Abstract
Objective
This study aimed to describe and compare the characteristics and clinical outcomes of patients with septic and non-septic acute kidney injury.
Methods
This study evaluated an open cohort of 117 critically ill patients with acute kidney injury who were consecutively admitted to an intensive care unit, excluding patients with a history of advanced-stage chronic kidney disease, kidney transplantation, hospitalization or death in a period shorter than 24 hours. The presence of sepsis and in-hospital death were the exposure and primary variables in this study, respectively. A confounding analysis was performed using logistic regression.
Results
No significant differences were found between the mean ages of the groups with septic and non-septic acute kidney injury [65.30±21.27 years versus 66.35±12.82 years, respectively; p=0.75]. In the septic and non-septic acute kidney injury groups, a predominance of females (57.4% versus 52.4%, respectively; p=0.49) and Afro-descendants (81.5% versus 76.2%, respectively; p=0.49) was observed. Compared with the non-septic patients, the patients with sepsis had a higher mean Acute Physiology and Chronic Health Evaluation II score [21.73±7.26 versus 15.75±5.98; p<0.001)] and a higher mean water balance (p=0.001). Arterial hypertension (p=0.01) and heart failure (p<0.001) were more common in the non-septic patients. Septic acute kidney injury was associated with a greater number of patients who required dialysis (p=0.001) and a greater number of deaths (p<0.001); however, renal function recovery was more common in this group (p=0.01). Sepsis (OR: 3.88; 95%CI: 1.51-10.00) and an Acute Physiology and Chronic Health Evaluation II score >18.5 (OR: 9.77; 95%CI: 3.73-25.58) were associated with death in the multivariate analysis.
Conclusion
Sepsis was an independent predictor of death. Significant differences were found between the characteristics and clinical outcomes of patients with septic versus non-septic acute kidney injury.
Keywords: Acute kidney injury, Sepsis, Critical illness
Abstract
Objetivo
Descrever e comparar as características e os desfechos clínicos de pacientes com lesão renal aguda séptica e não séptica.
Métodos
Coorte aberta com 117 pacientes graves com lesão renal aguda consecutivamente admitidos em unidade de terapia intensiva, sendo excluídos aqueles que apresentavam doença renal crônica em estágio avançado, transplante renal, internação ou morte em um período inferior a 24 horas. Presença de sepse e óbito intra-hospitalar representaram, respectivamente, a exposição e o desfecho principal. Análise de confundimento foi realizada com a regressão logística.
Resultados
Não houve diferenças na média de idade entre os grupos com lesão renal aguda séptica e não séptica [65,30±(21,27) anos versus 66,35±12,82 anos; p=0,75]. Nos dois grupos, similarmente, observou-se predomínio do sexo feminino (57,4% versus 52,4%; p=0,49) e de afrodescendentes (81,5% versus 76,2%; p=0,49). Os pacientes com sepse apresentaram maiores médias de escore Acute Physiology and Chronic Health Evaluation II [21,73±7,26 versus 15,75± (5,98; p<0,001)] e maiores médias de balanço hídrico (p=0,001). Hipertensão arterial (p=0,01) e insuficiência cardíaca (p<0,001) foram mais frequentes entre os não sépticos. A lesão renal aguda séptica foi associada à maior necessidade de diálise (p=0,001) e óbito (p<0,001); no entanto, a recuperação da função renal também foi mais frequente nesse grupo (p=0,01). Na análise multivariada, sepse (OR: 3,88; IC95%: 1,51-10,00) e escores Acute Physiology and Chronic Health Evaluation II >18,5 (OR: 9,77; IC95%: 3,73-25,58) foram associados ao óbito.
Conclusão
Sepse foi um preditor independente para óbito. Existem diferenças entre as características e desfechos clínicos dos pacientes com lesão renal aguda séptica versus não séptica.
INTRODUCTION
Acute kidney injury (AKI) comprises a broad spectrum of clinical manifestations that range from mild injury to severe damage, which may result in permanent and complete loss of kidney function.(1) Studies have demonstrated that AKI is associated with a high prevalence and mortality.(2-9) Additionally, the incidence of AKI continues to increase despite technological advances.(3,9) This increase has been attributed to demographic changes (the aging population and a higher incidence of comorbidities), disease severity (multiple organ dysfunction syndrome) and AKI that is associated with complex interventions (organ transplants).(10,11) These findings suggest that AKI has a multifactorial origin in critically ill patients;(5,8,10) however, the pathophysiology of this condition remains unclear.(2)
Several studies have analyzed the etiology of acute renal failure and have found that sepsis is a key contributing factor in AKI patients who are admitted to intensive care units (ICUs).(5,8,12) Scientific evidence has indicated that 35%-50% of critically ill patients with AKI have renal injury due to sepsis.(5,7,12) Patients who are diagnosed with septic AKI have a higher mortality risk than patients with non-septic AKI. In septic AKI patients, survival is associated with longer ICU and hospital stays.(4,5,10,13) Conversely, studies that initially focused on sepsis reported that 10%-50% of patients with sepsis subsequently developed AKI.(8,14) Published reports have found a significantly higher incidence of AKI and increased immune responses in non-critically ill patients, albeit with active infection.(15) Several authors have suggested that this finding may be due to the different pathophysiologies(10,11,15-20) of septic and non-septic AKI, which require specific treatments.
This study aimed to compare the etiology of septic versus non-septic AKI by analyzing the clinical and demographic characteristics of these patients. This study assessed Acute Physiology and Chronic Health Evaluation (APACHE) II scores and in-hospital outcomes, such as the need for dialysis, recovery of renal function; and death. Using patients from the local Brazilian population, this study aimed to validate the data that were previously found in North American, European and Australian populations. The incidence of death was the main outcome of this study.
METHODS
This study used data from a preexisting database that was derived from a cohort of critically ill patients with AKI who were admitted to the ICU of a tertiary hospital in Northeastern Brazil. Data were collected daily during patient hospitalization from the time of admission until discharge or death. The inclusion criteria were as follows: a diagnosis of AKI, consecutive admission to the ICU, an age over 18 years, and the time period of January 2010 to January 2011. The exclusion criteria were as follows: stage 3-5 chronic kidney disease, kidney transplantation, suspected brain death within 24 hours of admission, an ICU stay shorter than 24 hours and incomplete outcome data.
The main outcome of this study was in-hospital death, which was based on the hypothesis that patients with septic AKI would be more prone to death than patients with non-septic AKI. The secondary outcomes were the need for dialysis and renal function recovery.
AKI was defined and staged according to the Risk Injury Failure Loss End-Stage Kidney Disease (RIFLE) classification.(2) At the time of admission, the patients were allocated into two groups: septic and non-septic AKI. The criteria that were adopted to define sepsis and septic shock were based on the International Sepsis Definitions Conference report.(21)
Renal function recovery was defined as a final serum creatinine concentration within the limit of 20% or a baseline value of 44µmol/L. The definition included the discontinuation of renal replacement therapy for at least 3 days prior to death or hospital discharge for patients who required dialysis.(4)
The dependent variables were the need for dialysis, renal function recovery, and death. The independent variables were age; gender; ethnicity; patient origin (surgery center or the emergency ward); use of vasoactive drugs; water balance during the first 24 hours, which was defined as the volume acquired by the patient minus the volume lost to the external environment; APACHE II scores;(22) and the presence of diabetes mellitus (DM), systemic arterial hypertension (SAH), heart failure/cardiogenic shock, and sepsis/septic shock. The presence of sepsis was the main independent variable in the study (exposure variable), whereas the other independent variables were fit variables.
The sample size was calculated using OpenEpi, considering that the ratio of cases among exposed patients was 74.5%, whereas the ratio of cases among unexposed patients was 45.2%.(12) With a 5% significance level and an 80% statistical power of the test, a minimum of 44 patients per group were needed for this study.
Descriptive statistics were used to assess frequencies, measurements of central tendency and dispersion measurements related to the clinical, demographic, and laboratorial characteristics of the patients. The categorical variables were expressed as the absolute number (valid percentage), and the quantitative variables were expressed as the means and standard deviations in cases of normal distribution. The quantitative variables with non-Gaussian distribution were expressed as the medians and interquartile ranges. Student’s t-test and the chi-squared and Mann-Whitney statistical tests were used to compare the means, the frequencies and the medians between the septic versus non-septic AKI groups.
A receiver operating characteristics (ROC) curve was used to assess the discriminatory power of the APACHE II score as a predictor of death and to identify a cutoff point with better accuracy, which determined the composition of the groups for the multivariate analysis. Logistic regression was used to determine the independent predictors of death. The criterion of p<0.10 was used in the bivariate analysis to select the variables that composed the initial model of multivariate logistic regression. The final model was obtained using the stepwise backward method. The water balance was the only significant continuous variable in the multivariate analysis, which was stratified into tertiles using the upper tertile as the unexposed group. An alpha-type error of 5% was adopted for all of the statistical analyses. Statistical Package for Social Sciences (SPSS) version 17.0 was used for the data analysis.
The study was approved by the Research Ethics Committee of the Hospital Calixto Midlej Filho, Santa Casa de Misericórdia, in Itabuna (BA) under protocol number 01/2009. The next-of-kin of the patients signed the Informed Consent Form. The study followed the guidelines of the 1989 Declaration of Helsinki and resolution 196/96 of the National Health Council regarding research that involves humans.
RESULTS
This study assessed a sample of 117 patients with acute renal failure, including 54 (46.15%) patients with septic AKI and 63 (53.85%) patients with non-septic AKI. The clinical and demographic characteristics and the baseline and laboratory physiological variables of the study subjects were compared between the two groups and are outlined in table 1.
Table 1.
Demographic, clinical, and laboratory variables of patients with acute kidney injury who were admitted to an intensive care unit
| Variable | Septic AKI | Non-septic AKI | p value |
|---|---|---|---|
| N=54 | N=63 | ||
| Age (years) | 65.30±21.27 | 66.35±12.82 | 0.75 |
| Ethnicity | |||
| Afro-descendant | 44 (81.48) | 48 (76.19) | 0.49 |
| Non-Afro-descendant | 10 (18.52) | 15 (23.81) | |
| Gender | |||
| Female | 31 (57.41) | 33 (52.38) | 0.59 |
| Male | 23 (42.59) | 30 (47.62) | |
| Patient origin | |||
| Ward - EC | 38 (70.37) | 28 (44.44) | 0.12 |
| Surgical center | 16 (29.63) | 35 (55.55) | |
| Comorbidities | |||
| Hypertension | 27 (50.00) | 51 (80.95) | 0.01 |
| Diabetes mellitus | 15 (27.78) | 21 (33.33) | 0.52 |
| Heart failure | 12 (22.22) | 38 (60.32) | <0.001 |
| Use of vasoactive drugs | 17 (31.50) | 21 (33.33) | 0.83 |
| APACHE II score | 21.73±7.26 | 15.75±5.98 | 0.001 |
| Water balance | 2.212.25±1815.87 | 1.162.24±1338.21 | 0.001 |
AKI - acute kidney injury; EC - emergency care; APACHE II - Acute Physiology and Chronic Health Evaluation II. The categorical variables are expressed as the absolute n (valid percentage); the quantitative variables are expressed as the mean±standard deviation.
The mean age of the study subjects did not vary between the two groups. Most of the study patients in the sepsis AKI and non-sepsis AKI groups were Afro-descendants. The female gender was slightly predominant, albeit without statistical significance (Table 1). The patients with septic AKI were mainly from the emergency ward, whereas the main origin of the patients with non-septic AKI was the surgery center. However, the comparison of the different origins of the two groups did not reveal any significant differences (Table 1).
The most common diagnoses upon admission to the ICU were as follows: SAH (78/107; 72.9%), DM (36/107; 33.6%) and heart failure/cardiogenic shock (50/107; 46.7%). SAH (p=0.01) and heart failure (p<0.001) were the most prevalent in the non-septic AKI patients. However, no significant differences were found between the number of diabetic patients in the groups with and without sepsis (Table 1).
The mean APACHE II score was higher in the patients with septic AKI than in the non-septic AKI patients (p=0.001) (Table 1). The water balance, which is a marker of renal hemodynamics, was significantly higher in the group with sepsis (Table 1).
The patients with septic AKI were noticeably more prone to recover renal function, which was not observed in the non-septic AKI patients when the study subjects were allocated into groups according to outcome. In the septic AKI group, a greater number of patients required dialysis and a greater number of deaths occurred. Therefore, septic AKI was associated with a higher rate of in-hospital mortality than non-septic AKI (Table 2).
Table 2.
Bivariate analysis of sepsis and the clinical outcomes of patients with acute kidney injury who were admitted to an intensive care unit
| Outcome | Septic AKI | Non-septic AKI | p value |
|---|---|---|---|
| N=54 | N=63 | ||
| Recovery of renal function | 5 (9.26) | 0 (0) | 0.014 |
| Need for dialysis | 18 (33.33) | 6 (9.52) | 0.001 |
| Death | 35 (64.81) | 14 (22.22) | <0.001 |
AKI - acute kidney injury. The categorical variables are expressed as the absolute n (valid percentage).
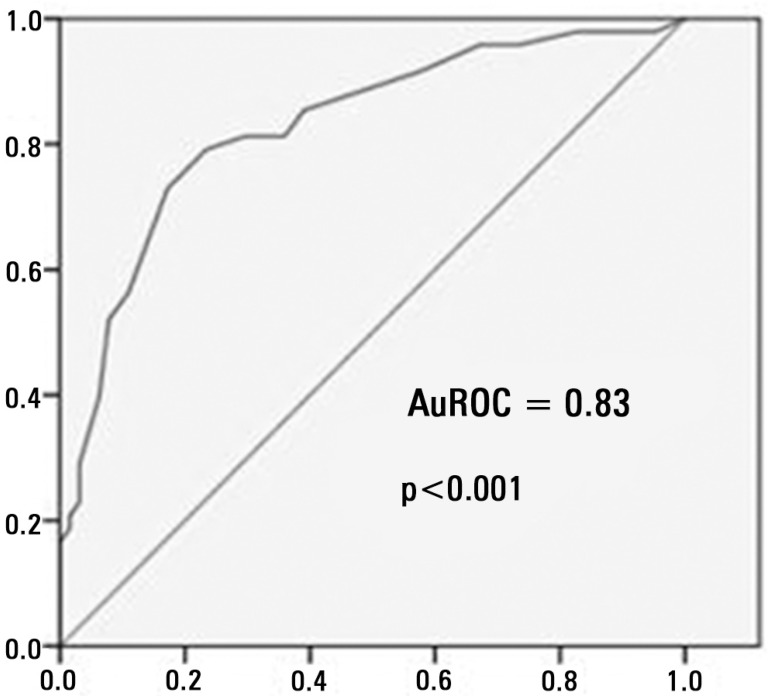
The area under the ROC curve (AuROC) for the APACHE II scores was 0.83 (p<0.001), and the cut-off point that was associated with the best sensitivity and specificity was 18.5 (Figure 1).
Figure 1.
Acute Physiology and Chronic Health Evaluation II score. The ROC curve for mortality in an intensive care unit.
AuROC - area under de curve ROC.
The multivariate analysis of death as an outcome indicated that sepsis (odds ratio, OR: 3.37; 95% confidence interval, CI: 1.20-9.50) and an APACHE II score >18.5 (OR: 8.66; 95% CI: 3.20-23.40) were predictors of in-hospital mortality using the complete model. In addition, these predictors were confirmed in the final reduced model (Table 3).
Table 3.
The predictors of hospital mortality in a cohort of patients with acute kidney injury who were admitted to an intensive care unit
| Predictor | OR (95%CI) | OR (95%CI) |
|---|---|---|
| Complete model | Final model | |
| Sepsis | 3.37 (1.20-9.50) | 3.88 (1.51-10.00) |
| Heart failure | 1.01 (0.34-2.97) | - |
| Hypertension | 1.04 (0.34-3.15) | - |
| APACHE II score >18.5 | 8.66 (3.20-23.40) | 9.77 (3.73-25.58) |
| Water balance in the first 24 hours (tertiles) | ||
| 1st (<842.64mL) | 0.48 (0.15-1.52) | - |
| 2nd (842.64-2,153.0mL) | 0.38 (0.11-1.34) | - |
OR - odds ratio; 95% CI - 95% confidence interval; APACHE II - Acute Physiology and Chronic Health Evaluation II.
DISCUSSION
This study was performed to analyze the characteristics and clinical outcomes of patients with septic and non-septic AKI in a context of critically ill patients. In this study, a higher ratio of renal function recovery, dialysis, and death was observed in the patients with septic AKI. In addition, a greater degree of disease severity was observed in the patients with septic AKI. Furthermore, the presence of sepsis and an APACHE II score >18.5 were predictors of in-hospital death. The patients with sepsis were approximately four times more likely to die than the patients with non-septic AKI. Additionally, septic AKI patients with an APACHE II score above 18.5 were approximately ten times more likely to die.
AKI is a common condition among critically ill patients,(4) and one of the primary etiological factors of AKI, if not the main factor, is sepsis.(5,8,12) The first large multicenter study to compare septic and non-septic AKI was conducted by Neveu et al.(12) who found a septic origin in 46% of the patients with AKI. Another large study was conducted by Bagshaw et al.(5) in which they found a septic etiology in 43% of the patients with acute renal injury. The patients with septic AKI in the present study accounted for approximately half of the cohort (46.15%), which corroborates previous data. This result suggests that the etiological factor is highly important in AKI. Sepsis has emerged as the most important and prevalent indicator of AKI in critically ill patients.(5,8,12)
The mean age of the patients in this study was high, which corroborates data from previous studies that were conducted on renal patients in the ICU setting.(7,23-27) Additionally, no significant differences were detected in the mean ages of both groups in this study.
An interesting finding in this study was the high prevalence of Afro-descendants. Previously published articles did not report on the ethnic profiles of AKI patients; therefore, this study is presumably the first to identify this population profile because previous studies were conducted in countries with a predominantly Caucasian population.(4,5,8,10,12,26,27) However, this important finding was not used to differentiate the groups but confirmed the similarity between the responses of the different ethnic subgroups.
The patients without sepsis had a higher prevalence of cardiovascular comorbidities, including SAH and heart failure. Hypertension increases the pressure throughout the vascular system, including renal glomerular arterioles and capillaries, thereby causing a reflex vasoconstriction that may lead to long-term nephrosclerosis and, consequently, to progressive and irreversible reduction of the glomerular filtration rate (GFR).(28) Heart failure predominantly results in low pressure, which favors low renal perfusion and results in a decreased GFR, especially in cases of acute decompensation. GFR reduction is the pre-renal mechanism of AKI.(29) SAH and heart failure were more frequent in the group without sepsis and therefore may represent other pathophysiologies of renal injury.
The different physiological characteristics of the groups in this study may be easily understood based on the sepsis process. The excessive production of inflammatory mediators and the exacerbated activation of inflammatory cells occur in systemic infection, thereby resulting in metabolic anarchy.(30) The main consequence of this inflammatory response is the involvement of multiple organs and systems. Therefore, the high APACHE II score in the septic AKI patients is a clinical manifestation of those metabolic changes.
The water balance consists of the volume that is acquired by the patient minus the volume lost to the external environment. The acquired volume represents the volume of solution that is infused into the patient, whereas the urine output accounts for the lost volume. Therefore, a high water balance may be due to a high volume of infused water and/or a low urine output. The septic patients with a higher water balance were hydrated with larger volumes of water and/or a lower urine output, which suggests a more severe clinical condition, regardless of the origin of the high water balance.(21)
Oliguria is a key marker of the septic process.(21,31-33) Previous studies have found that patients with sepsis-induced AKI were more likely to be oliguric than those with AKI due to other causes.(4,5)
The clinical manifestations of sepsis result from the initial infectious process, the implicit inflammatory process, and ongoing organ dysfunction. Hypovolemia is activated by arterial and venous dilatation, which is caused by inflammatory mediators that are released by the endothelium and a loss of fluids in the extravascular space due to endothelial dysfunction. Hypovolemia prevents good tissue perfusion, which causes ischemia and requires a greater volume of blood to be infused.(21,32)
Renal function recovery, dialysis, and death were more prevalent in the group of patients with septic AKI, which is similar to findings in other studies.(4,5,12) Renal function recovery was the first outcome analyzed in the present study and was only detected in the group of septic patients. Higher recovery rates have been previously reported following the discontinuation of renal replacement therapy in these patients despite the paucity of data on renal function recovery in septic patients.(5,34) The pathophysiological explanation for the recovery of renal function in septic AKI patients has not yet been provided. However, differences in the prognosis between septic and non-septic patients suggests a different pathophysiological mechanism for septic AKI. Therapeutic strategies may need to be redesigned based on the mechanism of septic AKI.
Renal function recovery was not observed in the group of patients with non-septic AKI most likely because of a chronic and irreversible renal impairment secondary to underlying SAH. Therefore, the GFR in these patients was most likely reduced to such an extent that they developed a clinical condition termed “acutized chronic kidney injury” because of exposure to a decompensation factor. However, these patients could not recover kidney function because the chronic kidney injury was irreversible. The low number of patients in this study may have contributed to the lack of renal function recovery among non-septic patients.
One of the secondary outcomes in this study was the need for dialysis, which is difficult to assess and compare with previous studies because no standardized definition of AKI has been used in studies. Therefore, the implementation of renal replacement therapy becomes a subjective outcome. The study by Bagshaw et al.(5) reported that approximately 70% of septic patients required dialysis, whereas the study by Neveu et al.(12) indicated that 47% of septic patients required dialysis. Mehta et al.(4) found that 71% of patients with sepsis before and after the onset of AKI required dialysis in contrast to 50% of patients without sepsis. The present study detected lower rates of patients who required dialysis than the rates published in the literature (33.33% of patients with septic AKI and 9.52% of patients with non-septic AKI). Even though the percentage of cases varies from previous studies, the difference between the groups in this study was statistically significant. A greater percentage of septic patients required dialysis than patients without sepsis, which confirms previously published data.
Death was the main outcome of this study, and a homogeneous pattern was observed for this outcome in the current study and previous studies. The number of deaths among patients with septic AKI in the current study was similar to those in previously published articles, which corroborates the validity of these numbers. The in-hospital mortality of patients with septic AKI reported by Neveu et al.(12) was 75%. In addition, Bagshaw et al.(5) found an in-hospital mortality rate of 70% in AKI patients with sepsis. However, Mehta et al.(4) found lower numbers: 48% for patients with sepsis before AKI and 44% for patients with sepsis after AKI. However, they demonstrated that patients with sepsis had an absolute mortality rate that was 20% higher than that in patients without sepsis. Therefore, these studies indicate an association between sepsis and mortality.
After adjusting for confounding factors, a significant association was observed between sepsis and in-hospital mortality in the present study. This result corroborate previous studies, which indicated that sepsis is an independent predictor of death among patients with AKI.(5,12) Another independent predictor of mortality in this study was an APACHE II score >18.5. This variable was significantly associated with mortality. Only one previous study has suggested that the APACHE II score is a predictor of death in AKI patients.(12) Other scores of severity have been reported to be predictors of mortality in previous studies.(4,5,12)
The present study had several limitations. First, the small sample size may affect the accuracy of the associations that were found by reducing the 95% CIs. Nevertheless, the findings were relevant and corroborated those in the literature. Another key limitation was the sepsis diagnosis, which was made by physicians who were in charge of the ICU using consensus criteria; however, the diagnostic agreement between the physicians was not assessed. Furthermore, the data on mortality were assessed from the time of admission until hospital discharge; therefore, long-term data are lacking.
The study only included acute renal injury patients without encompassing the entire population of critically ill patients, which may impact the external validity of this study. However, this study differed from previous studies precisely because it had a cohort of acute renal injury patients. Most studies have used a cohort of septic patients.(8,14,35-37) However, this study design precludes a comparison between septic and non-septic AKI because it assumes that all patients included in the study have sepsis. Therefore, the disease may only be described. The differences in the objectives and results between the present study and previous studies emphasizes the need for further studies.
CONCLUSION
The patients who developed septic acute kidney injury had a lower incidence of arterial hypertension and heart failure. These patients required greater fluid resuscitation within the first 24 hours of admission and had a lower urine output during this period, which resulted in a higher water balance and higher Acute Physiology and Chronic Health Evaluation II scores. Therefore, septic acute kidney injury was associated with a greater severity compared with non-septic acute kidney injury.
Septic acute kidney injury was associated with a higher percentage of patients who required dialysis and a higher percentage of in-hospital deaths. However, recovery of renal function was more common in this group of patients. Sepsis and an Acute Physiology and Chronic Health Evaluation II score >18.5 were independent predictors of in-hospital mortality.
Footnotes
Conflicts of interest: None.
Responsible editor: Thiago Costa Lisboa
REFERENCES
- 1.Case J, Khan S, Khalid R, Khan A. Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract. 2013;2013:479730. doi: 10.1155/2013/479730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committee Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007;11(3):R68. doi: 10.1186/cc5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta RL, Bouchard J, Soroko SB, Ikizler TA, Paganini EP, Chertow GM, Himmelfarb J, Program to Improve Care in Acute Renal Disease (PICARD) Study Group Sepsis as a cause and consequence of acute kidney injury: Program to Improve Care in Acute Renal Disease. Intensive Care Med. 2011;37(2):241–248. doi: 10.1007/s00134-010-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten HM, Ronco C, Kellum JA, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2(3):431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 6.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am SocNephrol. 2007;18(4):1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 7.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 8.Hoste EA, Lameire NH, Vanholder RC, Benoit DD, Decruyenaere JM, Colardyn FA. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol. 2003;14(4):1022–1030. doi: 10.1097/01.asn.0000059863.48590.e9. [DOI] [PubMed] [Google Scholar]
- 9.Waikar SS, Liu KD, Chertow GM. The incidence and prognostic significance of acute kidney injury. Curr Opin Nephrol Hypertens. 2007;16(3):227–236. doi: 10.1097/MNH.0b013e3280dd8c35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committee Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12(2):R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langenberg C, Wan L, Egi M, May CN, Bellomo R. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006;69(11):1996–2002. doi: 10.1038/sj.ki.5000440. [DOI] [PubMed] [Google Scholar]
- 12.Neveu H, Kleinknecht D, Brivet F, Loirat P, Landais P. Prognostic factors in acute renal failure due to sepsis. Results of a prospective multicentre study. The French Study Group on Acute Renal Failure. Nephrol Dial Transplant. 1996;11(2):293–299. doi: 10.1093/oxfordjournals.ndt.a027256. [DOI] [PubMed] [Google Scholar]
- 13.Piccinni P, Cruz DN, Gramaticopolo S, Garzotto F, Dal Santo M, Aneloni G, Rocco M, Alessandri E, Giunta F, Michetti V, Iannuzzi M, BelluomoAnello C, Brienza N, Carlini M, Pelaia P, Gabbanelli V, Ronco C, NEFROINT Investigators Prospective multicenter study on epidemiology of acute kidney injury in the ICU: a critical care nephrology Italian collaborative effort (NEFROINT) Minerva Anestesiol. 2011;77(11):1072–1083. [PubMed] [Google Scholar]
- 14.Lopes JA, Jorge S, Resina C, Santos C, Pereira A, Neves J, et al. Acute kidney injury in patients with sepsis: a contemporary analysis. Int J Infect Dis. 2009;13(2):176–181. doi: 10.1016/j.ijid.2008.05.1231. [DOI] [PubMed] [Google Scholar]
- 15.Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22(6):999–1006. doi: 10.1681/ASN.2010050484. Review. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs R, Honore PM, Joannes-Boyau O, Boer W, De Regt J, De Waele E, et al. Septic acute kidney injury: the culprit is inflammatory apoptosis rather than ischemic necrosis. Blood Purif. 2011;32(4):262–265. doi: 10.1159/000330244. [DOI] [PubMed] [Google Scholar]
- 17.Honore PM, Jacobs R, Joannes-Boyau O, De Regt J, Boer W, De Waele E, et al. Septic AKI in ICU patients. Diagnosis, pathophysiology, and treatment type, dosing, and timing: a comprehensive review of recent and future developments. Ann Intensive Care. 2011;1(1):32. doi: 10.1186/2110-5820-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chvojka J, Sýkora R, Karvunidis T, Raděj J, Kroužecký A, Novák I, et al. New developments in septic acute kidney injury. Physiol Res. 2010;59(6):859–869. doi: 10.33549/physiolres.931936. [DOI] [PubMed] [Google Scholar]
- 19.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351(2):159–169. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 20.Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R. Pathophysiology of septic acute kidney injury: what do we really know? Crit Care Med. 2008;36(4) Suppl:S198–S203. doi: 10.1097/CCM.0b013e318168ccd5. [DOI] [PubMed] [Google Scholar]
- 21.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, SCCM/ESICM/ACCP/ATS/SIS 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. Review. [DOI] [PubMed] [Google Scholar]
- 22.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 23.Levi TM, de Souza SP, de Magalhães JG, de Carvalho MS, Cunha AL, Dantas JG, et al. Comparison of the RIFLE, AKIN and KDIGO criteria to predict mortality in critically ill patients. Rev Bras Ter Intensiva. 2013;25(4):290–296. doi: 10.5935/0103-507X.20130050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang CH, Lin CY, Tian YC, Jenq CC, Chang MY, Chen YC, et al. Acute kidney injury classification: comparison of AKIN and RIFLE criteria. Shock. 2010;33(3):247–252. doi: 10.1097/SHK.0b013e3181b2fe0c. [DOI] [PubMed] [Google Scholar]
- 25.Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committee A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23(5):1569–1574. doi: 10.1093/ndt/gfn009. [DOI] [PubMed] [Google Scholar]
- 26.Lopes JA, Fernandes P, Jorge S, Resina C, Santos C, Pereira A, et al. Long-term risk of mortality after acute kidney injury in patients with sepsis: a contemporary analysis. BMC Nephrol. 2010;11:9. doi: 10.1186/1471-2369-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Biesen W, Yegenaga I, Vanholder R, Verbeke F, Hoste E, Colardyn F, et al. Relationship between fluid status and its management on acute renal failure (ARF) in intensive care unit (ICU) patients with sepsis: a prospective analysis. J Nephrol. 2005;18(1):54–60. [PubMed] [Google Scholar]
- 28.Szczech LA, Granger CB, Dasta JF, Amin A, Peacock WF, McCullough PA, Devlin JW, Weir MR, Katz JN, Anderson FA, Jr, Wyman A, Varon J, Studying the Treatment of Acute Hypertension Investigators Acute kidney injury and cardiovascular outcomes in acute severe hypertension. Circulation. 2010;121(20):2183–2191. doi: 10.1161/CIRCULATIONAHA.109.896597. [DOI] [PubMed] [Google Scholar]
- 29.Nunes TF, Brunetta DM, Leal CM, Pisi PC, Roriz-Filho JS. Insuficiência renal aguda. Medicina (Ribeirão Preto) 2010;43(3):272–282. [Google Scholar]
- 30.Bone RC. The pathogenesis of sepsis. Ann Intern Med. 1991;115(6):457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 31.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 32.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. Review. [DOI] [PubMed] [Google Scholar]
- 33.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL, International Surviving Sepsis Campaign Guidelines Committee. American Association of Critical-Care Nurses. American College of Chest Physicians. American College of Emergency Physicians. Canadian Critical Care Society. European Society of Clinical Microbiology and Infectious Diseases. European Society of Intensive Care Medicine. European Respiratory Society. International Sepsis Forum. Japanese Association for Acute Medicine. Japanese Society of Intensive Care Medicine. Society of Critical Care Medicine. Society of Hospital Medicine. Surgical Infection Society. World Federation of Societies of Intensive and Critical Care Medicine Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. Erratum in Crit Care Med. 2008 Apr;36(4):1394-6. [DOI] [PubMed] [Google Scholar]
- 34.Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9(6):R700–R709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suh SH, Kim CS, Choi JS, Bae EH, Ma SK, Kim SW. Acute kidney injury in patients with sepsis and septic shock: risk factors and clinical outcomes. Yonsei Med J. 2013;54(4):965–972. doi: 10.3349/ymj.2013.54.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oppert M, Engel C, Brunkhorst FM, Bogatsch H, Reinhart K, Frei U, Eckardt KU, Loeffler M, John S, German Competence Network Sepsis (Sepnet) Acute renal failure in patients with severe sepsis and septic shock-a significant independent risk factor for mortality: results from the German Prevalence Study. Nephrol Dial Transplant. 2008;23(3):904–909. doi: 10.1093/ndt/gfm610. [DOI] [PubMed] [Google Scholar]
- 37.Plataki M, Kashani K, Cabello-Garza J, Maldonado F, Kashyap R, Kor DJ, et al. Predictors of acute kidney injury in septic shock patients: an observational cohort study. Clin J Am Soc Nephrol. 2011;6(7):1744–1751. doi: 10.2215/CJN.05480610. [DOI] [PubMed] [Google Scholar]