Abstract
Extensive evidence suggests that the hypocretins/orexins influence cocaine reinforcement and dopamine signaling via actions at hypocretin receptor 1. By comparison, the involvement of hypocretin receptor 2 in reward and reinforcement processes has received relatively little attention. Thus, although there is some evidence that hypocretin receptor 2 regulates intake of some drugs of abuse, it is currently unclear to what extent hypocretin receptor 2 participates in the regulation of dopamine signaling or cocaine self-administration, particularly under high effort conditions. To address this, we examined the effects of hypocretin receptor 1, and/or hypocretin receptor 2 blockade on dopamine signaling and cocaine reinforcement. We used in vivo fast scan cyclic voltammetry to test the effects of hypocretin antagonists on dopamine signaling in the nucleus accumbens core and a progressive ratio schedule to examine the effects of these antagonists on cocaine self-administration. Results demonstrate that blockade of either hypocretin receptor 1 or both hypocretin receptor 1 and 2 significantly reduces the effects of cocaine on dopamine signaling and decreases the motivation to take cocaine. In contrast, blockade of hypocretin receptor 2 alone had no significant effects on dopamine signaling or self-administration. These findings suggest a differential involvement of the two hypocretin receptors, with hypocretin receptor 1 appearing to be more involved than hypocretin receptor 2 in the regulation of dopamine signaling and cocaine self-administration. When considered with the existing literature, these data support the hypothesis that hypocretins exert a permissive influence on dopamine signaling and motivated behavior via preferential actions on hypocretin receptor 1.
Keywords: Addiction, fast scan cyclic voltammetry, reward, drug abuse, motivation
The hypocretins/orexins (HCRT) consist of two peptides (hypocretin-1 and hypocretin-2) which are synthesized primarily in the lateral hypothalamus and adjacent perifornical regions. These peptides bind to two receptor subtypes, the hypocretin receptor 1 (HCRTr1) and hypocretin receptor 2 (HCRTr2), which are distributed widely throughout the brain.1 Although extensive evidence indicates that the HCRT system participates in arousal and arousal-related function,2 the HCRT system has also been heavily implicated in the regulation of mesolimbic dopamine (DA) signaling and reward and reinforcement processes. Indeed, HCRT neurons heavily innervate the DA-neuron containing ventral tegmental area (VTA), as well as the nucleus accumbens (NAc), both of which contain HCRTr1 and/or HCRTr2.3−6 Consistent with this, hypocretin-1 increases VTA cell firing, induces burst firing of DA neurons, and potentiates glutamate-mediated excitatory drive in DA neurons.7,8 Hypocretin-1 also enhances cocaine-induced elevations of DA in the NAc and promotes cocaine self-administration.9 By comparison, disruptions to HCRT neurotransmission result in dysregulated DA signaling. SB-334867, a HCRTr1 antagonist, reduces DA neuron firing10 and attenuates both cocaine-induced elevations in tonic and phasic DA signaling by altering the effects of cocaine on DA uptake inhibition.11 In terms of behavior, SB-334867 blocks reinstatement of cocaine-seeking12−17 and reduces the motivation to self-administer cocaine across schedules of reinforcement that require high-effort responding.11,18 These findings are in concert with reports involving genetic disruption to HCRT signaling which demonstrate that mice lacking the HCRT peptides show decreased DA and behavioral responses to morphine and cocaine.11,19,20 A similar finding is also observed in mice lacking HCRTr1, which show reduced cocaine self-administration.21,22 Together these observations indicate that the HCRT system participates in the regulation of reward and reinforcement processes and that these effects are mediated via signaling at HCRTr1.
Previous studies investigating the relative contribution of HCRT actions at HCRTr1 and/or HCRTr2 across physiological and behavioral processes have been limited largely to studies examining the influence of HCRT on sleep/wake activity.23−26 These studies indicate that HCRTr2 are particularly important for maintaining normal sleep/wake activity, while HCRTr1 appear to be less involved in these actions. Whether a functional distinction between signaling at HCRTr1 or HCRTr2 exists for processes associated with drugs of abuse is not clear. To date, only a few studies have reported on the contribution of HCRTr2 in drug reinforcement. In studies examining ethanol self-administration, HCRTr2 blockade reduces ethanol intake across schedules of reinforcement.27,28 Interestingly, however, in a cocaine study using low-effort schedules of reinforcement, blockade of HCRTr2 produced minimal reductions in cocaine intake.15 Therefore, to date, no study has compared the effects of HCRTr1 or HCRTr2 antagonists on high effort cocaine self-administration or the effects of these agents on DA signaling in the striatum. To address this issue, the present study examined the effects of HCRTr1, HCRTr2, and dual HCRTr1/HCRTr2 antagonists on DA signaling using in vivo fast scan cyclic voltammetry (voltammetry) and behavioral responses to cocaine using a progressive ratio (PR) schedule of reinforcement.
Results and Discussion
Blockade of HCRTr1 Reduces Cocaine-Induced DA Uptake Inhibition and Cocaine Self-Administration
We previously demonstrated that intra-VTA infusions of HCRT agents alter DA signaling in the NAc core under baseline conditions and in response to cocaine.9,11 To examine whether these effects extend to peripheral treatment with a HCRTr1 antagonist and to compare these effects to HCRTr2 and dual HCRTr1/HCRTr2 antagonists, we tested the effects of intraperitoneal (i.p.) vehicle (n = 10) or varying doses of SB-334867 (7.5 mg/kg, n = 6; 15 mg/kg, n = 6; and 30 mg/kg, n = 6). Following establishment of a stable baseline of DA signaling, rats were treated with SB-334867 and 30 min later received a single 1.5 mg/kg intravenous (i.v.) cocaine injection. Relative to vehicle, SB-334867 did not significantly affect DA uptake (F(3,24) = 1.05, P < 0.39) or peak height (F(3,24) = 2.41, P < 0.092) prior to cocaine treatment, although there was a trend for reduced peak height. This finding is in agreement with our previous microdialysis report indicating that intra-VTA, but not systemic, SB-334867 reduced baseline DA signaling in the absence of cocaine.11 Similar to previous work,29,30 i.v. delivery of cocaine elicited robust DA uptake inhibition (F(21,504) = 63.59, P < 0.0001) and increased DA peak height (F(21,504) = 21.163, P < 0.0001) across groups tested (Figure 1A–C). DA uptake inhibition was calculated as a change in the apparent affinity (Km) of DA for the DA transporter as described below (see Methods). Despite these effects, SB-334867 pretreatment reduced the magnitude of cocaine-induced DA uptake inhibition (F(3,24) = 4.08, P < 0.01). Further, a significant interaction indicated that, depending on dose, SB-334867 differentially altered the effects of cocaine on DA uptake inhibition over time (F(63,504) = 2.61, P = 0.000001). As shown in Figure 1C, post hoc analyses demonstrated that both the 15 and 30 mg/kg doses of SB-334867 significantly reduced the effects of cocaine on DA uptake inhibition at the 30 s and 1 min post-cocaine time point, as well as at other time points following cocaine. SB-334867 did not significantly affect cocaine-induced elevations in DA peak height (data not shown).
Figure 1.
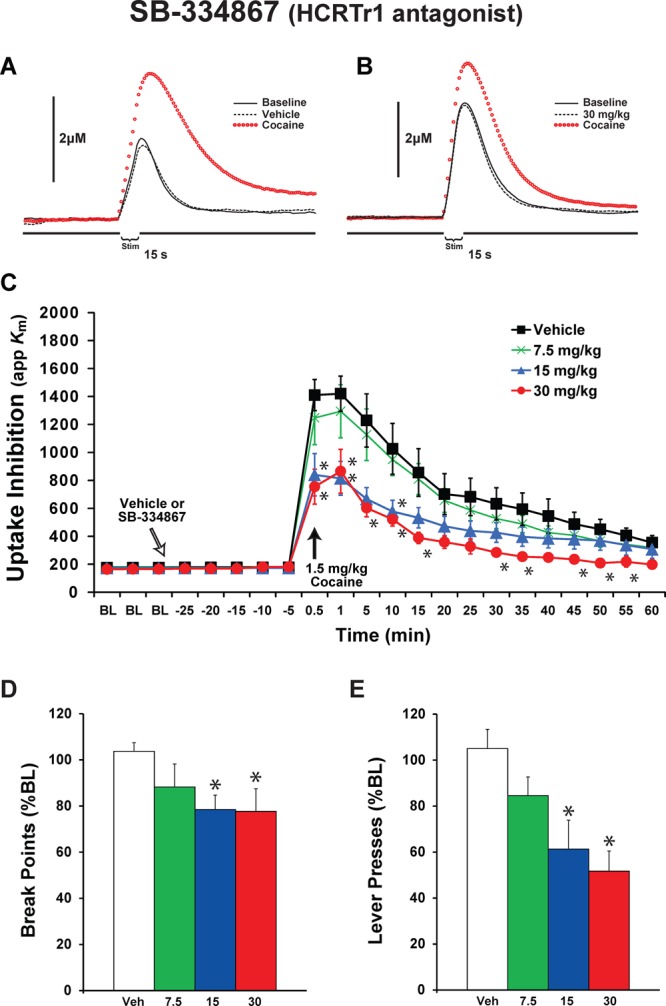
Blockade of HCRTr1 attenuates cocaine-induced DA uptake inhibition and reduces cocaine self-administration. Representative concentration–time plots of DA responses from rats that received i.p. injections of (A) vehicle or (B) 30 mg/kg SB-334867. Stim represents the time of electrical stimulation. (C) Mean ± SEM of DA uptake inhibition (apparent affinity, Km) following i.p. injections of vehicle (n = 10) or 7.5, 15, or 30 mg/kg of SB-334867 (n = 6) each. White arrow indicates time of SB-334867 delivery immediately after the last baseline (BL) collection. Black arrow indicates time of cocaine delivery. Mean ± SEM (D) break points (number of cocaine injections) and (E) lever presses, following i.p. injections of vehicle (n = 9) or SB-334867 (7.5, 15, or 30 mg/kg, n = 7 each).
We previously demonstrated that SB-334867 dose-dependently reduced the motivation to take 0.75 mg/kg cocaine under a PR schedule of reinforcement when SB-334867 was delivered i.p. or bilaterally into the VTA.11 To compare the effects of HCRTr1 blockade to that observed following antagonists that target HCRTr2, we tested the effects of SB-334867 on 1.5 mg/kg cocaine responding on a PR schedule. Rats were pretreated 30 min prior to testing with vehicle (n = 9) or varying doses of SB-334867 (7.5 mg/kg, n = 7; 15 mg/kg, n = 7; and 30 mg/kg, n = 7). Under baseline conditions, rats displayed an average break point (number of injections taken) of 17.4 ± 0.9, which was associated with 1011.7 ± 136.0 lever presses. Vehicle injections did not significantly alter break points (105.6 ± 3.1%; F(1,16) = 0.09, P = 0.78) or lever presses (105.0 ± 9.2%; P = 0.59). In contrast, SB-334867 produced a significant overall reduction in break points (F(4,20) = 6.0, P < 0.005) and lever presses (F(4,20) = 3.8, P < 0.05). As shown in Figure 1D, the 15 mg/kg (78.5 ± 6.2%, P = 0.025) and 30 mg/kg (77.6 ± 9.8%, P = 0.02) doses of SB-334867 significantly reduced break points. Additionally, the 15 mg/kg (61.3 ± 12.5%, P = 0.012) and 30 mg/kg (51.3 ± 9.7%, P < 0.002) doses significantly reduced lever presses (Figure 1E).
Blockade of Both HCRTr1 and HCRTr2 Reduces Cocaine-Induced DA Uptake Inhibition and Cocaine Self-Administration
To examine the effects of dual HCRTr1/HCRTr2 blockade, rats were treated with i.p. vehicle (n = 10) or varying doses of almorexant (25 mg/kg, n = 6; 50 mg/kg, n = 6; and 100 mg/kg, n = 6). Following establishment of a stable baseline of DA signaling, rats were treated with almorexant and 30 min later received a 1.5 mg/kg i.v. cocaine injection. Similar to SB-334867, almorexant did not significantly affect DA uptake (F(1,3) = 1.33, P = 0.29) or peak height (F(1,3) = 1.72, P = 0.19) prior to cocaine treatment. As expected, cocaine elicited robust DA uptake inhibition (F(21,504) = 59.53, P < 0.000001) and increased DA peak height (F(21,504) = 32.39, P < 0.000001) across groups tested (Figure 2A–C). Unlike what was observed with SB-334867, almorexant pretreatment did not produce a general attenuation in cocaine-induced DA uptake inhibition, possibly suggesting less robust effects (F(1,3) = 1.38, P = 0.272). Nevertheless, a significant interaction indicated that, depending on dose, almorexant differentially altered the effects of cocaine on DA uptake inhibition over time (F(63,504) = 1.63, P = 0.00267). As shown in Figure 2C, post hoc analyses demonstrated that the 50 and 100 mg/kg doses of almorexant significantly reduced the effects of cocaine on DA uptake inhibition at the 30 s post-cocaine time point. Moreover, the 100 mg/kg dose reduced the effects of cocaine on DA uptake inhibition at the 1 min post-cocaine time point. Similar to SB-334867, almorexant did not significantly affect cocaine-induced elevations in DA peak height (data not shown).
Figure 2.
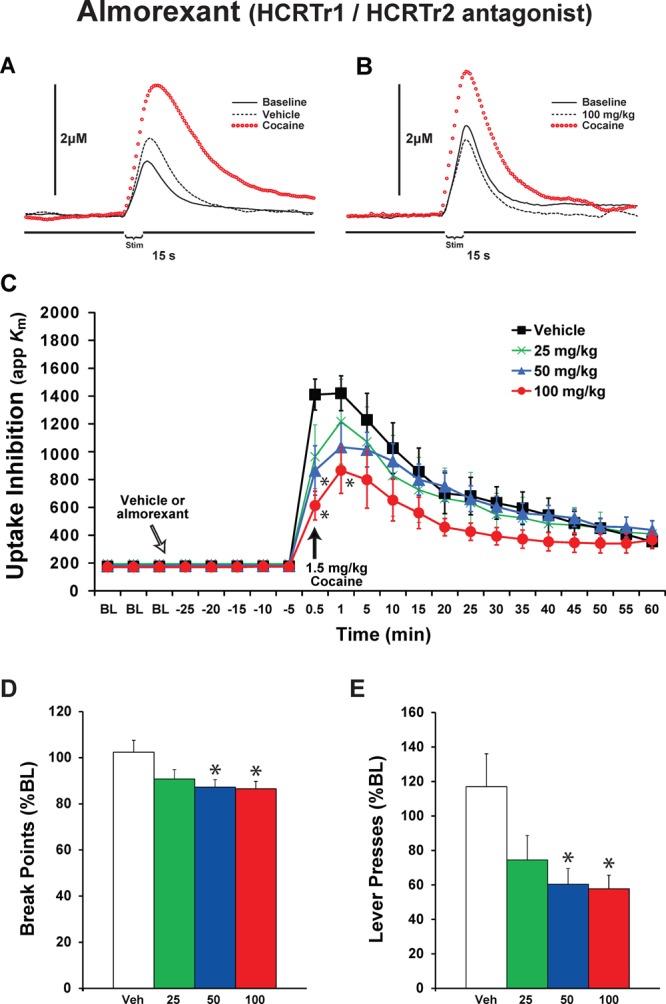
Blockade of both HCRTr1 and HCRTr2 attenuates cocaine-induced DA uptake inhibition and reduces cocaine self-administration. Representative concentration–time plots of DA responses from rats that received i.p. injections of (A) vehicle or (B) 100 mg/kg of almorexant. Stim represents the time of electrical stimulation. (C) Mean ± SEM of DA uptake inhibition (apparent affinity, Km) following i.p. injections of vehicle (n = 10) or 25, 50, or 100 mg/kg almorexant (n = 6) each. White arrow indicates time of almorexant delivery immediately after the last baseline (BL) collection. Black arrow indicates time of cocaine delivery. Mean ± SEM (D) break points (number of cocaine injections) and (E) lever presses, following i.p. injections of vehicle (n = 7) or almorexant (25, 50, and 100 mg/kg, n = 7 each).
To examine the effects of dual HCRTr1/HCRTr2 blockade on cocaine responding, rats were pretreated with vehicle (n = 7) or varying doses of almorexant (25 mg/kg, n = 7; 50 mg/kg, n = 7; and 100 mg/kg, n = 7) 30 min prior to the onset of the PR session. Under baseline conditions, rats displayed an average break point of 19.7 ± 0.99, which was associated with 1792.9 ± 359.7 lever presses. Vehicle injections did not significantly alter break points (102.4 ± 5.1%; F(1,12) = 0.14, P = 0.71) or lever presses (116.0 ± 19.7%; P = 0.76). Similar to that observed following SB-334867 treatment, almorexant produced a dose-dependent reduction in break points (F(3,24) = 3.8, P = 0.023) and lever presses (F(3,24) = 4.0, P = 0.02). As shown in Figure 2D, the 50 mg/kg (87.3 ± 3.8%, P = 0.033) and 100 mg/kg (86.6 ± 3.2%, P = 0.017) doses of almorexant significantly reduced break points. Moreover, 50 mg/kg (60.4 ± 9.2%, P = 0.024) and 100 mg/kg (57.8 ± 7.9, P = 0.012) almorexant significantly reduced lever presses (Figure 2E).
Blockade of HCRTr2 Does Not Reduce Cocaine-Induced DA Uptake Inhibition or Cocaine Self-Administration
To examine the extent to which HCRTr2 are involved in regulating DA signaling, rats were treated with i.p. vehicle (n = 10) or varying doses of 4PT (7.5 mg/kg, n = 6; 15 mg/kg, n = 6; and 30 mg/kg, n = 6). Following establishment of a stable baseline of DA signaling, rats were treated with 4PT and 30 min later received an i.v. cocaine injection (1.5 mg/kg). Similar to what was observed with antagonists that blocked HCRTr1, there were no significant changes in either DA uptake (F(3,24) = 0.994 P = 0.412) or peak height (F(3,24) = 0.812 P = 0.5) prior to cocaine when pretreating with 4PT. As expected, cocaine produced robust (F(21,504) = 55.596, P = 0.000001) DA uptake inhibition and increased DA peak height (F(21,504) = 23.268, P = 0.000001) across all groups tested (Figure 3A–C). In contrast to SB-334867 and almorexant, 4PT did not significantly reduce cocaine-induced DA uptake inhibition (F(3,24) = 1.83, P = 0.169) though a modest reduction was observed. Additionally, no interaction between dose and time was observed (F(63,504) = 1.11, P = 0.260). Finally, as was the case with SB-334867 and almorexant, 4PT had no effect on cocaine-induced elevations in DA peak height (data not shown).
Figure 3.
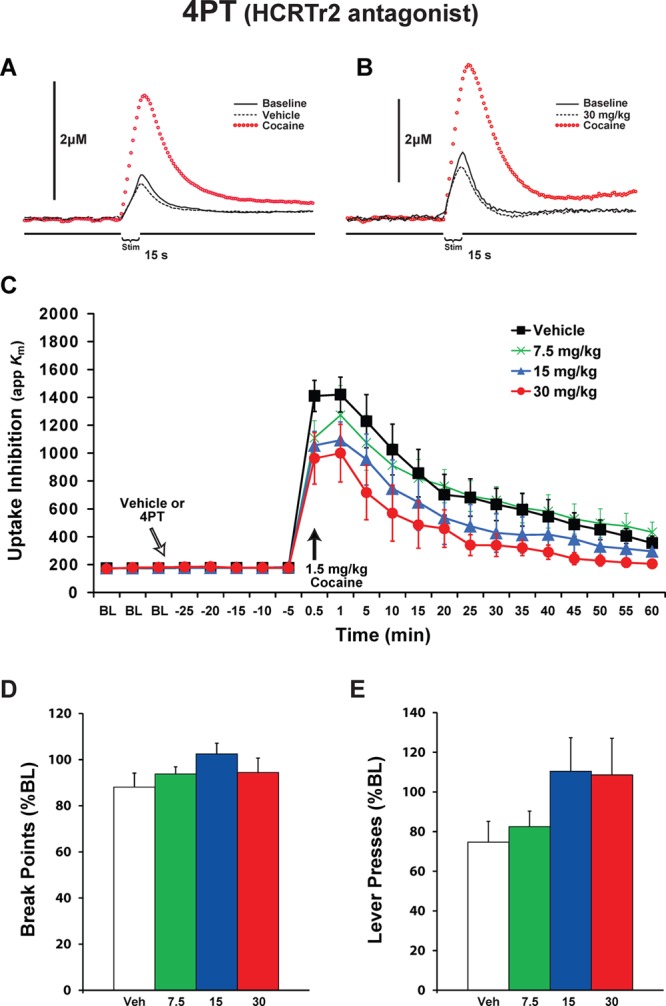
Blockade of HCRTr2 does not attenuate cocaine-induced DA uptake inhibition and does not reduce cocaine self-administration. Representative concentration–time plots of DA responses from rats that received i.p. injections of (A) vehicle or (B) 30 mg/kg of 4PT. Stim represents the time of electrical stimulation. (C) Mean ± SEM of DA uptake inhibition (apparent affinity, Km) following i.p. injections of vehicle (n = 10) or 7.5, 15, or 30 mg/kg 4PT (n = 6) each. White arrow indicates time of 4PT delivery immediately after the last baseline (BL) collection. Black arrow indicates time of cocaine delivery. Mean ± SEM (D) break points (number of cocaine injections) and (E) lever presses, following i.p. injections of vehicle (n = 7) or 4PT (7.5, 15, or 30 mg/kg, n = 7 each.
To examine the effects of HCRTr2 blockade on cocaine responding, rats were pretreated with vehicle (n = 7) or varying doses of 4PT (7.5 mg/kg, n = 6; 15 mg/kg, n = 7; and 30 mg/kg, n = 7) 30 min prior to testing on the PR session. Under baseline conditions, rats displayed an average break point of 19.6 ± 0.6, which was associated with 1495.9 ± 202.9 lever presses. Vehicle injections did not significantly alter break points (88.1 ± 6.1%; F(1,12) = 1.7, P = 0.21) or lever presses (68.6 ± 8.3%; P = 0.11), although there was a trend for decreased lever presses. In contrast to that observed with SB-334867 and almorexant, 4PT did not significantly alter break points (F(3,23) = 2.9, P = 0.06) or lever presses (F(3,23) = 2.1, P = 0.10) although there was a trend for an increase in both of these measures relative to vehicle treatment (Figure 3D and E).
In summary, the current studies compared the contributions of HCRTr1 and/or HCRTr2 blockade on DA signaling in the NAc and cocaine self-administration behavior. Results demonstrated significant reductions in the effects of cocaine on DA uptake inhibition and cocaine self-administration following blockade of HCRTr1 alone or dual blockade of both HCRTr1 and HCRTr2. When blocking just HCRTr2, no significant changes were observed for either DA signaling or cocaine self-administration. Together, these observations suggest that signaling at HCRTr1 is preferentially involved in maintaining motivation for cocaine and that these actions likely involve DA signaling in the NAc core.
HCRTr1 Mediate the Effects of Cocaine on DA Signaling
Previous reports suggest that the attenuation of cocaine self-administration observed following HCRTr1 antagonists may be related to reduced DA signaling in the NAc core.11 For example, neurochemical studies examining both tonic and phasic changes in DA signaling demonstrate that SB-334867, when delivered into the VTA, reduces baseline DA activity and attenuates the effects of cocaine on DA uptake and evoked DA release in the NAc core.11 Additionally, HCRT knockout mice show disrupted DA signaling, with reduced DA uptake rates under baseline conditions and attenuated uptake inhibition following cocaine.11 Consistent with a role of HCRT in regulating DA signaling, we have observed increased evoked DA release and cocaine-induced uptake inhibition when animals are treated with hypocretin-1 directly into the VTA.9 The present studies extend these observations by demonstrating that HCRT neurotransmission at HCRTr1, but likely not HCRTr2, is important in the regulation of mesolimbic DA signaling. Indeed, the current voltammetry findings indicate that SB-334867 and almorexant produce significant attenuation of cocaine effects on DA signaling in the NAc core. While the effects of SB-334867 were significant at both the intermediate and highest dose, the effects for almorexant were comparably less robust and only significant at the highest dose. In contrast, 4PT did not significantly reduce the effects of cocaine on DA signaling, although at the highest dose there was a modest reduction.
In addition to modulating the effects of cocaine on DA transmission, it appears that the HCRT system may also participate in the regulation of DA signaling as it relates to other drugs of abuse, as one report shows that HCRT knockout mice display decreased DA responses to morphine in the NAc.20 When combined with previous reports, these observations provide substantial evidence to support the hypothesis that HCRT neurotransmission is necessary for the regulation of DA signaling and that this may be preferentially mediated via actions at HCRTr1.
HCRTr1 Regulate Cocaine Self-Administration
A series of reports demonstrate that HCRT manipulations influence cocaine self-administration under conditions that require effortful responding or that limit access to cocaine.9,11,18 Specifically, we previously demonstrated that SB-334867, when injected i.p. or directly into the VTA, reduces the motivation to take cocaine under a PR or threshold schedule of reinforcement and reduces cocaine intake under a discrete trials schedule of reinforcement.11 In the current studies, rats were tested on a PR schedule to assess HCRT influences on the motivation to take cocaine. In the early portions of a PR session, cocaine is obtained with few lever presses, indicating relatively low effort requirements. During this phase of the session, we have shown that SB-334867 has little effect on cocaine intake.11 As the PR session progresses, lever press requirements to obtain single injections of cocaine increase exponentially, thereby necessitating greater numbers of lever presses and, thus, greater effort. Under these conditions, i.p. injections of SB-334867 or almorexant reduce break points and lever presses. In contrast, 4PT did not reduce these measures of self-administration. In fact, when compared to vehicle treatment, which by itself produced a modest, nonsignificant reduction in self-administration, 4PT appeared to restore normal levels of cocaine intake. The current observations are in agreement with an emerging literature indicating that the HCRT system regulates the reinforcing effects of cocaine. In particular, it has been shown that blockade of HCRTr1, systemically or directly in the VTA, blocks reinstatement of cocaine-seeking12−17 and decreases behavioral sensitization to cocaine,8 while blockade of HCRTr2 has negligible effects on cocaine intake.15
Similar to what was observed for the DA experiments, the effects of HCRT manipulations appear to extend beyond stimulant self-administration since previous work shows an involvement of HCRT in mediating the behavioral effects of nicotine, opiates, and ethanol.19,20,31−33 When combined with previous reports, the present results indicate that HCRT neurotransmission at HCRTr1 is necessary to support drug self-administration and that HCRTr2 may be less important in these actions. Further, these data provide ample evidence that systemic treatment with antagonists that target HCRTr1 is sufficient to reduce cocaine intake, a finding that provides further support for the utility of HCRT-based therapies to treat cocaine addiction.
Hypocretin Alters Cocaine Effects via Actions on the Mesolimbic DA System
The neural processes underlying the effects of disrupted HCRT neurotransmission on DA signaling currently remain unclear. Nevertheless, experiments employing voltammetry, microdialysis, and electrophysiology have provided an initial perspective as to the possible mechanisms that may be involved. One view is that the HCRT system exerts a facilitatory influence on DA neurons of the VTA, such that under normal conditions DA neurons are primed to respond to drug-related cues and rewards. This view is supported by the observation that HCRT peptides increase burst and tonic firing of DA neurons in the VTA,7,34 increase DA levels in the NAc,9,20 and regulate cocaine-induced changes in glutamate-mediated excitatory drive of DA neurons.8,35 Blockade of HCRTr1 in the VTA produces the opposite effects with reduced DA cell firing in the VTA10 and attenuated tonic and phasic DA activity in the NAc following cocaine.11 By regulating DA activity, the HCRT system is able to influence DA neurotransmission directly, which could result in altered sensitivity of DA systems to cocaine or other drugs of abuse. Indeed, recent evidence indicates that in addition to blocking DA uptake, cocaine stimulates glutamate release in the VTA36 and increases the incidence and/or magnitude of DA release events in the NAc shell and core,37 the latter observation likely being associated with a synapsin-mediated recruitment of vesicular stores of DA.38 Therefore, by altering DA release dynamics, independent of DA transporter (DAT) function, the HCRT system may induce DA neurons to display a differential sensitivity to cocaine and possibly other drugs of abuse.
A second perspective is that the HCRT system influences DA signaling by altering the state of DA terminals, likely via changes to DAT sensitivity. Given that baseline uptake rates are dependent on functional DATs, modifications that alter cell surface DAT expression can result in changes to baseline DA uptake rates, which can alter psychostimulant potency. Several observations indicate that the presence of the DAT in the cell membrane can be modulated by numerous signaling cascades that result in phosphorylation and glycosylation39−41 and that trafficking can occur within the time frame of the HCRT manipulations discussed herein.42 By altering DA neuronal activity state and influencing DA levels at the terminal, the HCRTs may effectively modulate DA D2 autoreceptors, which have been shown to regulate DAT cell surface expression and DA uptake rate activity.43−46 In this manner it is possible that, by regulating DA neuronal firing and subsequently changes to synaptic levels of DA, the HCRTs are poised to impact D2 autoreceptor activity thereby influencing D2 mediated DAT function and related sensitivity to cocaine.
Another alternative, particularly for studies using systemic delivery of HCRT receptor antagonists, is that HCRT manipulations could also be acting outside of the VTA. Indeed, the paraventricular thalamus (PVT), insular cortex, and the NAc contain HCRTr1 and/or HCRTr24,6 and participate in the regulation of reward and reinforcement processing. For example, delivery of hypocretin-1 into the PVT increases DA levels in the NAc,47 and increases ethanol intake.48 Moreover, blockade of HCRTr2 in the PVT reduces ethanol intake.48 HCRT may also be regulating drug reinforcement via actions on the cortex, as blockade of HCRTr1 in the insular cortex reduces nicotine self-administration.32 Finally, there is also evidence that the HCRT system regulates DA signaling via actions in the NAc. Both hypocretin-1 and -2 have been shown to increase firing of medium spiny neurons in the NAc shell49,50 and hypocretin-1 promotes local DA release in this region.51 Together with extensive evidence that the HCRT system exerts its effects on reward and reinforcement process via the VTA, these observations suggests broad modulatory effects of HCRT which likely extend beyond the regions discussed herein.
Differential Physiological Effects of HCRTr1 and HCRTr2 Antagonists
Although most research on HCRT regulation of reward and reinforcement has focused on HCRTr1 manipulations, several studies investigating the involvement of HCRT systems in sleep/wake regulation have compared the involvement of HCRTr1 and HCRTr2. In studies testing the effects of specific knockout of HCRT receptors, mice lacking either HCRTr2 or both HCRTr1 and HCRTr2 display qualitatively similar disruptions to sleep/wake behavior.23 By comparison, HCRTr1 knockout mice display only slight disruptions to sleep/wake behavior.23 Consistent with this, pharmacological blockade of HCRTr2 or dual blockade of HCRTr1 and HCRTr2 promotes sleep in animals and humans;24,25 however, blockade of only HCRTr1 appears to produce minimal effects on sleep/wake behavior.25,26 These observations indicate that actions at HCRTr2, but likely not HCRTr1, are especially important to maintain normal sleep/wake activity, which raises an interesting issue when considering the lack of self-administration effects following 4PT treatment. As an HCRTr2 antagonist, it is expected that 4PT would produce sedation and generalized disruptions to arousal that could manifest as reduced self-administration behavior. Nevertheless, it is important to note that in our experiments rats are actively self-administering cocaine and, thus, would be affected by the stimulant effects of this drug. Therefore, it is likely that any sedative effects produced by 4PT treatment might be easily overwhelmed by the arousal-enhancing, stimulatory effects of cocaine.
Despite this issue, the current observations showing that blockade of HCRTr2 on its own has little effect on DA signaling and cocaine-associated behavior, and that blockade of both HCRTr1 and HCRTr2 produces qualitatively similar effects to those observed following blockade of just HCRTr1, indicates that reduced cocaine self-administration and reduced cocaine effects on DA signaling, may largely be mediated via the HCRTr1. Future studies comparing the effects of HCRTr1 and HCRTr2 involvement in additional aspects of reward and reinforcement or in different physiological processes altogether, may provide further information that will help identify the unique contribution that these receptors provide to the regulation of behavior.
Hypocretin and Arousal
The HCRT system has repeatedly been recognized to participate in the regulation of sleep/wake function and locomotor activity.52−56 Despite initial concerns that HCRT-based drugs exert their effects on reinforcement processes indirectly through gross disruption of sleep/wake function and locomotor activity, a series of reports over the past decade have largely assuaged these reservations. For example, several reports indicate that SB-334867 does not elicit sleep,25,57 does not affect responding for food and water,31,32 and does not alter the motivation to lever press for highly palatable foods in food restricted rats.11,18 Moreover, across several reports, it has been demonstrated that neither hypocretin-1 nor blockade of HCRTr1 affects responding for cocaine under fixed ratio 1 conditions9,11,14 or during the early portions of a PR or threshold schedule of reinforcement,9,11 conditions in which maintaining preferred blood levels of cocaine requires relatively low effort. Finally, we recently showed that SB-334867 does not alter cocaine-induced elevations in locomotor activity,58 which demonstrates that animals are capable of responding to reward signals and display no motor deficits that could explain a reduced ability to respond on a lever. When considered together, these data suggest that the pharmacological effects of SB-334867 cannot be readily explained by generalized effects on sedation or motor activity.
Conclusions
The current studies demonstrate that blockade of HCRTr1, but not HCRTr2, reduces cocaine-induced DA responses in the NAc core and cocaine self-administration behavior. Together with a developing literature, these observations provide strong evidence in support of the hypothesis that the HCRT system is involved in reward and reinforcement processes through HCRTr1 actions that likely involve the mesolimbic DA system.
Methods
Animals
Male Sprague–Dawley rats (380–440g, Harlan, Frederick, MD) were given ad libitum access to food and water and kept on a reverse 12/12 h light/dark cycle (lights on at 1500 h). All protocols and animal care procedures were maintained in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals: Eighth Edition (The National Academies Press, Washington, DC, 2011) and approved by the Institutional Animal Care and Use Committee at Drexel University College of Medicine.
Surgery
After a minimum 7 day acclimation period, rats used for self-administration experiments were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and implanted with an i.v. silicone catheter (ID, 0.012 in OD, 0.025 in Access Technologies, Skokie, IL) inserted into the jugular vein that exited through the skin of the dorsal scapulae region. Rats received postsurgical antibiotic (Neo-Predef, Pharmacia & Upjohn Company, New York, NY) and analgesic (5 mg/kg; Ketoprofen, Patterson Veterinary, Devens, MA) and recovered for 3 days prior to training.
For voltammetry experiments, rats were anesthetized with intraperitoneal (i.p.) urethane (1.5 g/kg) and implanted with a jugular vein catheter before being placed in a stereotaxic apparatus. Once in the apparatus, rats were implanted with a bipolar stimulating electrode (Plastics One, Roanoke, VA) aimed at the VTA (+5.2 P, +1.1 L, −7.5 to −8.0 V). A carbon fiber microelectrode was implanted within the core of the NAc (−1.3 A, +1.3 L, −6.5 to −7.0 V), and a reference electrode was implanted in the contralateral cortex (−2.5 A, −2.5 L, −2.0 V).
Self-Administration
After surgery, rats were individually housed in chambers equipped with a counterbalanced swivel that held a stainless steel spring that was connected to the back plate of the catheter. Rats were trained to self-administer cocaine on an FR schedule, in which a single lever press resulted in a single cocaine injection of 0.75 mg/kg cocaine (in saline; National Institute on Drug Abuse) over approximately 5 s followed by a 20 s intertrial interval. FR training sessions were concluded once 20 injections were reached. A stable pattern of cocaine self-administration was defined as 3 days of 20 injections per day, after which the rat was switched to a PR schedule.
The PR schedule assesses the reinforcing effects of cocaine and therefore is a useful procedure to measure motivation.9,11,59 Rats on the PR schedule were given access to the lever for a 6 h period (10:00h-16:00h). The number of injections obtained before a 1 h unrewarded period had elapsed was defined as the “break point” and the number of lever presses made to obtain the last injection received, regardless of elapsed time was defined as “lever presses”. Stable responding was defined as 3 consecutive days of ±2 break points that did not show an ascending or descending trend. Once stable, rats were given an i.p. injection of vehicle or one of three doses of HCRT receptor antagonists 30 min prior to the session beginning (9:30h) in a counterbalanced design.
In Vivo Fast Scan Cyclic Voltammetry
To examine pharmacologically induced changes in DA release and uptake, voltammetry studies were conducted. After catheterization and placement in the stereotaxic apparatus, a stimulating electrode was lowered into the VTA. The carbon fiber electrode was first lowered into the caudate putamen (+1.3 A, +1.3 L, −4.5 V), until a 1 s, 60 Hz monophasic (4 ms; 300–500 μA) stimulation train elicited a robust DA signal. The caudate putamen region has been shown to produce higher levels of DA release and faster uptake (4 μm/s) than the NAc core (2.5 μm/s),29,60−62 which makes it a useful region for optimizing recording conditions. Once adequate levels of release were observed in the caudate putamen, the carbon fiber electrode was lowered 2–2.5 mm further into the NAc core, which yields lower DA release levels and slower DA uptake. After collecting stable baselines in the NAc core at 5 min intervals, rats received an i.p. injection of vehicle or one of the three HCRT receptor antagonists and DA signaling continued to be monitored. Cocaine (1.5 mg/kg) was delivered i.v. 30 min after antagonist injection. Electrically stimulated DA responses were recorded at 30 s, 1 min, and 5 min after cocaine injection, followed by every 5 min thereafter.
Data Acquisition
The electrode potential was linearly scanned from −0.4 to 1.2 V and back to −0.4 V vs Ag/AgCl. Cyclic voltammograms were recorded at the carbon fiber electrode every 100 ms with a scan rate of 400 V/s, using a voltammeter/amperometer (Chem-Clamp; Dagan Corporation, Minneapolis, MN). The magnitude of electrically evoked DA release and transporter-mediated uptake kinetics, including Vmax and Km were monitored. Extracellular concentrations of DA were assessed by comparing the current at the peak oxidation potential for DA in consecutive voltammograms with electrode calibrations of known concentrations of DA (1–10 μm). DA overflow curves were fitted to a Michaelis–Menten-based kinetic model,62 using Demon Voltammetry and Analysis software63 written in Labview language (National Instruments, Austin, TX). DA uptake rates prior to any drug treatment were modeled by setting the affinity of DA for the DA transporter to between 0.16 and 0.2 μm and then fitting the overflow curve to establish a baseline Vmax (maximal uptake rate) individually for each subject. Following cocaine injection, Vmax was held constant for the remainder of the experiment, and changes in DA uptake rate, due to cocaine-induced uptake inhibition, were calculated as a change in the apparent affinity for the DA transporter and defined as Km.
Hypocretin Receptor Antagonists
HCRT antagonists were used for both in vivo voltammetry and self-administration experiments via i.p. injections and were a gift from Eli Lilly and Company, Indianapolis, IN. The HCRTr1 antagonist, SB-334867, was given at 7.5, 15, and 30 mg/kg (Eli Lilly or Tocris R & D, Minneapolis, MN), doses which have previously been shown to alter cocaine self-administration across various schedules of reinforcement.11 Almorexant was used as the dual HCRTr1/HCRTr2 antagonist at 25, 50, and 100 mg/kg based on preliminary results showing similar reductions in cocaine self-administration as those observed following SB-334867. SB-334867 and almorexant were dissolved in 10% β-cyclodextran + 4% dimethyl sulfoxide (DMSO) in distilled H2O. The HCRTr2 antagonist, 4PT, was dissolved in 5% Solutol in 25 mM phosphate buffer and delivered at 7.5, 15, and 30 mg/kg based on previous reports.15,64
Data Analysis and Statistics
Voltammetry
Stimulated DA release following vehicle or HCRT antagonist treatment was calculated as the percent change from baseline, with “baseline” (100%) defined as the average of three samples that occurred prior to the injection of the antagonist. Stimulated DA release (peak height) following cocaine was calculated as the percent change from the “post-antagonist” release that preceded the cocaine injection. Changes in maximal uptake rate following antagonist injections were expressed as Vmax, and changes in uptake inhibition following cocaine were expressed as apparent Km. To examine the effects of antagonists on DA signaling prior to cocaine, stimulated DA release and Vmax were assessed using a two-way mixed design ANOVA comparing DA release or Vmax from the three baseline recordings prior to antagonist injection and DA release or Vmax for the 30 min following antagonist treatment (average baseline vs pre cocaine). In this manner, drug dose (vehicle or antagonists) was the between subjects variable and time was the repeated subjects variable. To examine the effects of antagonists on cocaine-induced changes in DA signaling, stimulated DA release and uptake inhibition (apparent Km) were assessed using a two-way mixed design ANOVA over the course of the experiment such that drug dose (vehicle or antagonist) was the between subjects variable and time was the repeated measures variable. Where appropriate, Dunnett’s post hoc analyses using vehicle as the control were conducted to examine differences between drug treatments across time.
Self-Administration
For figure presentation and statistical analysis, break points and lever presses were expressed as a percentage change relative to the previous 3 days of baseline responding. The effects of HCRT antagonists were assessed using one-way repeated-measures ANOVA (vehicle and each dose of antagonist). When statistical significance was obtained, Dunnett’s post hoc tests, using vehicle as the control, were conducted.
Acknowledgments
We would like to thank Jessica K. Shaw and Selina J. Eckert for their expert technical assistance with fast scan cyclic voltammetry and self-administration experiments and Eli Lilly and Company for their kind gift of SB-334867, almorexant, and 4PT.
Author Contributions
C.D.P. collected self-administration and fast scan cyclic voltammetry data, analyzed data, and contributed to writing the manuscript. A.R.R. collected fast scan cyclic voltammetry data. J.T.Y. programmed necessary software for experiments and contributed to the writing of the manuscript. R.A.E. conceived and designed the study, collected fast scan cyclic voltammetry data, analyzed data, and contributed to writing the manuscript. All authors read and approved the final manuscript.
National Institute on Drug Abuse K01DA025279 (R.A.E.) and R01DA031900 (R.A.E.); National Institute on Alcohol abuse and Alcoholism T32DA007262 (J.T.Y.)
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Sakurai T.; Amemiya A.; Ishii M.; Matsuzaki I.; Chemelli R. M.; Tanaka H.; Williams S. C.; Richarson J. A.; Kozlowski G. P.; Wilson S.; Arch J. R.; Buckingham R. E.; Haynes A. C.; Carr S. A.; Annan R. S.; McNulty D. E.; Liu W. S.; Terrett J. A.; Elshourbagy N. A.; Bergsma D. J.; Yanagisawa M. (1998) Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 1. [DOI] [PubMed] [Google Scholar]
- de Lecea L.; Sutcliffe J. G. (2005) The hypocretins and sleep. FEBS J. 272, 5675–5688. [DOI] [PubMed] [Google Scholar]
- Peyron C.; Tighe D. K.; van Den Pol A. N.; de Lecea L.; Heller H. C.; Sutcliffe J. G.; Kilduff T. S. (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus J. N.; Aschkenasi C. J.; Lee C. E.; Chemelli R. M.; Saper C. B.; Yanagisawa M.; Elmquist J. K. (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol. 435, 6–25. [DOI] [PubMed] [Google Scholar]
- Fadel J.; Deutch A. Y. (2002) Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience 111, 379–387. [DOI] [PubMed] [Google Scholar]
- Martin G.; Fabre V.; Siggins G. R.; de Lecea L. (2002) Interaction of the hypocretins with neurotransmitters in the nucleus accumbens. Regul. Pept. 104, 111–117. [DOI] [PubMed] [Google Scholar]
- Korotkova T. M.; Sergeeva O. A.; Eriksson K. S.; Haas H. L.; Brown R. E. (2003) Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J. Neurosci. 23, 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland S. L.; Taha S. A.; Sarti F.; Fields H. L.; Bonci A. (2006) Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49, 589–601. [DOI] [PubMed] [Google Scholar]
- España R. A.; Melchior J. R.; Roberts D. C. S.; Jones S. R. (2011) Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology (Berlin, Ger.) 214, 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman D. E.; Aston-Jones G. (2010) Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. J. Neurosci. 30, 15585–15599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España R. A.; Oleson E. B.; Locke J. L.; Brookshire B. R.; Roberts D. C. S.; Jones S. R. (2010) The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur. J. Neurosci. 31, 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B.; Kenny P. J.; Specio S. E.; Martin-Fardon R.; Markou A.; Koob G. F.; de Lecea L. (2005) Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc. Natl. Acad. Sci. U. S. A. 102, 19168–19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G. C.; Wimmer M.; Aston-Jones G. (2005) A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437, 556–559. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G.; Smith R. J.; Moorman D. E.; Richardson K. A. (2009) Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology 56(Suppl 1), 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J.; See R. E.; Aston-Jones G. (2009) Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur. J. Neurosci. 30, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J.; Tahsili-Fahadan P.; Aston-Jones G. (2010) Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology 58, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S. V.; Smith R. J.; Aston-Jones G. (2013) Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berlin, Ger.) 226, 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland S. L.; Chang S. J.; Bowers M. S.; Thompson J. L.; Vittoz N.; Floresco S. B.; Chou J.; Chen B. T.; Bonci A. (2009) Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J. Neurosci. 29, 11215–11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D.; Zachariou V.; Barrot M.; Mieda M.; Willie J. T.; Eisch A. J.; Yanagisawa M.; Nestler E. J.; DiLeone R. J. (2003) Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J. Neurosci. 23, 3106–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M.; Nagumo Y.; Hashimoto S.; Narita M.; Khotib J.; Miyatake M.; Sakurai T.; Yanagisawa M.; Nakamachi T.; Shioda S.; Suzuki T. (2006) Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J. Neurosci. 26, 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander J. A.; Pham D.; Fowler C. D.; Kenny P. J. (2012) Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: Pharmacological and behavioral genetics evidence. Front Behav. Neurosci. 6, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp J. W.; Hollander J. A.; Thompson J. L.; Voren G.; Hassinger L. C.; Onvani S.; Kamenecka T. M.; Borgland S. L.; Kenny P. J.; Carlezon W. A. Jr. (2014) Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc. Natl. Acad. Sci. U. S. A. 111, E1648–E1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie J. T.; Chemelli R. M.; Sinton C. M.; Yanagisawa M. (2001) To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu. Rev. Neurosci. 24, 429–458. [DOI] [PubMed] [Google Scholar]
- Brisbare-Roch C.; Dingemanse J.; Koberstein R.; Hoever P.; Aissaoui H.; Flores S.; Mueller C.; Nayler O.; van G. J.; de Haas S. L.; Hess P.; Qiu C.; Buchmann S.; Scherz M.; Weller T.; Fischli W.; Clozel M.; Jenck F. (2007) Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat. Med. 13, 150–155. [DOI] [PubMed] [Google Scholar]
- Dugovic C.; Shelton J. E.; Aluisio L. E.; Fraser I. C.; Jiang X.; Sutton S. W.; Bonaventure P.; Yun S.; Li X.; Lord B.; Dvorak C. A.; Carruthers N. I.; Lovenberg T. W. (2009) Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J. Pharmacol. Exp. Ther. 330, 142–151. [DOI] [PubMed] [Google Scholar]
- Morairty S. R.; Revel F. G.; Malherbe P.; Moreau J. L.; Valladao D.; Wettstein J. G.; Kilduff T. S.; Borroni E. (2012) Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS One 7, e39131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. M.; Khoo S. Y.; Lawrence A. J. (2013) Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int. J. Neuropsychopharmacol. 16, 2067–2079. [DOI] [PubMed] [Google Scholar]
- Anderson R. I.; Becker H. C.; Adams B. L.; Jesudason C. D.; Rorick-Kehn L. M. (2014) Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models. Front. Neurosci. 8, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España R. A.; Roberts D. C. S.; Jones S. R. (2008) Short-acting cocaine and long-acting GBR-12909 both elicit rapid dopamine uptake inhibition following intravenous delivery. Neuroscience 155, 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason J. T.; Jones S. R.; España R. A. (2011) Low and high affinity dopamine transporter inhibitors block dopamine uptake within 5 sec of intravenous injection. Neuroscience 182, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence A. J.; Cowen M. S.; Yang H. J.; Chen F.; Oldfield B. (2006) The orexin system regulates alcohol-seeking in rats. Br. J. Pharmacol. 148, 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander J. A.; Lu Q.; Cameron M. D.; Kamenecka T. M.; Kenny P. J. (2008) Insular hypocretin transmission regulates nicotine reward. Proc. Natl. Acad. Sci. U. S. A. 105, 19480–19485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage M. G.; Perry J. L.; Kotz C. M.; Shelley D.; Corrigall W. A. (2010) Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology (Berlin, Ger.) 209, 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova T. M.; Brown R. E.; Sergeeva O. A.; Ponomarenko A. A.; Haas H. L. (2006) Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur. J. Neurosci. 23, 2677–2685. [DOI] [PubMed] [Google Scholar]
- Borgland S. L.; Storm E.; Bonci A. (2008) Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur. J. Neurosci. 28, 1545–1556. [DOI] [PubMed] [Google Scholar]
- Wise R. A.; Wang B.; You Z. B. (2008) Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS One 3, e2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona B. J.; Cleaveland N. A.; Stuber G. D.; Day J. J.; Carelli R. M.; Wightman R. M. (2008) Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J. Neurosci. 28, 8821–8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton B. J.; Seipel A. T.; Phillips P. E.; Wetsel W. C.; Gitler D.; Greengard P.; Augustine G. J.; Wightman R. M. (2006) Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J. Neurosci. 26, 3206–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. B.; Chen N.; Ramamoorthy S.; Chi L.; Cui X. N.; Wang L. C.; Reith M. E. (2004) The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J. Biol. Chem. 279, 21012–21020. [DOI] [PubMed] [Google Scholar]
- Johnson L. A.; Furman C. A.; Zhang M.; Guptaroy B.; Gnegy M. E. (2005) Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacology 49, 750–758. [DOI] [PubMed] [Google Scholar]
- Mortensen O. V.; Larsen M. B.; Prasad B. M.; Amara S. G. (2008) Genetic complementation screen identifies a mitogen-activated protein kinase phosphatase, MKP3, as a regulator of dopamine transporter trafficking. Mol. Biol. Cell 19, 2818–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman C. A.; Chen R.; Guptaroy B.; Zhang M.; Holz R. W.; Gnegy M. (2009) Dopamine and amphetamine rapidly increase dopamine transporter trafficking to the surface: Live-cell imaging using total internal reflection fluorescence microscopy. J. Neurosci. 29, 3328–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiergerd S. M.; Patterson T. A.; Schenk J. O. (1993) D2 receptors may modulate the function of the striatal transporter for dopamine: Kinetic evidence from studies in vitro and in vivo. J. Neurochem. 61, 764–767. [DOI] [PubMed] [Google Scholar]
- Cass W. A.; Gerhardt G. A. (1994) Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neurosci. Lett. 176, 259–263. [DOI] [PubMed] [Google Scholar]
- Wu Q.; Reith M. E.; Walker Q. D.; Kuhn C. M.; Carroll F. I.; Garris P. A. (2002) Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: An in vivo voltammetric study. J. Neurosci. 22, 6272–6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F. J.; Pei L.; Moszczynska A.; Vukusic B.; Fletcher P. J.; Liu F. (2007) Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 26, 2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. L.; Davis J. F.; Magrisso I. J.; Fitzgerald M. E.; Lipton J. W.; Benoit S. C. (2012) Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience 210, 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson J. R.; Ho H. T.; Leibowitz S. F. (2014) Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict. Biol. 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai K.; Kim J.; Nakajima K.; Oomura Y.; Wayner M. J.; Sasaki K. (2009) Electrophysiological effects of orexin/hypocretin on nucleus accumbens shell neurons in rats: An in vitro study. Peptides 30, 1487–1496. [DOI] [PubMed] [Google Scholar]
- Mori K.; Kim J.; Sasaki K. (2011) Electrophysiological effects of orexin-B and dopamine on rat nucleus accumbens shell neurons in vitro. Peptides 32, 246–252. [DOI] [PubMed] [Google Scholar]
- Patyal R.; Woo E. Y.; Borgland S. L. (2012) Local hypocretin-1 modulates terminal dopamine concentration in the nucleus accumbens shell. Front. Behav. Neurosci. 6, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan J. J.; Leslie R. A.; Patel S.; Evans M. L.; Wattam T. A.; Holmes S.; Benham C. D.; Taylor S. G.; Routledge C.; Hemmati P.; Munton R. P.; Ashmeade T. E.; Shah A. S.; Hatcher J. P.; Hatcher P. D.; Jones D. N.; Smith M. I.; Piper D. C.; Hunter A. J.; Porter R. A.; Upton N. (1999) Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc. Natl. Acad. Sci. U. S. A. 96, 10911–10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin P.; Huitron-Resendiz S.; Spier A. D.; Fabre V.; Morte B.; Criado J. R.; Sutcliffe J. G.; Henriksen S. J.; de Lecea L. (2000) Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J. Neurosci. 20, 7760–7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper D. C.; Upton N.; Smith M. I.; Hunter A. J. (2000) The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur. J. Neurosci. 12, 726–730. [DOI] [PubMed] [Google Scholar]
- España R. A.; Baldo B. A.; Kelley A. E.; Berridge C. W. (2001) Wake-promoting and sleep-suppressing actions of hypocretin (orexin): Basal forebrain sites of action. Neuroscience 106, 699–715. [DOI] [PubMed] [Google Scholar]
- España R. A.; Plahn S.; Berridge C. W. (2002) Circadian-dependent and circadian-independent behavioral actions of hypocretin/orexin. Brain Res. 943, 224–236. [DOI] [PubMed] [Google Scholar]
- Deng B. S.; Nakamura A.; Zhang W.; Yanagisawa M.; Fukuda Y.; Kuwaki T. (2007) Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J. Appl. Physiol. 103, 1772–1779. [DOI] [PubMed] [Google Scholar]
- Calipari E. S.; España R. A. (2012) Hypocretin/orexin regulation of dopamine signaling: implications for reward and reinforcement mechanisms. Front. Behav. Neurosci. 6, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson N. R.; Roberts D. C. S. (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods 66, 1–11. [DOI] [PubMed] [Google Scholar]
- Kuczenski R.; Segal D. S.; Aizenstein M. L. (1991) Amphetamine, Cocaine, and Fencamfamine - Relationship Between Locomotor and Stereotypy Response Profiles and Caudate and Accumbens-Dopamine Dynamics. J. Neurosci. 11, 2703–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. R.; Garris P. A.; Kilts C. D.; Wightman R. M. (1995) Comparison of dopamine uptake in the basolateral amygdaloid nucleus, caudate-putamen, and nucleus accumbens of the rat. J. Neurochem. 64, 2581–2589. [DOI] [PubMed] [Google Scholar]
- Wu Q.; Reith M. E.; Wightman R. M.; Kawagoe K. T.; Garris P. A. (2001) Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J. Neurosci. Methods 112, 119–133. [DOI] [PubMed] [Google Scholar]
- Yorgason J. T.; España R. A.; Jones S. R. (2011) Demon Voltammetry and Analysis software: Analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J. Neurosci. Methods 202, 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose M.; Egashira S.; Goto Y.; Hashihayata T.; Ohtake N.; Iwaasa H.; Hata M.; Fukami T.; Kanatani A.; Yamada K. (2003) N-Acyl 6,7-Dimethoxy-1,2,3,4-tetrahydroisoquinoline: The first orexin-2 receptor selective non-peptidic antagonist. Bioorg. Med. Chem. Lett. 13, 4497–4499. [DOI] [PubMed] [Google Scholar]


