Abstract
Objective
To test the effectiveness of using a cuff pressure relief valve technique to maintain cuff pressure levels within the normal in vitro range (Phase 1) in patients admitted to the intensive care unit (Phase 2) and to test the reproducibility of the technique using different syringes.
Methods
In Phase 1, a tracheal tube was inserted into a trachea model. Ten- and 20mL syringes were used to inflate the cuff through the tracheal tube. The cuff was slowly and steadily inflated until the syringe plunger would move in the opposite direction of the application. After the plunger stopped, the cuff pressures were recorded. In Phase 2, the same maneuvers for inflating the cuff were performed on 20 patients using 5, 10, and 20mL syringes and were compared with manometer measurements. The intraclass correlation coefficient and Bland-Altman analysis were employed to determine the reproducibility and agreement between syringes. Data were expressed as medians (interquartile range).
Results
There was no reproducibility between syringes with an intraclass correlation coefficient ranging between -0.33 and 0.8 (p>0.05). The pressures generated with the syringes were higher than the pressures generated using a standard manometer: the 5mL syringe pressure was 105cmH2O (82.5-120cmH2O), the 10mL syringe pressure was 69cmH2O (47.5-111.3cmH2O), and the 20mL syringe pressure was 45cmH2O (35-59.5cmH2O). The Bland-Altman analysis confirmed the large bias and variability between the syringes used, compared with the manometer.
Conclusion
The use of syringes is not an effective technique for determining the cuff pressure in patients admitted to the intensive care unit.
Keywords: Transducers, pressure; Respiration, artificial; Airway management; Intubation, intratracheal; Respiratory therapy; Intensive care units
Abstract
Objetivo
Testar a eficácia da técnica de alívio de pressão de cuff por meio de uma válvula em manter níveis de pressão de cuff dentro da normalidade in vitro (Fase 1) e em pacientes internados em unidade de terapia intensiva (Fase 2), bem como testar a reprodutibilidade da técnica utilizando diferentes seringas.
Métodos
Na Fase 1, uma cânula orotraqueal foi inserida em um modelo de traqueia. Seringas de 10 e 20mL foram utilizadas para insuflar o cuff da cânula. O cuff foi insuflado lenta e progressivamente até que o êmbolo da seringa se deslocasse em direção contrária da aplicação. Após a pausa do êmbolo, as pressões do balonete foram registradas. Na Fase 2, a mesma manobra de insuflação do cuff foi realizada em 20 pacientes, utilizando-se seringas de 5, 10 e 20mL, e foi comparada com as medidas de um manômetro. O índice de correlação intraclasse e a análise de Bland-Altman foram realizados para verificar a reprodutibilidade e a concordância entre as seringas. Os dados foram expressos como mediana (intervalo interquartil).
Resultados
A reprodutibilidade entre as seringas foi nula, com índice de correlação intraclasse variando entre -0,33 e 0,8 (p>0,05). As pressões geradas com as seringas foram superiores à pressão obtida com o manômetro padrão: seringa de 5mL teve 105cmH2O (82,5-120cmH2O); seringa de 10mL teve 69cmH2O (47,5-111,3cmH2O) e seringa de 20mL teve 45cmH2O (35-59,5cmH2O). O teste de Bland-Altman verificou grandes vieses e variabilidade entre as seringas utilizadas, quando estas foram comparadas ao manômetro.
Conclusão
O uso de seringas não é eficaz em determinar valores de pressão de cuff seguros em pacientes internados em unidade de terapia intensiva.
INTRODUCTION
Tracheal prostheses are important for pulmonary ventilation of patients in intensive care units (ICU), but they can also damage the histological structure of the trachea when the pressure exerted by the cuff is greater than the tracheal perfusion.(1,2) The severity of the injury depends on both the contact time and the pressure exerted between the cuff and the tracheal wall,(3,4) which may result in the loss of cilia, epithelial erosion, rupture of blood capillaries with tracheal ulceration, stenosis, and tracheoesophageal fistulas.(5,6)
Conversely, cuff pressures below 20cmH2O may lead to the aspiration of contaminated oropharyngeal contents into the lower respiratory tract. According to the American Thoracic Society and the Infectious Diseases Society of America, the primary route of bacterial entry into the lower respiratory tract is the aspiration of oropharyngeal pathogens through the cuff, predisposing patients to mechanical ventilator-associated pneumonia,(1,7) the prevalence of which varies between 10% and 27% in critically ill patients.(8-10)
To avoid injuries caused by the hyperinflation or underinflation of the cuff, many authors recommend that the intra-cuff pressures be maintained at between 20 and 30cmH2O.(11-13) However, maintaining these pressures within these levels has been a challenge in clinical practice because many factors influence the variation in cuff pressures, such as changes in the tracheal muscle tone, hypothermia, and the position of both the patient and cuff,(14-16) reinforcing the necessity of frequently monitoring and adjusting the cuff pressures.
Although numerous techniques have been described to measure cuff pressure, it is believed that the most effective methods for achieving the recommended pressures are those that use an aneroid manometer (usually portable) specifically designed for this function.(17) However, in Brazil, many hospitals do not have such a device because of its high cost; instead, pressures are checked using indirect measurements.
The cuff pressure relief valve technique was described by Somri et al. as an economic alternative for measuring and maintaining the cuff pressure in patients undergoing anesthesia. This technique uses a 20mL (P20) syringe to inflate the pilot balloon with 15mL of air. Tracheal distension caused by air insufflation generates an opposing force of resistance, which acts on the cuff and is then transferred to the syringe plunger, moving it in the opposite direction of the application. When the syringe plunger stops moving, the tracheal wall pressure is considered equal to the cuff pressure, thus maintaining it at safe levels.(18)
In a surgical environment where the gases used in inhalation anesthetics directly influence cuff pressure, it has been demonstrated that the cuff pressure relief valve technique can maintain pressures within the recommended limits.(18) Because this technique is an economical alternative to a manometer, it has spread beyond the surgical center. However, its effectiveness has not yet been established in patients admitted to ICU, where cuff pressure is not affected by anesthetic gases. Therefore, the aim of this study was to test the effectiveness of the cuff pressure relief valve technique in maintaining adequate cuff pressure levels in ICU patients using 20mL (P20), 10mL (P10), and 5mL (P5) syringes. Furthermore, we analyzed the reproducibility of this technique using different P20 and P10 syringes in a trachea model.
METHODS
This study was a prospective cross-sectional investigation that was conducted in two phases: (1) in vitro and (2) in vivo. The study was approved by the Research Ethics Committee of the Hospital das Clínicas at the Universidade de São Paulo (1070/09). Family members of all the patients agreed to participate in the study and signed an informed consent form.
The first phase assessed the reproducibility of five P10 and P20 syringes (BD, Becton, Dickinson and Company, USA) using a trachea model. For this purpose, a PVC tube was used that had an inner diameter of approximately 4cm, into which a tracheal tube with an inner diameter of 8mm was inserted until the cuff portion of the tracheal tube reached the middle third of the tube. A three-way connector was attached to the pilot balloon, to a manometer specifically designed to measure cuff pressures (VBM Medizintechnik GmbH, Germany) and to the syringe being used in the experiment.
The syringes were prefilled with air, and the cuff was slowly and steadily inflated until the syringe plunger moved in the opposite direction of the application. Small pauses were taken while inflating the cuff to allow passive recoil of the syringe plunger until it reached pressure equilibrium. The cuff pressure was measured with the manometer as soon as the syringe plunger stopped moving. For this phase, five P10 and five P20 syringes from the same manufacturer were used, and five measurements were performed for each syringe.
The second phase consisted of an in vivo experiment, in which the maneuver described in Phase 1 was conducted on a convenience sample of 20 patients intubated less than 48 hours who were ≥18 years of age. Patients who had a prior history of intubation and/or tracheostomy, head and neck surgery (previous or current), or mechanical ventilation at high peak airway pressures (positive end-expiratory pressure (PEEP) >10cmH2O) were excluded. In this phase, a three-way connector was connected to the pilot balloon of the endotracheal tube of each patient, to the manometer, and to a syringe. After a bronchial clearance session and suctioning the upper airways and supra-cuff region, the initial cuff pressure (P-initial) was evaluated. Then, the pilot balloon was completely emptied, and the same maneuver performed in Phase 1 was sequentially and randomly performed with P5, P10, and P20 syringes. When the syringe plunger stopped moving (as in Phase 1), the cuff pressure was measured.
Cuff pressure measurements with a manometer were performed during the expiratory phase. In this phase, a single cuff pressure measurement was performed with each syringe on each patient. After collecting the data, the initial cuff pressure was reestablished. The calculation of the sample power (1-β) with a confidence interval (CI) of 99% (two-sided) indicated that our sample had a power of 100%.
New syringes with less than one year since the manufacturing date and stored at room temperature in a dry place were used in all phases of the study. Prior to the experiments, the syringes were “tested”; in other words, we moved the plunger within the syringe barrel two or three times to unstick the rubber plunger from the barrel (due to general storage of the syringe).
Statistical analysis
In Phase 1, we used a simple analysis of variance (one-way ANOVA) to determine the variability between the five P10 syringes and between the five P20 syringes used. At this stage, the pressures between the P10 and P20 syringes were not compared. The intraclass correlation coefficient (ICC) with a 95% CI was used to determine the reproducibility of each P10 and P20 syringe. The ICC can range from -1 to +1, where values >0.75 indicate excellent reproducibility, values between 0.4 and 0.75 indicate reasonable reproducibility, and values <0.4 indicate poor reproducibility. Negative values indicate cases with an extreme lack of consistency between the data analyzed.(19)
In Phase 2 of the study, the Wilcoxon test was used to compare the initial cuff pressures to the pressures generated using maneuvers involving the P5, P10, and P20 syringes. This test was selected because the data were not normally distributed. A Bland-Altman plot was used to analyze the degree of agreement between the P5, P10, and P20 syringes compared with the P-initial. This method assesses the level of agreement between two different instruments. To apply this method, the mean difference between the two measurement methods and the 95% limits of agreement were calculated, which generates a scatterplot that can be used to visualize the bias (how the differences deviate from zero), the error (dispersion of the individual differences around the mean difference), and the outliers and trends.(20)
The level of significance was set at p<0.05, and the results are expressed as the median (interquartile range), mean±standard deviation (SD), or as specified. Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS, Chicago, USA) software, version 15.0 and GraphPad Prism 5 (GraphPad, San Diego, California, USA).
RESULTS
In the tracheal model used in Phase 1, the cuff pressures for all of the syringes during maneuvers with the P10 and P20 syringes exhibited high levels. Upon comparing the different syringes (the five P10 and five P20 syringes), we observed a statistically significant difference between P10 syringes and between P20 syringes (Table 1).
Table 1.
Cuff pressures observed after inflating the pilot balloon in Phase 1 of the study
| Syringe 1 | Syringe 2 | Syringe 3 | Syringe 4 | Syringe 5 | p value | |
|---|---|---|---|---|---|---|
| P10 | 45.2±7.5 | 54.8±10.1 | 48.2±4.3 | 47±6.1 | 59.6±9.2 | p=0.043* |
| P20 | 84±16.5 | 53.2±5 | 48.8±4.6 | 51.6±14.5 | 35±2.4 | p<0.001** |
Values are expressed as the mean±standard deviation of the five measurements performed with each syringe. P10 - maneuver performed with a 10mL syringe; P20 - maneuver performed with a 20mL syringe.
comparisons between 10mL syringes;
comparisons between 20mL syringes.
The ICC between the five tests performed with the syringes in Phase 1 was not significant, and the 95% CI ranged from a low negative value to a high positive value, which implies that the reproducibility of each syringe was zero (Table 2).
Table 2.
Intraclass correlation coefficient between the five tests performed with each syringe in Phase 1.
| Syringe | P10 | p value | P20 | p value |
|---|---|---|---|---|
| 1 | 0.03 (-8.2-0.89) | 0.4 | 0.3 (-5.6-0.92) | 0.3 |
| 2 | -0.33 (-11.7-0.86) | 0.6 | 0.49 (-3.8-0.94) | 0.2 |
| 3 | 0.12 (-7.3-0.9) | 0.4 | 0.63 (-2.5-0.96) | 0.2 |
| 4 | 0.15 (-7-0.91) | 0.4 | 0.33 (-5.3-0.93) | 0.3 |
| 5 | 0.16 (-6.9-0.91) | 0.4 | 0.8 (-0.6-0.98) | 0.06 |
P10 - maneuver performed with a 10mL syringe; P20 - maneuver performed with a 20mL syringe. Values for the intraclass correlation coefficients and the 95% confidence intervals (95%CI).
Of the 20 patients included in Phase 2 of this study, 11 (55%) were male and had a median height of 163cm (153-170cm) and a median age of 57 years (27-74 years). The patients were intubated with endotracheal tubes of various sizes: 7 (n=3), 7.5 (n=3), 8 (n=12), and 8.5 (n=2). Most patients (n=15) were not sedated at the time the data were collected.
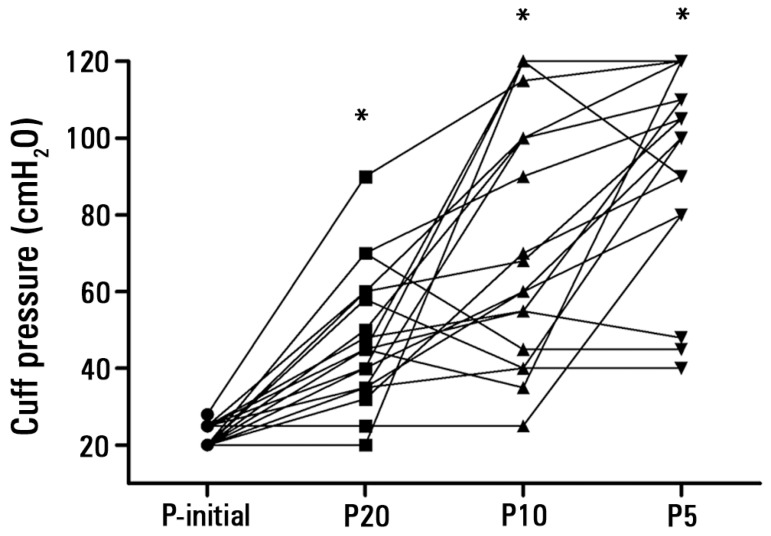
The cuff pressures generated with the P5 [105 (82.5-120) cmH2O], P10 [69 (47.5-111.3) cmH2O], and P20 [45 (35-59.5) cmH2O] syringes were higher than the P-initial values [20 (20-25) cmH2O] (p<0.001) (Figure 1).
Figure 1.
Comparisons between the initial cuff pressures (cmH2O) (P-initial) and the cuff pressures obtained during maneuvers with 5mL (P5), 10mL (P10), and 20mL (P20) syringes in Phase 2 of the study; in vivo experiments (individual data for all patients). * p<0.001 compared with the P-initial.
The Bland-Altman plots revealed large biases between the P-initial and P5 (75±26.2cmH2O), P10 (54.8±33.1cmH2O), and P20 (25.3±16.5cmH2O) values. The variability in the values, as determined using the 95% upper and lower limits of agreement (bias±1.96 SD), ranged from 23.6 to 126.4cmH2O for P5, -10.1 to 119.6cmH2O for P10, and -7.1 to 57.5cmH2O for P20, compared to the P-initial (Figure 2).
Figure 2.
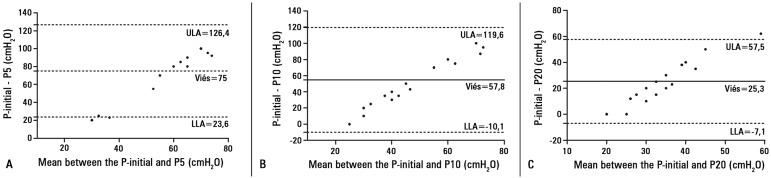
Bland-Altman plot of the differences and means between the initial cuff pressures (P-initial) and the cuff pressures obtained during maneuvers with the 5mL (P5) (A), 10mL (P10) (B), and 20mL (P20) (C) syringes. The differences are listed on the y-axis, and the mean of the two values are on the x-axis. Bias is shown as a solid line, and the 95% limits of agreement are shown as dotted lines.
ULA - 95% upper limit of agreement (bias+1.96 * standard deviation); LLA - 95% lower limit of agreement (bias-1.96 * standard deviation).
DISCUSSION
This study examined the effectiveness of the cuff pressure relief valve technique in maintaining the cuff pressure within the recommended limits in ICU patients and the reproducibility of P20 and P10 syringes in an experimental trachea model. The reproducibility was tested using five tests for each of the syringes. The ICC, which indicates the degree of agreement between measurements, indicated a lack of reproducibility between tests for each syringe. The cuff pressure values observed in the maneuver with the P5, P10, and P20 syringes exceeded the limit considered safe (20-30cmH2O) and exhibited significant variability and bias in the Bland-Altman analysis, compared with the initial pressure.
The use of a syringe to measure cuff pressure (cuff pressure relief valve technique) is an alternative, inexpensive, and rapid method to ensure that the pressure on the trachea is not too high (thereby injuring the trachea) or too low (to prevent microaspiration) in hospitals that do not have a manometer specifically designed to perform such measurements. However, our findings demonstrate that this technique is not effective at maintaining the cuff pressure within the recommended limits and is therefore not safe to use. The use of this technique may cause the loss of cilia or even the formation of tracheoesophageal fistulas due to high pressures exerted on the trachea.(5,6)
The use of syringes with different volumes and from different manufacturers for determining the cuff pressure using the pressure relief valve technique was previously tested by Mac et al. These authors used a trachea model to analyze three different brands of P20 syringes and found that only one brand was able to maintain the cuff pressure within safe limits. Upon comparing syringes with different volumes (P10 and 60mL), the authors found cuff pressures of 57 and 23cmH2O, respectively, demonstrating not only that the syringe brand is important for determining cuff pressure using this technique but also that the syringe volume is paramount.(21) Our study is consistent with and complements this previous study, as we observed large variations between the same brand of syringe with the same volume in sequential tests.
Testing the cuff pressure relief valve technique in patients who were not anesthetized, we found that as the size of the syringe decreased, cuff pressure increased. This observation can be explained by the physical principle in which the pressure is determined by the ratio of the force applied and the surface area on which the force is applied. The increased surface area of the larger syringe plungers implies that less pressure is required to overcome the force resulting from the dynamic friction.(21)
Substituting one instrument or evaluation technique for another is only possible if the new device is equivalent to the previous one and has been tested prior to its clinical use.(20) It is very unlikely that a clinical measurement evaluated using two different devices will yield identical results. Bland et al. therefore proposed that the differences between instruments be as small as possible to provide equivalent, accurate, and reliable clinical measurements.(20) However, the acceptable degree of divergence between instruments depends on the clinical measurement being examined.(22) In the case of cuff pressures in tracheal tubes for which the normal range is extremely narrow (20 to 30cmH2O), the variability between measurements should also be as small as possible. Using the Bland-Altman method, we observed biases of 75cmH2O, 57.8cmH2O, and 25.3cmH2O using P5, P10, and P20 syringes, respectively. Furthermore, the 95% lower and upper limits of agreement were excessively high (between -10.1cmH2O and 126.4cmH2O), showing clinically impractical values.
Our study does have some limitations. In Phase 1 of the study, we used a PVC tube to simulate the trachea. Due to its rigidity, this material does not accurately simulate the resistance and elasticity of the trachea. However, in Phase 1, only the reproducibility of the technique was tested, without making comparisons between the different volumes of syringes and the standard manometer. In both phases of the study, the pauses taken while inflating the pilot balloon with the syringes were not standardized, and the perceived recoil of the plunger was always “researcher-dependent.” Furthermore, the design of our study did not allow us to “blind” the researcher for the data collection. However, the same researcher performed all of the measurements, and the order in which the syringes were used during the data collection was random, which might have partially minimized these effects. Our study employed a convenience sample that consisted of only 20 patients. Although it was calculated after the data were collected, the power of our sample was 100%; therefore, we do not believe that increasing the sample size would change the results. Finally, different brands of syringes can have different plunger sizes and thicknesses, although they have the same overall volume. Thus, we cannot conclude whether the results would be different if other brands of syringes were used. Moreover, regardless of the dimensional characteristics (thickness and size of the plunger), the concepts of static and dynamic resistance (where the static resistance is always greater than the dynamic resistance) would have a predominant effect; consequently, we believe that our results would be similar for any brand of syringe.
CONCLUSION
The present study demonstrates that the cuff pressure relief valve technique is not reproducible in a trachea model and is not effective for using 5mL, 10mL, or 20mL syringes to determine the pressure of cuffs secured in intubated intensive care unit patients. Therefore, the use of a manometer specifically designed to measure the cuff pressure of endotracheal tubes is recommended for this population.
Footnotes
Conflicts of interest: None.
Responsible editor: Flávia Ribeiro Machado
REFERENCES
- 1.Rello J, Soñora R, Jubert P, Artigas A, Rué M, Vallés J. Pneumonia in intubated patients: role of respiratory airway care. Am J Respir Crit Care Med. 1996;154(1):111–115. doi: 10.1164/ajrccm.154.1.8680665. [DOI] [PubMed] [Google Scholar]
- 2.Seegobin RD, van Hasselt GL. Endotracheal cuff pressure and tracheal mucosal blood flow: endoscopic study of effects of four large volume cuffs. Br Med J (Clin Res Ed) 1984;288(6422):965–968. doi: 10.1136/bmj.288.6422.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbosa PM, Santos BM. Alterações morfológicas em traqueias de pacientes intubados em função do tempo de intubação. Rev Latinoam Enferm. 2003;11(6):727–733. doi: 10.1590/s0104-11692003000600005. [DOI] [PubMed] [Google Scholar]
- 4.Curiel García JA, Guerrero-Romero F, Rodríguez-Morán M. Cuff pressure in endotracheal intubation: should it be routinely measured? Gac Med Mex. 2001;137(2):179–182. Spanish. [PubMed] [Google Scholar]
- 5.Castilho EC, Braz JR, Catâneo AJ, Martins RH, Gregório EA, Monteiro ER. Efeitos da pressão limite (25cmH2O) e mínima de “selo” do balonete de tubos traqueais sobre a mucosa traqueal do cão. Rev Bras Anestesiol. 2003;53(6):743–755. doi: 10.1590/s0034-70942003000600006. [DOI] [PubMed] [Google Scholar]
- 6.Nseir S, Duguet A, Copin MC, De Jonckheere J, Zhang M, Similowski T, et al. Continuous control of endotracheal cuff pressure and tracheal wall damage: a randomized controlled animal study. Crit Care. 2007;11(5):R109. doi: 10.1186/cc6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Thoracic Society. Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 8.Resende MM, Monteiro SG, Callegari B, Figueiredo PM, Monteiro CR, Monteiro-Neto V. Epidemiology and outcomes of ventilator-associated pneumonia in northern Brazil: an analytical descriptive prospective cohort study. BMC Infect Dis. 2013;13:119. doi: 10.1186/1471-2334-13-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33(10):2184–2193. doi: 10.1097/01.ccm.0000181731.53912.d9. Review. [DOI] [PubMed] [Google Scholar]
- 10.Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28(2):108–121. doi: 10.1007/s00134-001-1143-z. Erratum in: Intensive Care Med. 2002;28(4):525-6. [DOI] [PubMed] [Google Scholar]
- 11.Bernhard WN, Yost L, Joynes D, Cothalis S, Turndorf H. Intracuff pressures in endotracheal and tracheostomy tubes. Related cuff physical characteristics. Chest. 1985;87(6):720–725. doi: 10.1378/chest.87.6.720. [DOI] [PubMed] [Google Scholar]
- 12.Nseir S, Zerimech F, De Jonckheere J, Alves I, Balduyck M, Durocher A. Impact of polyurethane on variations in tracheal cuff pressure in critically ill patients: a prospective observational study. Intensive Care Med. 2010;36(7):1156–1163. doi: 10.1007/s00134-010-1892-7. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez P, Bassi GL, Torres A. Measures to prevent nosocomial infections during mechanical ventilation. Curr Opin Crit Care. 2012;18(1):86–92. doi: 10.1097/MCC.0b013e32834ef3ff. Review. [DOI] [PubMed] [Google Scholar]
- 14.Godoy AC, Vieira RJ, Capitani EM. Endotracheal tube cuff pressure alteration after changes in position in patients under mechanical ventilation. J Bras Pneumol. 2008;34(5):294–297. doi: 10.1590/s1806-37132008000500008. [DOI] [PubMed] [Google Scholar]
- 15.Souza EP, Neto, Piriou V, Durand PG, George M, Evans R, Obadia JF, et al. Influence of temperature on tracheal tube cuff pressure during cardiac surgery. Acta Anaesthesiol Scand. 1999;43(3):333–337. doi: 10.1034/j.1399-6576.1999.430315.x. [DOI] [PubMed] [Google Scholar]
- 16.Sultan P, Carvalho B, Rose BO, Cregg R. Endotracheal tube cuff pressure monitoring: a review of the evidence. J Perioper Pract. 2011;21(11):379–386. doi: 10.1177/175045891102101103. [DOI] [PubMed] [Google Scholar]
- 17.Galinski M, Tréoux V, Garrigue B, Lapostolle F, Borron SW, Adnet F. Intracuff pressures of endotracheal tubes in the management of airway emergencies: the need for pressure monitoring. Ann Emerg Med. 2006;47(6):545–547. doi: 10.1016/j.annemergmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Somri M, Fradis M, Malatskey S, Vaida S, Gaitini L. Simple on-line endotracheal cuff pressure relief valve. Ann Otol Rhinol Laryngol. 2002;111(2):190–192. doi: 10.1177/000348940211100215. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Diehr P, Patrick DL. Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Control Clin Trials. 1991;12(4) Suppl:142S–158S. doi: 10.1016/s0197-2456(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 21.Mac Murdo SD, Buffington CW. Brand and size matter when choosing a syringe to relieve pressure in a tracheal tube cuff. Anesth Analg. 2004;99(5):1445–1449. doi: 10.1213/01.ANE.0000134799.36294.E5. table of contents. [DOI] [PubMed] [Google Scholar]
- 22.Myles PS, Cui J. Using the Bland-Altman method to measure agreement with repeated measures. Br J Anaesth. 2007;99(3):309–311. doi: 10.1093/bja/aem214. [DOI] [PubMed] [Google Scholar]