result
MiR-199a-5p level was higher in human ADPKD tissues and rat model
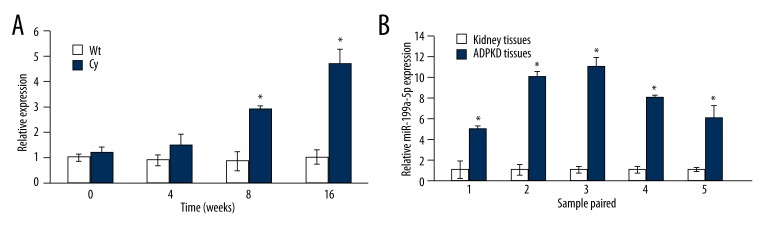
MiR-199a-5p was detected in Han: SPRD rat model and human tissues by using a realtime PCR method. We found that the expression of miR-199a-5p increased with the disease progression in Han: SPRD cystic rats (Figure 1A). We also found that miR-199a-5p level increased in 5 paired human ADPKD tissues compared with the adjacent normal control tissues (Figure 1B).
Figure 1.
Increase of miR-199a-5p in animal models and tissue in patients with ADPKD. Bar graphs of increased levels of miR-199a-5p in AKPKD rat models (A) and in tissues of patients with ADPKD (B). * P<0.05 vs. the control group.
MiR-199a-5p inhibitor inhibited the proliferation rate of human renal ox161 cells
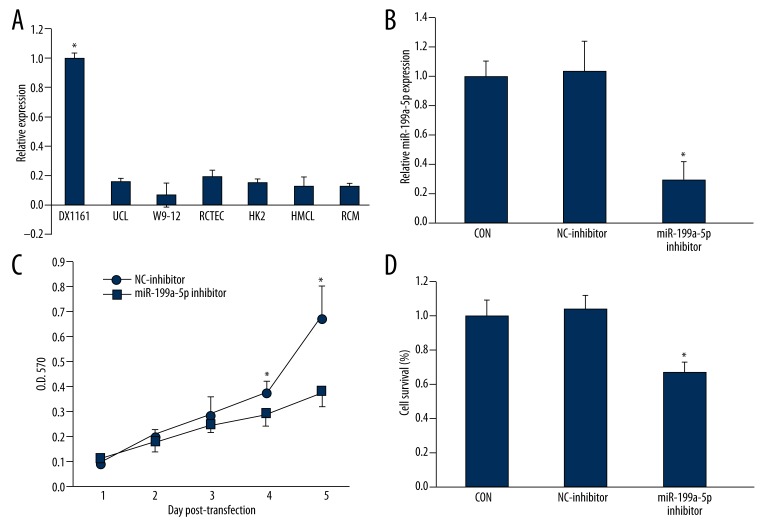
The expression level of miR-199a-5p was determined in 7 kinds of human renal cells and we found miR-199a-5p was dramatically higher than that in other cells (Figure 2A). The expression is similar to that in cyst tissues, and ox161 was selected for the following experiments. We found that our inhibitor effectively inhibited the level of miR-199a-5p in ox161 cells (Figure 2B). By using an MTT assay (Figure 2C) and a colony assay (Figure 2D), we found that miR-199a-5p inhibitor significantly inhibited ox161 cell proliferation.
Figure 2.
miR-199a-5p inhibitor inhibited the proliferation of human PKD1 cystic ox161 cell line. A: Bar graphs of expression of miR-199a-5p in many human renal cell lines (A) and cells treated with miR-199a-5p inhibitor (B). Cell proliferation and survival in different treated groups were conducted with MTT assay (C) and colony formation assay (D), respectively. * P<0.05 vs. the control group.
Inhibition of miR-199a-5p induced cell apoptosis
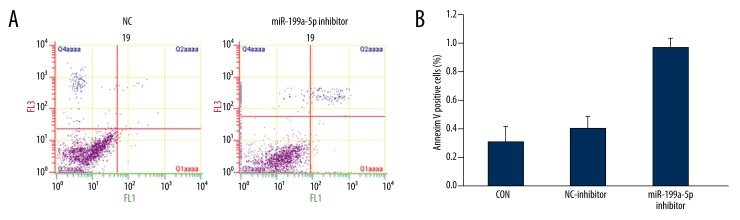
After transfection, apoptosis in ox161 cells was determined by Annexin V and PI staining method. We found that miR-199a-5p inhibitor induced cells apoptosis (Figure 3A, 3B). These data indicated that miR-199a-5p plays a role in cell apoptosis regulation.
Figure 3.
Inhibition of miR-199a-5p induced apoptosis in ox161 cells. (A) Representative images of apoptosis in ox161 cells after transfection of miR-199a-5p inhibitor. (B) a bar graph of Annexin V positive cells in different groups. * P<0.05 vs. the control group.
miR-199a-5p directly targeted CDKN1C/p57
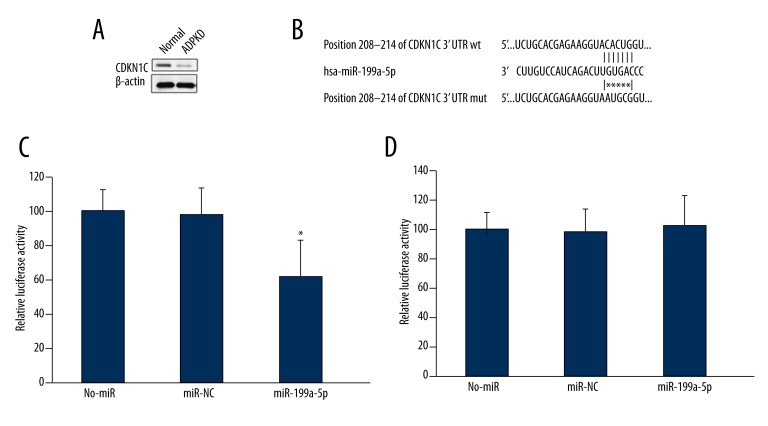
By using a TargetScan method, wild type of human CDKN1C 3′UTR was found to contain a putative miR-199a-5p binding site, but the mutated mouse BMI1 3′ UTR was unable to bind (Figure 4B). We also demonstrated that CDKN1C level is downregulated in ADPKD tissues (Figure 4A). We also found that miR-199a-5p significantly reduced the luciferase activity of the wild type CDKN1C 3′UTR (Figure 4C) but had no effect on the luciferase activity of the mutated CDKN1C 3′UTR (Figure 4D).
Figure 4.
miR-199a-5p directly targeted CDKN1C/p57. (A) Western blot assay of CDKN1C in ADPKD tissues and normal tissues. (B) miR-199a-5p targets CDKN1C 3′UTR site predicted by TargetScan. Bar graphs of firefly luciferase activities in HEK293 cells transfected with wild type PGL3-CDKN1C (C) or mutated PGL3-CDKN1C (D) vector and miR-NC or miR-199a-5p. * P<0.05 vs. the control group.
Discussion
Previous studies have demonstrated that miR-199a-5p has important roles in cancer cell proliferation, fibrosis, cardiac hypertrophy, and angiogenesis [23–27]. In the present study, we reported the up-regulation of miR-199a-5p in ADPKD animal models and human tissues. Inhibition of miR-199a-5p also reduced the proliferation of cyst cells and induced apoptosis and cell cycle arrest. Finally, we identified CDKN1C/p57 as a direct target of miR-199a-5p.
MiRNAs participate in many physiological and pathological processes through a mechanism of post-transcriptionally suppressing target genes [28–30]. Numerous miRs have been found to be up-regulated and down-regulated in many diseases [31], indicating that miRs act as a very critical network in vivo and provide important clues for therapy target identification. In our study, we found that miR-199a-5p increased dramatically in animal models and in human ADPKD tissues, which provides important information that the abnormal expression of miR-199a-5p exerts critical roles in the genesis and progression of ADPKD. Next, we found that this miR was extremely high in a human cyst cell line, OX161, compared to other renal cell lines. This prompted us to study the effects of miR-199a-5p inhibitor on cyst cell proliferation and the progression of ADPKD. Our data showed that miR-199a-5p inhibitor suppressed the proliferation of OX161 cells, suggesting a role of miR-199a-5p in the high rates of proliferation of cyst epithelial cells. At the same time, WT9–12 is also an ADPKD-related cell line, which was also expected to have a high level of miR-199a-5p; however, we did not see a huge difference in miR-199a-5p level between WT9–12 and other renal cell lines. The underlying mechanism still needs to be clarified.
Cell proliferation suppression has 2 possible causes: apoptosis and cell cycle arrest. We demonstrated that miR-199a-5p inhibition induced apoptosis in OX161 cells. Increased cell apoptosis is an important feature of ADPKD [32,33], and we found that miR-199a-5p inhibitor induced cell apoptosis, which is not consistent with the increase of apoptosis in the disease model. It might be that apoptosis induction is not dominant in this process, but the underlying mechanism still needs further studies. Then we predicted potential targets for miR-199a-5p with a bioinformatics tool, TargetScan 5.1, and examined by a dual reporter gene, we confirmed CDKN1C as a directly target of miR-199a-5p. Down-regulation of miR-199a-5p increased the CDKN1C/p57 gene expression. CDKN1C/p57 is very important in cell cycle regulation, and cells deficient in this gene exhibit high rates of cell growth and decreased differentiation [34,35]. CDKN1C/p57 was down-regulated in many proliferation diseases, including cancer [36,37]. In the miR-199a-5p inhibitor group, CDKN1C expression is obviously up-regulated, suggesting CDKN1C as a mediator of the proliferation inhibitory effect of miR-199a-5p inhibitor and as a target of this miR.
Conclusions
In conclusion, we demonstrated the up-regulation of miR-199a-5p and found that inhibition of miR-199a-5p suppressed the cyst cells proliferation and induced cell apoptosis. Finally, we found miR-199a-5p can exert a proliferation inhibitory effect through targeting CDKN1C/p57, which is also lower in AKPKD tissues compared with normal tissues. Our study provides some clues for a novel target in the clinical treatment of ADPKD.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
Source of support: The paper was supported by National Natural Science Foundation of China (NSFC) (No: 811000487; 810000117)
References
- 1.Bible E. Polycystic kidney disease: Periostin is involved in cell proliferation and interstitial fibrosis in polycystic kidney disease. Nat Rev Nephrol. 2014;10(2):66. doi: 10.1038/nrneph.2013.270. [DOI] [PubMed] [Google Scholar]
- 2.Sweeney WE, Jr, Avner ED. Pathophysiology of childhood polycystic kidney diseases: new insights into disease-specific therapy. Pediatr Res. 2014;75(1–2):148–57. doi: 10.1038/pr.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torra R. [Treatment of autosomal dominant polycystic kidney disease]. Med Clin (Barc) 2014;142(2):73–79. doi: 10.1016/j.medcli.2013.09.018. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 4.Carney EF. Polycystic kidney disease: TGF-beta signalling and vascular complications in ADPKD. Nat Rev Nephrol. 2013;9(12):694. doi: 10.1038/nrneph.2013.214. [DOI] [PubMed] [Google Scholar]
- 5.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13(9):2384–98. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 6.Koptides M, Deltas CC. Autosomal dominant polycystic kidney disease: molecular genetics and molecular pathogenesis. Hum Genet. 2000;107(2):115–26. doi: 10.1007/s004390000347. [DOI] [PubMed] [Google Scholar]
- 7.Büscher R, Büscher AK, Weber S, et al. Clinical manifestations of autosomal recessive polycystic kidney disease (ARPKD): kidney-related and non-kidney-related phenotypes. Pediatr Nephrol. 2013 doi: 10.1007/s00467-013-2634-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Saito Y, et al. Epigenetic Alterations and MicroRNA Misexpression in Cancer and Autoimmune Diseases: a Critical Review. Clin Rev Allergy Immunol. 2013 doi: 10.1007/s12016-013-8401-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seton-Rogers S. microRNA: Self-regulated transcription. Nat Rev Cancer. 2013;14(1):9. [Google Scholar]
- 10.Thum T, Mayr M. Review focus on the role of microRNA in cardiovascular biology and disease. Cardiovasc Res. 2012;93(4):543–44. doi: 10.1093/cvr/cvs085. [DOI] [PubMed] [Google Scholar]
- 11.Chen F, Hu SJ. Effect of microRNA-34a in cell cycle, differentiation, and apoptosis: a review. J Biochem Mol Toxicol. 2012;26(2):79–86. doi: 10.1002/jbt.20412. [DOI] [PubMed] [Google Scholar]
- 12.Yendamuri S, Calin GA. The role of microRNA in human leukemia: a review. Leukemia. 2009;23(7):1257–63. doi: 10.1038/leu.2008.382. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y1, Zhao J, Zhang PY, et al. MicroRNA-10b targets E-cadherin and modulates breast cancer metastasis. Med Sci Monit. 2012;18(8):BR299–308. doi: 10.12659/MSM.883262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel V1, Williams D, Hajarnis S, et al. miR-17~92 miRNA cluster promotes kidney cyst growth in polycystic kidney disease. Proc Natl Acad Sci USA. 2013;110(26):10765–70. doi: 10.1073/pnas.1301693110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang T1, Wang L, Xiong X, et al. Mycophenolate mofetil versus Rapamycin in Han: SPRD rats with Polycystic Kidney Disease. Biol Res. 2009;42(4):437–44. [PubMed] [Google Scholar]
- 16.Jing W, Chen Y, Lu L, et al. Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Producing IL15 Eradicate Established Pancreatic Tumor in Syngeneic Mice. Mol Cancer Ther. 2014;13(8):2127–37. doi: 10.1158/1535-7163.MCT-14-0175. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Liu C, Gao F, et al. miR-200c enhances radiosensitivity of human breast cancer cells. J Cell Biochem. 2013;114(3):606–15. doi: 10.1002/jcb.24398. [DOI] [PubMed] [Google Scholar]
- 18.Peng TS, et al. [The role of CD44 in the proliferation, adhesiveness and invasiveness of osteosarcoma cell lines]. Zhonghua Bing Li Xue Za Zhi. 2005;34(6):362–66. [in Chinese] [PubMed] [Google Scholar]
- 19.Hu H, Zhang Y, Huang F, Deng H. [Inhibition of proliferation and induction of apoptosis by tanshinone II A in NCI-H460 cell]. Zhong Yao Cai. 2005;28(4):301–4. [in Chinese] [PubMed] [Google Scholar]
- 20.Zhang QL, Yang SH, Liu HY, Wu CH. [Inhibition of osteosarcoma cell proliferation by a short hairpin RNA targeting proliferation cell nuclear antigen]. Zhonghua Bing Li Xue Za Zhi. 2005;34(3):167–70. [in Chinese] [PubMed] [Google Scholar]
- 21.Wang DT, Ma ZL, Li YL, et al. miR-150, p53 protein and relevant miRNAs consist of a regulatory network in NSCLC tumorigenesis. Oncol Rep. 2013;30(1):492–98. doi: 10.3892/or.2013.2453. [DOI] [PubMed] [Google Scholar]
- 22.Gao XN1, Lin J, Li YH, et al. [Construction of FLT3 3′-UTR-luciferase reporter vector and evaluation of its activity]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18(3):694–97. [in Chinese] [PubMed] [Google Scholar]
- 23.Monastyrskaya K, Sánchez-Freire V, Hashemi Gheinani A, et al. miR-199a-5p regulates urothelial permeability and may play a role in bladder pain syndrome. Am J Pathol. 2013;182(2):431–48. doi: 10.1016/j.ajpath.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Xu N, Zhang J, Shen C, et al. Cisplatin-induced downregulation of miR-199a-5p increases drug resistance by activating autophagy in HCC cell. Biochem Biophys Res Commun. 2012;423(4):826–31. doi: 10.1016/j.bbrc.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 25.Sakurai K, Furukawa C, Haraguchi T, et al. MicroRNAs miR-199a-5p and -3p target the Brm subunit of SWI/SNF to generate a double-negative feedback loop in a variety of human cancers. Cancer Res. 2011;71(5):1680–89. doi: 10.1158/0008-5472.CAN-10-2345. [DOI] [PubMed] [Google Scholar]
- 26.Shen Q, Cicinnati VR, Zhang X, et al. Role of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion. Mol Cancer. 2010;9:227. doi: 10.1186/1476-4598-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rane S, He M, Sayed D, et al. An antagonism between the AKT and beta-adrenergic signaling pathways mediated through their reciprocal effects on miR-199a-5p. Cell Signal. 2010;22(7):1054–62. doi: 10.1016/j.cellsig.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai Y, Yu X, Hu S, Yu J. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics. 2009;7(4):147–54. doi: 10.1016/S1672-0229(08)60044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miao KR, Xu W, Li JY. [New member miRNA in p53 gene signal pathway – review]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17(2):500–3. [in Chinese] [PubMed] [Google Scholar]
- 30.Shang C, Lu YM, Meng LR. MicroRNA-125b down-regulation mediates endometrial cancer invasion by targeting ERBB2. Med Sci Monit. 2012;18(4):BR149–55. doi: 10.12659/MSM.882617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang JF, Zha YF, Li HW, et al. Screening plasma miRNAs as biomarkers for renal ischemia-reperfusion injury in rats. Med Sci Monit. 2015;21:283–89. doi: 10.12659/MSM.889937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnaout MA. Molecular genetics and pathogenesis of autosomal dominant polycystic kidney disease. Annu Rev Med. 2001;52:93–123. doi: 10.1146/annurev.med.52.1.93. [DOI] [PubMed] [Google Scholar]
- 33.Bolster F, O’Dywer E, Ryan J, Geoghegan T. Megaseminal vesicles in adult polycystic kidney disease. ANZ J Surg. 2013 doi: 10.1111/ans.12305. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Hildebrand J, Spörl F, Korge S, et al. Cell cycle regulator Cdkn1c (p57/KIP2) shows distinct expression in epidermal differentiation. Eur J Dermatol. 2012;22(5):694–96. doi: 10.1684/ejd.2012.1828. [DOI] [PubMed] [Google Scholar]
- 35.Sun K, Wang W, Lei ST, et al. [MicroRNA-221 promotes colon carcinoma cell proliferation in vitro by inhibiting CDKN1C/p57 expression]. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31(11):1885–89. [in Chinese] [PubMed] [Google Scholar]
- 36.Larson PS, Schlechter BL, King CL, et al. CDKN1C/p57kip2 is a candidate tumor suppressor gene in human breast cancer. BMC Cancer. 2008;8:68. doi: 10.1186/1471-2407-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmann MJ, Florl AR, Seifert HH, Schulz WA. Multiple mechanisms downregulate CDKN1C in human bladder cancer. Int J Cancer. 2005;114(3):406–13. doi: 10.1002/ijc.20749. [DOI] [PubMed] [Google Scholar]