Abstract
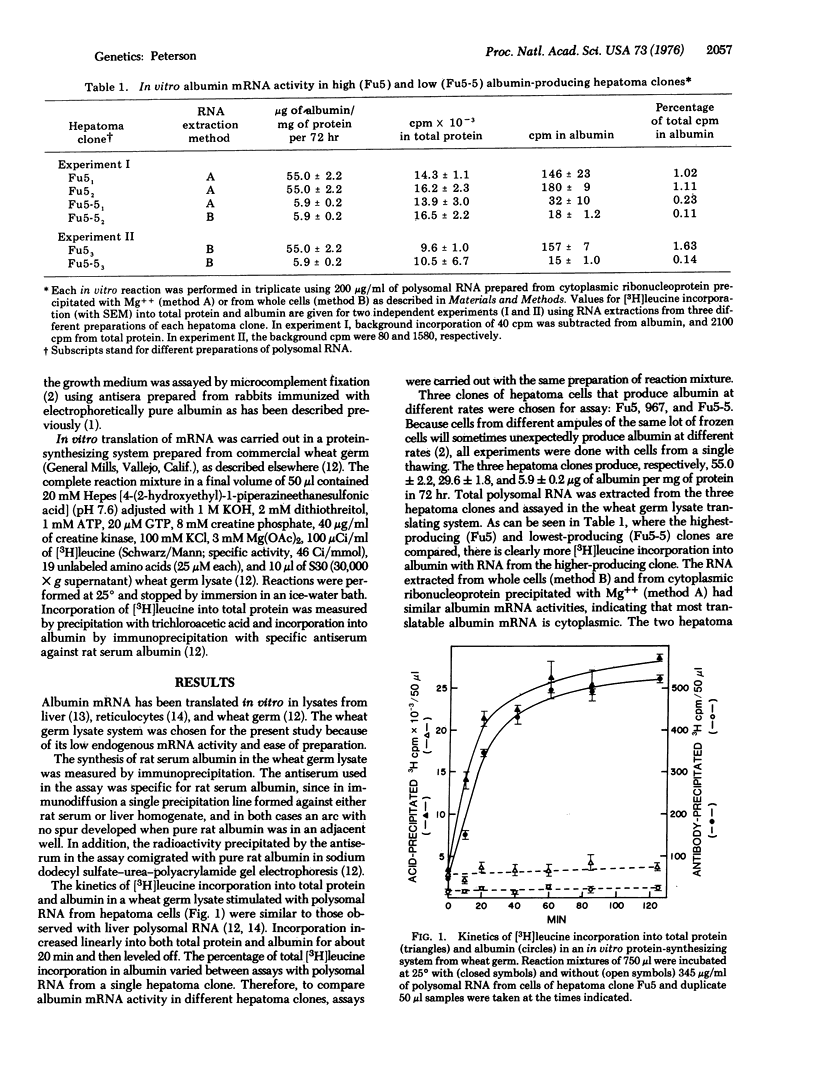
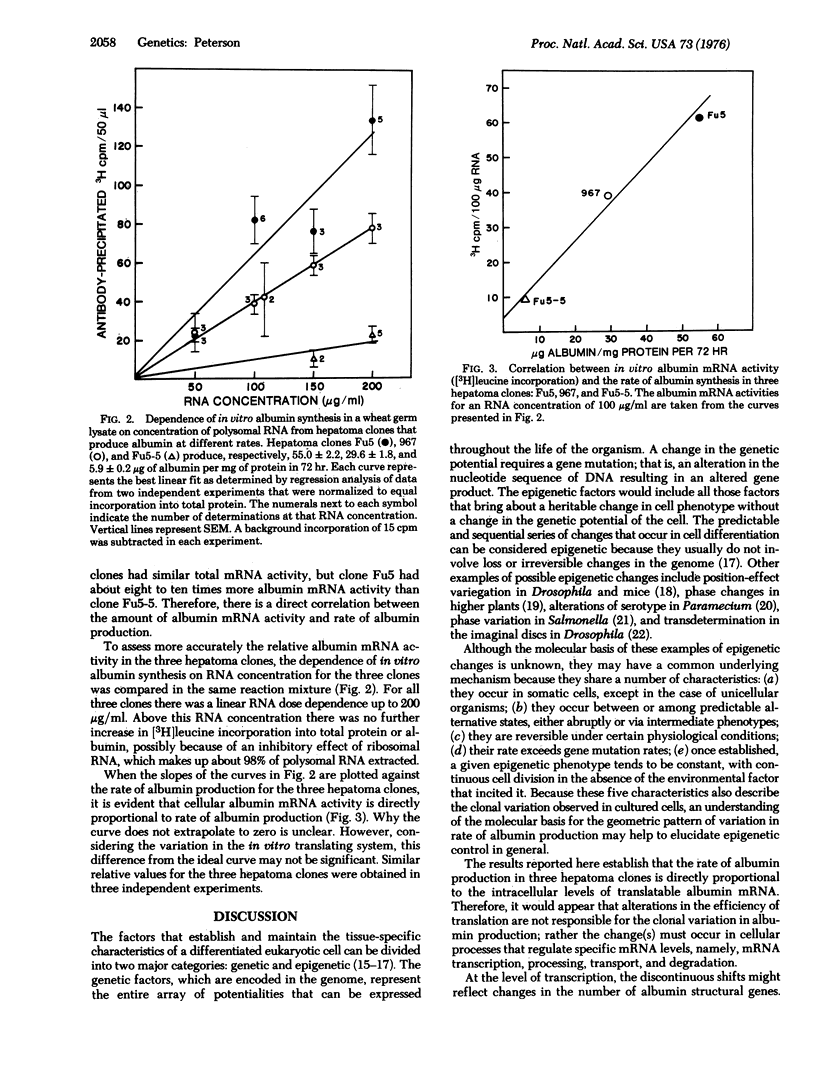
The clonal variation in rate of albumin synthesis in hepatoma cells is described as a tool for the study of epigenetic control of differentiation. Previous studies have demonstrated that, from a population of hepatome cells, variant subclones can be readily isolated that produce albumin at different rates. Each clonal variant had a characteristic rate of albumin production, and the clones clustered around discrete values that formed a geometric progression. The present experiments, using a cell-free protein-synthesizing system from wheat germ; show that albumin messenger RNA activity is directly proportional to the rate of albumin synthesis in three different hepatoma clones, thus suggesting a pretranslational control of albumin production. Possible hypotheses to explain the geometric pattern of clonal variation are discussed with respect to the organization and control of the transcriptional unit.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaldi F., Lava-Sanchez P. A., Buongiorno-Nardelli M. Nuclear DNA content variability in Xenopus laevis: a redundancy regulation common to all gene families. Nature. 1973 Apr 27;242(5400):615–617. doi: 10.1038/242615a0. [DOI] [PubMed] [Google Scholar]
- Aviv D., Thompson E. B. Variation in tyrosine aminotrasferase induction in HTC cell clones. Science. 1972 Sep 29;177(4055):1201–1203. doi: 10.1126/science.177.4055.1201. [DOI] [PubMed] [Google Scholar]
- BEALE G. H. The role of the cytoplasm in antigen determination in Paramecium aurelia. Proc R Soc Lond B Biol Sci. 1958 Mar 18;148(932):308–314. doi: 10.1098/rspb.1958.0022. [DOI] [PubMed] [Google Scholar]
- Baker W. K. Position-effect variegation. Adv Genet. 1968;14:133–169. [PubMed] [Google Scholar]
- Baltimore D. Expression of animal virus genomes. Bacteriol Rev. 1971 Sep;35(3):235–241. doi: 10.1128/br.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Chipchase M., Speirs J. The ribosomal RNA cistrons. Prog Nucleic Acid Res Mol Biol. 1971;11:351–389. doi: 10.1016/s0079-6603(08)60332-3. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Freeman K. B. DNA sequences neighboring the duck hemoglobin genes. Cold Spring Harb Symp Quant Biol. 1974;38:707–716. doi: 10.1101/sqb.1974.038.01.076. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Pemberton R., Baglioni C. Reiteration frequency of haemoglobin genes in the duck. Nat New Biol. 1972 Feb 23;235(60):231–234. doi: 10.1038/newbio235231a0. [DOI] [PubMed] [Google Scholar]
- Buongiorno-Nardelli M., Amaldi F., Lava-Sanchez P. A. Amplification as a rectification mechanism for the redundant rRNA genes. Nat New Biol. 1972 Aug 2;238(83):134–137. doi: 10.1038/newbio238134a0. [DOI] [PubMed] [Google Scholar]
- Callan H. G. The organization of genetic units in chromosomes. J Cell Sci. 1967 Mar;2(1):1–7. doi: 10.1242/jcs.2.1.1. [DOI] [PubMed] [Google Scholar]
- Coffino P., Scharff M. D. Rate of somatic mutation in immunoglobulin production by mouse myeloma cells. Proc Natl Acad Sci U S A. 1971 Jan;68(1):219–223. doi: 10.1073/pnas.68.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse H. V., Keyl H. G. Extra replications in the "DNA-puffs" of Sciara coprophila. Chromosoma. 1968 Nov;25(3):357–364. doi: 10.1007/BF01183126. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Hough B. R., Klein W. H., Britten R. J. Structural genes adjacent to interspersed repetitive DNA sequences. Cell. 1975 Mar;4(3):217–238. doi: 10.1016/0092-8674(75)90170-1. [DOI] [PubMed] [Google Scholar]
- Delovitch T. L., Baglioni C. Estimation of light-chain gene reiteration of mouse immunoglobulin by DNA-RNA hybridization. Proc Natl Acad Sci U S A. 1973 Jan;70(1):173–178. doi: 10.1073/pnas.70.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber A. J., Miall S. H., Tamaoki T. Synthesis of albumin with exogenous mouse-liver messenger RNA in a homologous cell-free system. Can J Biochem. 1974 May;52(5):429–432. doi: 10.1139/o74-064. [DOI] [PubMed] [Google Scholar]
- Gage L. P. Polyploidization of the silk gland of Bombyx mori. J Mol Biol. 1974 Jun 15;86(1):97–108. doi: 10.1016/s0022-2836(74)80010-0. [DOI] [PubMed] [Google Scholar]
- Harris M. Mutation rates in cells at different ploidy levels. J Cell Physiol. 1971 Oct;78(2):177–184. doi: 10.1002/jcp.1040780204. [DOI] [PubMed] [Google Scholar]
- Hourcade D., Dressler D., Wolfson J. The amplification of ribosomal RNA genes involves a rolling circle intermediate. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2926–2930. doi: 10.1073/pnas.70.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedes L. H., Birnstiel M. L. Reiteration and clustering of DNA sequences complementary to histone messenger RNA. Nat New Biol. 1971 Apr 7;230(14):165–169. doi: 10.1038/newbio230165a0. [DOI] [PubMed] [Google Scholar]
- Keyl H. G. A demonstrable local and geometric increase in the chromosomal DNA of Chironomus. Experientia. 1965 Apr 15;21(4):191–193. doi: 10.1007/BF02141878. [DOI] [PubMed] [Google Scholar]
- Lederberg J, Iino T. Phase Variation in Salmonella. Genetics. 1956 Sep;41(5):743–757. doi: 10.1093/genetics/41.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezger-Freed L. Effect of ploidy and mutagens on bromodeoxyuridine resistance in haploid and diploid frog cells. Nat New Biol. 1972 Feb 23;235(60):245–246. doi: 10.1038/newbio235245a0. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr Structure and composition of peripheral nucleoli of salamander oocytes. Natl Cancer Inst Monogr. 1966 Dec;23:53–66. [PubMed] [Google Scholar]
- Nanney D. L. EPIGENETIC CONTROL SYSTEMS. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):712–717. doi: 10.1073/pnas.44.7.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paigen K., Swank R. T., Tomino S., Ganschow R. E. The molecular genetics of mammalian glucuronidase. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):379–392. doi: 10.1002/jcp.1040850406. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Paul J. General theory of chromosome structure and gene activation in eukaryotes. Nature. 1972 Aug 25;238(5365):444–446. doi: 10.1038/238444a0. [DOI] [PubMed] [Google Scholar]
- Peterson J. A. Discontinuous variability, in the form of a geometric progression, of albumin production in hepatoma and hybrid cells. Proc Natl Acad Sci U S A. 1974 May;71(5):2062–2066. doi: 10.1073/pnas.71.5.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. A., Weiss M. C. Expression of differentiated functions in hepatoma cell hybrids: induction of mouse albumin production in rat hepatoma-mouse fibroblast hybrids. Proc Natl Acad Sci U S A. 1972 Mar;69(3):571–575. doi: 10.1073/pnas.69.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickett M. S., Coultrip L., Patterson M. K., Morrow J. Effect of ploidy on spontaneous mutation rate to asparagine non-requirement in cultured cells. J Cell Physiol. 1975 Jun;85(3):621–626. doi: 10.1002/jcp.1040850314. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Taylor J. M., McKnight G. S., Palacios R., Gonzalez C., Kiely M. L., Schimke R. T. Isolation of hen oviduct ovalbumin and rat live albumin polysomes by indirect immunoprecipitation. J Biol Chem. 1974 Jun 25;249(12):3665–3671. [PubMed] [Google Scholar]
- Sharp J. D., Capecchi N. E., Capecchi M. R. Altered enzymes in drug-resistant variants of mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3145–3149. doi: 10.1073/pnas.70.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D., Palacios R., Stavnezer J., Taylor J. M., Faras A. J., Kiely M. L., Summers N. M., Bishop J. M., Schimke R. T. Synthesis of a deoxyribonucleic acid sequence complementary to ovalbumin messenger ribonucleic acid and quantification of ovalbumin genes. J Biol Chem. 1973 Nov 10;248(21):7530–7539. [PubMed] [Google Scholar]
- Suzuki Y., Gage L. P., Brown D. D. The genes for silk fibroin in Bombyx mori. J Mol Biol. 1972 Oct 14;70(3):637–649. doi: 10.1016/0022-2836(72)90563-3. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Schimke R. T. Synthesis of rat liver albumin in a rabbit reticulocyte cell-free protein-synthesizing system. J Biol Chem. 1973 Nov 25;248(22):7661–7668. [PubMed] [Google Scholar]



