Abstract
The authors report a rare case of shock in a patient without significant clinical history, admitted to the intensive care unit for suspected septic shock. The patient was initially treated with fluid therapy without improvement. A hypothesis of systemic capillary leak syndrome was postulated following the confirmation of severe hypoalbuminemia, hypotension, and hemoconcentration - a combination of three symptoms typical of the disease. The authors discussed the differential diagnosis and also conducted a review of the diagnosis and treatment of the disease.
Keywords: Capillary permeability; Hypotension; Shock, septic; Hemoconcentration; Edema; Case reports
Abstract
Os autores apresentam um caso raro de choque em doente sem antecedentes pessoais significativos, admitido na unidade de terapia intensiva por suspeita de choque séptico. Inicialmente, foi tratado com fluidoterapia sem melhoria, tendo sido aventada a hipótese de síndrome de hiperpermeabilidade capilar, após confirmação de hipoalbulinemia paradoxal grave, hipotensão e hemoconcentração exuberante - tríade característica da doença. Os autores discutiram o diagnóstico diferencial e ainda realizaram uma revisão do diagnóstico e do tratamento da doença.
INTRODUCTION
Systemic capillary leak syndrome (SCLK), also known as Clarkson’s disease, was first described in 1960.(1) The authors described the case of a patient with recurrent episodes of distributive shock and generalized edema, resulting from the passage of fluid and proteins from the intravascular space into the extravascular space.(1)
Few reports are available in the literature, with an approximate total of 150 published cases.(2,3) SCLK may be fatal in extreme situations, when there is no treatment or measures to reverse shock.
The pathophysiology of this syndrome is associated with vascular endothelial dysfunction, allowing the passage of fluids and macromolecules from the vascular space into the interstitium.(4) That alteration results in the depletion of fluid and therefore in a state of tissue hypoperfusion and hypoxia characteristic of shock. Cellular elements remain in the vascular territory, resulting in elevated leukocyte, erythrocyte, and platelet counts.(4)
CASE REPORT
A 53-year-old Caucasian woman with a history of hypercholesterolemia, tobacco smoking, and degenerative spinal disease, treated with clopidogrel, rosuvastatin, and ethyl loflazepate began to develop symptoms of asthenia and generalized edema (feet, forearms, and face) five months before admission. Hypothyroidism was diagnosed following observation and brief analytical assessment (8.67μg/mL thyroid stimulating hormone - TSH, 0.20-4.20μg/mL reference values - RV), which improved with levothyroxine. A new episode of sudden generalized edema, which culminated in syncope, occurred three months later, with subsequent recovery. She was observed at the Emergency Room (ER) at that time, wherein she received complementary diagnostic tests. The following results were observed: 16.5g/dL hemoglobin (Hg), 16.500U/L leukocytosis, 0.21mg/dL C-reactive protein (CRP; RV<0.30mg/dL), renal failure with 1.6mg/dL creatinine (Cr), and leukocyturia (Table 1). The patient was treated with ciprofloxacin and reassessed at a later consultation, wherein normalization of blood count and of renal function and protein electrophoresis with monoclonal peak in the gamma-globulin region were observed.
Table 1.
Progression of analytical parameters during hospitalization
| 1st syncope | Admission to the ER | 12 hours later | Day 1 | Day 2 | Day 3 | Day 5 | Day 12 | Hospital discharge | |
|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 16.5 | 20.7 | 18.1 | 15.4 | 12 | 8.2 | 7.8 | 9.1 | 9.8 |
| Hematocrit (%) | 48.1 | 62.4 | 54.4 | 47 | 35.8 | 23.7 | 23.0 | 27.5 | 30.3 |
| Leukocytes x 109/L | 16.50 | 25.40 | 33.30 | 43.80 | 25.80 | 10.50 | 11.70 | 16.00 | 10.50 |
| Urea (mg/dL) | 59 | 54 | 45 | 32 | 22 | 85 | 53 | 67 | |
| Creatinine (mg/dL) | 1.60 | 1.54 | 1.25 | 1.27 | 1.2 | 0.89 | 1.75 | 1.01 | 0.99 |
| Potassium (mEq/dL) | 4.6 | 4.6 | 4.3 | 3.6 | 3.5 | 3.6 | 3.9 | 3.7 | 4.1 |
| Calcium (mg/dL) | 5.5 | 7.4 | 8.7 | ||||||
| Total Proteins (g/dL) | 2.73 | 3.72 | 4.03 | ||||||
| Albumin (g/dL) | 0.9 | 2.5 | 3.2 | 3.6 | |||||
| Creatine phosphokinase (U/L) | 87 | 129 | 2.658 | 8.406 | 6.412 | 3.176 | 355 | ||
| Myoglobin | 130 | >4.026 | >4.026 | 3.080 | 404.3 | 39.9 |
ER - Emergency Room.
A new episode of generalized edema, profuse sweating, and malaise, followed by syncope, occurred on the day of admission. The ER assessment showed unmeasurable blood pressure, preserved consciousness, no response to fluid therapy (3,000ml of crystalloid administered), and oligo-anuria. The laboratory tests showed hemoconcentration (Table 1) (20.7g/dL Hb, 62.4% hematocrit - Htc and 25.400 leukocyte count) and acute renal failure (1.56mg/dL Cr) with metabolic acidemia (50% FiO2 blood gas analysis: 7.33 pH; 22.2 partial pressure of carbon dioxide - pCO2; 130.3 partial pressure of oxygen - pO2; 11.5 bicarbonate - HCO3; 2.55 lactate; and 20 anion gap). The electrocardiogram was negative for acute myocardial ischemia. The patient underwent thoracic-abdominal-pelvic computed tomography with contrast, which excluded aortic dissection, and she was transferred to the intensive care unit (ICU).
The patient was anxious, sweaty, and with cold extremities at ICU admission. She was also afebrile and had unmeasurable blood pressure, sinus tachycardia, and 98% oxygen saturation with 50% Venturi mask. The cardiopulmonary auscultation was normal, the abdomen was free from tenderness on pressure, and the lower extremities showed no edema. Central venous pressure was 1cmH2O. The serum albumin level was 0.9g/dL (3.6 to 5.5g/dL RV) with 2.73g/dL total proteins (6.6 to 8.3g/dL RV).
Given the severe hypoalbuminemia, blood pressure, and diuresis (70cc urine output in 10 hours) unresponsiveness to fluid resuscitation with crystalloids and worsening of metabolic acidosis, 20% albumin replenishment and dialysis were started, reaching hemodynamic stability and fluid and electrolyte balance. The transthoracic echocardiogram showed a hyperdynamic left ventricular function with no further alterations.
The onset of severe lower limb edema, pain in the calf muscle region, and questionable foot pulses by palpation occurred three hours after admission and fluid therapy with crystalloids. The hypothesis of compartment syndrome was postulated and assessed by vascular surgery and plastic surgery, and the need for decompression fasciotomy was ruled out after performing Doppler ultrasound. Rhabdomyolysis with a creatine kinase peak of 8406U/L was observed in the laboratory tests. Clinical improvement with progressive decrease of leg perimeter and return of palpable pulses occurred 24 hours later.
On the 2nd day of hospitalization, the patient was orotracheally intubated due to respiratory exhaustion associated with fluid overload and was mechanically ventilated for 48 hours. On the 3rd day of hospitalization, diuresis resumed with spontaneous negativation of water balance and gradual reduction of peripheral edema, and dialysis was suspended on the 4th day. Hemoglobin levels were normalized in the first hours of fluid therapy with progressive normalization of serum albumin levels.
Treatment with aminophylline was started upon suspected SCLK diagnosis. The patient was transferred to the internal medicine ward on the 8th day of hospitalization.
The following results were obtained in the study and etiological assessment: normal prothrombin and activated partial thromboplastin times, transaminases, normal gamma glutamyl transferase, and alkaline phosphatase levels, 8mg/dL corrected calcium, 3.8mg/dL CRP (RV<5.0). Urine II with 50mg/dL proteinuria and 1.56g total 24-hour urine protein, 50.3KUI/L total IgE (RV 0 to 100KUI/L), 2mm/h sedimentation rate, sterile blood cultures (two) and urine culture, and normal thyroid function and serum cortisol levels; negative viral serology (human immunodeficiency virus (HIV) 1/2, hepatitis B and C).
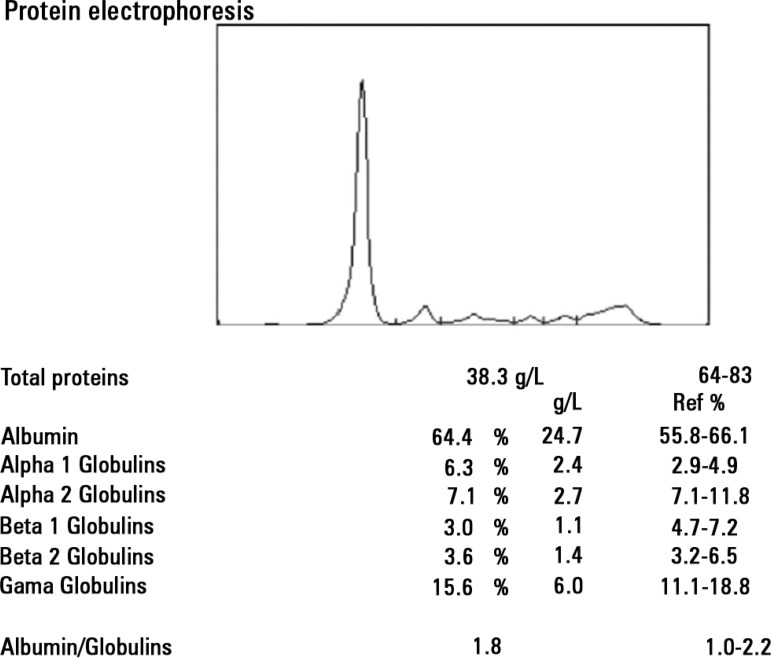
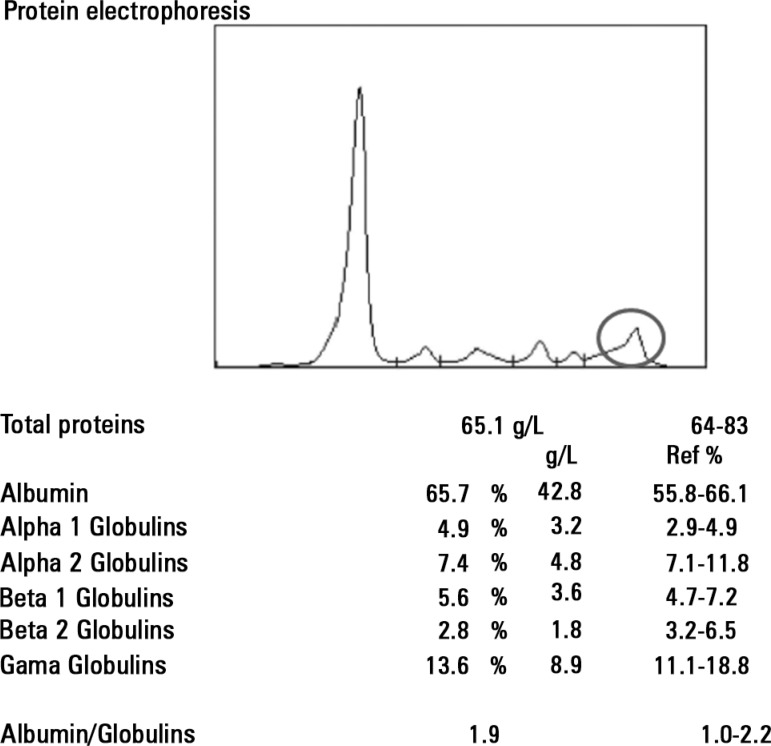
Analysis through gel electrophoresis of proteins and assessment of the levels of immunoglobulins was performed for immunological analysis. Decreased IgG and IGA levels were observed, with 0.79 kappa/lambda free light chains ratio (1.4 to 2.7 RV), and immunofixation showed IgG lambda-type monoclonal component and high serum levels, 185.0mg/L (5.71 to 26.30 RV). The 24-hour urinary excretion showed a significant increase in lambda light chains (133.0mg/L; RV<4.0) and Bence Jones protein positive for lambda free light chains. It should be noted that protein electrophoresis performed during the leak phase showed no monoclonal bands (Figure 1), although the replicate, after solving the acute episode, showed previously reported gammopathy (Figure 2). The patient was subjected to bone biopsy with description of interstitial and perivascular plasma cells, without cytologic atypia, monotypic for lambda-chains, which accounted for 5% of the medullary population.
Figure 1.
Protein electrophoresis in the acute phase, showing no alterations regarding the gamma-globulin region.
Figure 2.
Protein electrophoresis performed during hospitalization following the stabilization of an acute episode, showing alterations regarding the gamma-globulin region.
The patient was discharged on the 20th day of hospitalization without peripheral edema, with normal renal function, 3.6g/dL albumin, normal thyroid function without levothyroxine, and maintaining treatment with aminophylline. The patient was referred to a hematology and internal medicine consultation.
The patient returned to the hospital one month after discharge, reporting blood pressure values lower than the usual pattern. However, hypotension was not detected during the medical observation. The patient showed swelling of the face and was admitted to the intermediate care unit of the internal medicine department. Laboratory tests showed normal albumin on admission (3.9g/L), which decreased to 3.36g/L 24 hours later. Accordingly, immunoglobulin therapy was started, with regression of clinical symptoms. The patient has maintained the monthly treatment with 2g/kg immunoglobulin for 2 days, without relapse of the disease since then.
DISCUSSION
Several diagnoses may mimic SCLK, and the frequent causes of rapidly progressing shock must first be excluded.
The elements to be considered in the differential diagnosis of shock, contextualized with this clinical case, are as follows: severe hypoalbuminemia, hemoconcentration, and development of edema without evidence of fluid overload. That triad is not characteristic of cardiogenic, septic, or anaphylactic shock or angioedema, and cardiac dysfunction is not observed on the echocardiogram, in the clinical case. The CRP levels were normal; the culture tests were sterile; there was no history of allergies, urticaria, or eosinophilia; and the total IgE levels were normal.
Simultaneously, we may list kidney, liver, or nutritional factors as the possible etiology of hypoalbuminemia.
No clinical signs of chronic liver disease were found. The laboratory tests showed no alterations, especially regarding the prothrombin time, and the abdominal computed tomography showed no changes in liver structure. Acute renal injury was observed, although the urine protein levels were outside the nephrotic range. There was no clinical history information available that would indicate nutritional causes for hypoalbuminemia, the patient had a diverse diet, and the protein electrophoresis excluded protein-losing enteropathy.
There are endocrine causes that relate to shock and fluid retention in the third space, especially hypothyroidism. However, the analytical parameters of thyroid function were normal. Generalized edema may also be associated with Cushing’s syndrome with secondary hyperaldosteronism. However, the patient showed no stigmata of hypercortisolism or of elevated serum cortisol.
For this clinical case, all those differential diagnoses may be generally excluded due to the elevated hematocrit, which is consistent with decreased effective circulating volume.
The pathophysiology of SCLK may be divided into two phases.(1,2) The first results from an increase in capillary permeability that favors the passage of fluid and albumin into the extravascular space, which is manifested by generalized edema, hemoconcentration, and hypoproteinemia. This phase is associated with distributive shock in the most severe cases, typically attended by preservation of consciousness.(5) The second phase is characterized by vascular repletion with hypervolemia.(1,2)
The etiology of SCLK is still poorly understood. However, most cases are associated with monoclonal gammopathy, predominantly IgG kappa.(6) Despite studies with mixed results, many authors advocate a direct action of IgG paraprotein, with subsequent endothelial injury and capillary hyperpermeability.(5)
The formation of edema may result from an alteration in the function of endothelial cells induced by cytokines(7) because cases associated with interleukin-2 therapy used in the treatment of disseminated renal cell carcinoma and melanoma(8) and with interferon beta used in the treatment of multiple sclerosis(9,10) have been reported.
Given the rarity of this situation, the data available in the literature are scarce and refer only to case series and a few observational studies.
The treatment of the acute phase of SCLK is empirical and based on supportive therapy, wherein cautious fluid resuscitation is preferred,(5) given the risk of compartment syndrome and respiratory failure due to acute pulmonary edema during the repletion phase resulting from initial aggressive fluid resuscitation.(5) The next phase consists of aminergic support organ therapy with dialysis and ventilatory support when needed.
The existence of any clinical benefit from replenishing a severe deficit of albumin is questionable because the administered albumin may be diverted into the interstitial space due to alterations in the capillary barrier.(6) However, the initial treatment during the acute phase of SCLK with crystalloid administration may not suffice to maintain adequate intravascular volume status.(6) Thus, the potential clinical benefits of a trial of volume expansion with albumin as hemodynamic stabilizer and intravascular oncotic pressure regulator for rescue therapy should be assessed.
SCLK is a potentially relapsing disease. The relapses can have different degrees of severity, and each of them may be eventually fatal, leading to particular interest in treatment to prevent recurrences.
Treatment with beta-2 agonists, including terbutaline, and with xanthines, including theophylline and aminophylline, reduces SCLK relapse. A possible explanation is the decreased capillary permeability induced by histamine and bradykinin,(11) and the inhibition of apoptosis of endothelial cells resulting from the increased intracellular cAMP.(5) Based on studies with promising results, some authors recommend the empirical use of monthly immunoglobulins (2g/kg) for relapse prevention in refractory cases.(5,12)
In our specific case, a controlled initial fluid resuscitation had to be performed, given the risk of pulmonary and soft tissue edema with subsequent development of compartment syndrome. Albumin was administered to preserve the intravascular oncotic pressure, given the severe hypoalbuminemia and refractoriness to fluid resuscitation with crystalloids. Despite subsequent progression to acute pulmonary edema and severe lower limb edema, clinical stability was reached after 48 hours.
Aminophylline was initially administered due to the triad typical of SCLK with relapsing symptoms; however, relapse of the syndrome occurred under this treatment. Therefore, it was decided to begin immunoglobulin therapy. No new episodes of relapse occurred for 22 months following the introduction of this experimental therapy. It should be noted that the diagnosis of monoclonal gammopathy, which strengthens the diagnosis of SCLK, could have been overlooked during the SCLK phase, wherein no evidence of M band was found in the electrophoresis, most likely because of protein losses through urine.
CONCLUSION
Strong clinical suspicion, confirmation of hemoconcentration with paradoxical hypoalbuminemia, and exclusion of other causes of shock associated with generalized edema are necessary to diagnose systemic capillary leak syndrome.
The clinical case is paradigmatic of frequent disease relapse episodes, and the 3rd relapse is a serious and potentially fatal complication. No new relapses have been observed thus far with the monthly immunoglobulin regimen.
The progression of systemic capillary leak syndrome may be lethal at an early phase of vascular leakage due to distributive shock, and death may occur due to pulmonary edema at a later stage of vascular repletion with hypervolemia. Knowledge of this syndrome is important to prevent treatment that worsens the prognosis.
Footnotes
Conflicts of interest: None.
Responsible editor: Rui Moreno
REFERENCES
- 1.Clarkson B, Thompson D, Horwith M, Luckey EH. Cyclical edema and shock due to increased capillary permeability. Trans Assoc Am Physicians. 1960;73:272–282. [PubMed] [Google Scholar]
- 2.Druey KM, Greipp PR. Narrative review: the systemic capillary leak syndrome. Ann Intern Med. 2010;153(2):90–98. doi: 10.1059/0003-4819-153-2-201007200-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gousseff M, Arnaud L, Lambert M, Hot A, Hamidou M, Duhaut P, Papo T, Soubrier M, Ruivard M, Malizia G, Tieulié N, Rivière S, Ninet J, Hatron PY, Amoura Z, Capillary Leak Syndrome Registry The systemic capillary leak syndrome: a case series of 28 patients from a European registry. Ann Intern Med. 2011;154(7):464–471. doi: 10.7326/0003-4819-154-7-201104050-00004. [DOI] [PubMed] [Google Scholar]
- 4.Marks J, Shuster S. Disorders of capillary permeability. Br J Dermatol. 1973;88(6):619–621. doi: 10.1111/j.1365-2133.1973.tb08029.x. Review. [DOI] [PubMed] [Google Scholar]
- 5.Gousseff M, Amoura Z. Idiopathic capillary leak syndrome. Rev Med Interne. 2009;30(9):754–768. doi: 10.1016/j.revmed.2009.01.005. French. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson JP, Waldmann TA, Stein SF, Gelfand JA, Macdonald WJ, Heck LW, et al. Systemic capillary leak syndrome and monoclonal IgG gammopathy: studies in a sixth patient and a review of the literature. Medicine (Baltimore) 1977;56(3):225–239. doi: 10.1097/00005792-197705000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman U, Fontana A, Steurer J, Bollinger A. Idiopathic oedema with increased cytokine production: a pathogenetic link? J Intern Med. 1998;244(2):179–182. doi: 10.1046/j.1365-2796.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 8.Vial T, Descotes J. Clinical toxicity of interleukin-2. Drug Saf. 1992;7(6):417–433. doi: 10.2165/00002018-199207060-00004. Review. [DOI] [PubMed] [Google Scholar]
- 9.Walther EU, Hohlfeld R. Multiple sclerosis: side effects of interferon beta therapy and their management. Neurology. 1999;53(8):1622–1627. doi: 10.1212/wnl.53.8.1622. [DOI] [PubMed] [Google Scholar]
- 10.Niederwieser G. Lethal capillary leak syndrome after a single administration of interferon beta-1b. Neurology. 2000;54(7):1545–1546. doi: 10.1212/wnl.54.7.1542-d. [DOI] [PubMed] [Google Scholar]
- 11.Svensjö E, Adamski SW, Su K, Grega GJ. Quantitative physiological and morphological aspects of microvascular permeability changes induced by histamine and inhibited by terbutaline. Acta Physiol Scand. 1982;116(3):265–273. doi: 10.1111/j.1748-1716.1982.tb07140.x. [DOI] [PubMed] [Google Scholar]
- 12.Gousseff M, Arnaud L, Lambert M, Hot A, Hamidou M, Duhaut P, Papo T, Soubrier M, Ruivard M, Malizia G, Tieulié N, Rivière S, Ninet J, Hatron PY, Amoura Z, Capillary Leak Syndrome Registry The systemic capillary leak syndrome: a case series of 28 patients from a European registry. Ann Intern Med. 2011;154(7):464–471. doi: 10.7326/0003-4819-154-7-201104050-00004. [DOI] [PubMed] [Google Scholar]