Abstract
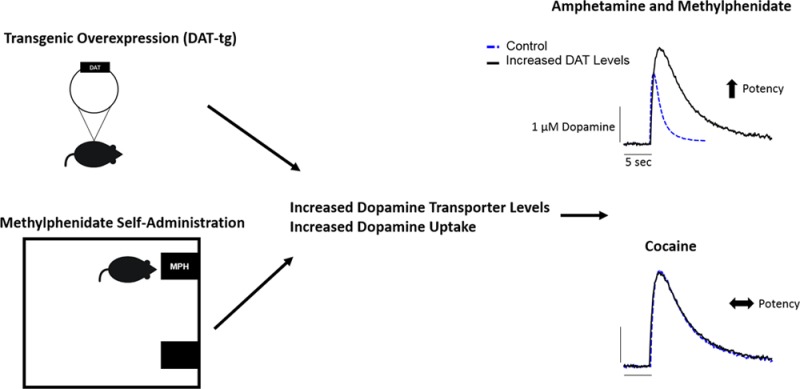
Dopamine transporter (DAT) levels vary across brain regions and individuals, and are altered by drug history and disease states; however, the impact of altered DAT expression on psychostimulant effects in brain has not been systematically explored. Using fast scan cyclic voltammetry, we measured the effects of elevated DAT levels on presynaptic dopamine parameters as well as the uptake inhibition potency of the blockers cocaine and methylphenidate (MPH) and the releaser amphetamine (AMPH) in the nucleus accumbens core. Here we found that increases in DAT levels, resulting from either genetic overexpression or MPH self-administration, caused markedly increased maximal rates of uptake (Vmax) that were positively correlated with the uptake inhibition potency of AMPH and MPH, but not cocaine. AMPH and MPH were particularly sensitive to DAT changes, with a 100% increase in Vmax resulting in a 200% increase in potency. The relationship between Vmax and MPH potency was the same as that for AMPH, but was different from that for cocaine, indicating that MPH more closely resembles a releaser with regard to uptake inhibition. Conversely, the effects of MPH on stimulated dopamine release were similar to those of cocaine, with inverted U-shaped increases in release over a concentration–response curve. This was strikingly different from the release profile of AMPH, which showed only reductions at high concentrations, indicating that MPH is not a pure releaser. These data indicate that although MPH is a DAT blocker, its uptake-inhibitory actions are affected by DAT changes in a similar manner to releasers. Together, these data show that fluctuations in DAT levels alter the potency of releasers and MPH but not blockers and suggest an integral role of the DAT in the addictive potential of AMPH and related compounds.
Keywords: Dopamine transporter, methylphenidate, cocaine, amphetamine, addiction, voltammetry
There has been considerable debate in the literature as to how variability in dopamine transporter (DAT) levels alter the potencies of psychostimulant drugs.1−5 This is particularly clinically relevant as the DAT is the primary site of action for the euphorigenic, rewarding, and reinforcing properties of psychostimulants such as cocaine, methylphenidate (MPH), and amphetamine (AMPH).6−9 Thus, the determination of how DAT levels alter drug effects could allow for the identification of individuals who are “at risk” for the development of a substance use disorder in the human population. Further, there seems to be a distinction between psychostimulant class (blocker versus releaser) and how DAT levels alter drug potency. Blockers act by binding to the DAT and inhibiting its uptake function, while releasers actively release dopamine from terminals by entering terminals, via the DAT, and depleting vesicular dopamine stores. While cell culture studies have suggested that increasing DAT levels reduces the potency of blockers, but not releasers,1 in vivo studies have shown that increasing DAT levels increases the potency of releasers, but not blockers, to inhibit dopamine uptake.2 Thus, currently, the relationships between different stimulant drug classes and DAT levels/uptake rates are unclear.
Although MPH is categorized as a DAT blocker, a number of studies have shown that it is distinct from both blockers and releasers in the way in which it interacts with the DAT.10,11 MPH is not a substrate for the DAT, is not transported into cells, and thus cannot directly interact with vesicles, although these actions are integral components of releaser mechanisms.12 However, at higher concentrations, MPH produces nonexocytotic dopamine release,13,14 which is the sine qua non effect of releasers.15,16 Recent experiments using voltammetry in brain slices have shown that MPH is unique, with aspects of its acute effects at the DAT resembling releasers, but not blockers,17−19 particularly in animals with a history of psychostimulant self-administration. Further, the compensatory alterations that occur within the dopamine system following MPH self-administration are distinct from the alterations that occur following either cocaine or AMPH self-administration.17−21 Thus, one aim of this study was to determine if MPH is more similar to blockers or releasers in regard to the effects of DAT levels on drug potencies.
Here we describe a number of findings: (1) Dopamine release and uptake rates are positively correlated, suggesting that they fluctuate together. (2) Drug-induced dopamine release is not correlated with the effects of stimulants at the DAT, suggesting that they occur via separate mechanisms. (3) MPH is a unique compound in the way that it interacts with the presynaptic dopamine terminal and the way in which MPH self-administration alters dopamine neurochemistry, as compared to other DAT blockers. (4) Uptake rates are positively correlated with releaser and MPH, but not blocker, potency. These findings differ from what was previously hypothesized by cell culture work, and suggest that current theories on the relationship between DAT levels and drug potencies should be revisited.
1. Results and Discussion
1.1. DAT Levels Correlate with the Potency of Psychostimulants at the DAT
In order to determine the effects of DAT level on psychostimulant effects at the DAT, we used two models: DAT-tg and MPH self-administration. We chose both a mouse genetic model and a rat pharmacological model of elevated Vmax in order to increase the likelihood of observing reproducible effects by minimizing potential flaws/changes in a single model, such as developmental alterations in the genetic model, off-target modifications in the pharmacological model, or species effects that could be driving the observed changes. DAT-tg animals are genetically altered to contain four additional copies of the DAT gene and as a result have increased surface expression of DAT as well as increased uptake rates (Vmax).2 We chose to use MPH self-administration as a nongenetic model, since previously published work shows that MPH self-administration induces a marked increase in Vmax and increased DAT expression levels.18,19 Thus, MPH self-administering animals can be used here as a nongenetically manipulated group for comparison with higher Vmax/DAT levels within the same region of interest. Further, because MPH abuse is prevalent in both the adult and adolescent population, understanding how MPH-induced changes in DAT function can enhance the effects of other stimulants could lead to the identification of MPH-exposed individuals “at risk” for subsequent psychostimulant abuse/addiction. In previously published grouped results, MPH self-administering and DAT-tg animals were shown to exhibit increased potency at the DAT for releaser compounds;2,18,19 however, no correlations have been examined until now, to compare individual magnitudes of change in Vmax and drug potencies within the same animal and establish the strength of the relationship between the two variables.
Using voltammetry in brain slices from two distinct models that lead to DAT overexpression (DAT-tg and MPH self-administration), we determined dopamine release (peak amplitude of the evoked signal) and uptake dynamics by fitting dopamine efflux curves to a Michaelis–Menten-based model. The model was fit to all of the raw data curves with a r2 value of 0.9 or greater. Using a repeated measures two-way analysis of variance (ANOVA), previous work reported that MPH self-administration and transgenic DAT overexpression caused a leftward shift in MPH and AMPH effects, but that cocaine effects on the dopamine system were not significantly altered.18,19
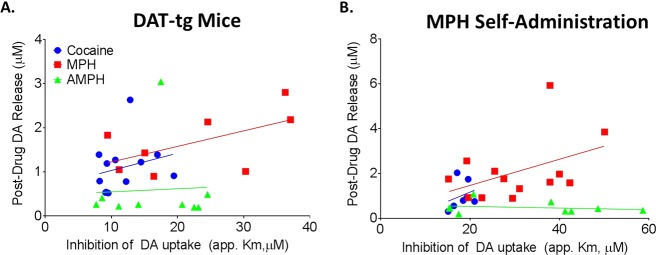
Here, to more explicitly examine the relationship between drug effects and DAT levels, we correlated the variable “apparent Km” (app. Km), which has been shown to accurately represent uptake inhibition effects at the DAT, with a functional measure of DAT levels (Vmax). Shifts in values of app Km across drug concentrations are representative of changes in the potency of drugs to inhibit the DAT. Potency is operationally defined here as the magnitude of drug effects on uptake inhibition across concentrations, where an increase in potency is characterized by increased magnitude of uptake inhibition (greater app. Km) at lower doses of drug. Correlations were determined by comparing baseline Vmax and drug-induced uptake inhibition at concentrations with equivalent DAT affinity for MPH (30 μM), cocaine (30 μM), and AMPH (10 μM). Vmax positively correlated with app. Km for AMPH (DAT-tg, r = 0.73, p < 0.05; MPH self-administration, r = 0.93, p < 0.001) and MPH (DAT-tg, r = 0.88, p < 0.001; MPH self-administration, r = 0.91, p < 0.0001). The potency of cocaine and Vmax for dopamine uptake were correlated following MPH self-administration, but not in DAT-tg animals (MPH self-administration, r = 0.85, p < 0.01; DAT-tg, r = 0.54, ns) (Figure 1). However, because correlations could be inflated in the cocaine group due to the restricted range of app. Km (relative to AMPH and MPH), we analyzed the slope of the regression lines (Table 1). The regression line for cocaine was not significantly different than a slope of zero in either group (Figure 1), highlighting that the correlation, although statistically significant, is only relevant in nonphysiological conditions. This suggests that only changes in DAT levels that far exceed the possible range in an in vivo system would have an effect on the potency of cocaine on the dopamine system. Further, the slope of the regression line for cocaine (DAT-tg, β = 1.37 ± 0.74; MPH self-administration, β = 1.54 ± 0.37) was not as steep as that for MPH (DAT-tg, β = 7.76 ± 1.47, vs cocaine: p < 0.0001; MPH self-administration, β = 11.14 ± 1.59, vs cocaine: p < 0.0001) and AMPH (DAT-tg, β = 8.17 ± 2.86, vs cocaine p < 0.0001; MPH self-administration, β = 8.90 ± 1.38, vs cocaine p < 0.0001), indicating that cocaine was differentially affected by DAT levels as compared to the other two stimulants tested. The regression lines for MPH and AMPH were not significantly different from one another for either DAT-tg or MPH self-administration (Figure 1), indicating that the extent to which increased Vmax results in increased uptake inhibition for MPH and AMPH does not differ. This indicates that MPH-induced uptake inhibition is affected by baseline changes in Vmax in a fashion that is not different from a releaser (AMPH), but is different from a blocker (cocaine). Together, these data indicate that fluctuations in DAT levels/Vmax are capable of altering the potency of all psychostimulants tested; however, the extent to which uptake inhibition is affected by Vmax differs across drugs.
Figure 1.
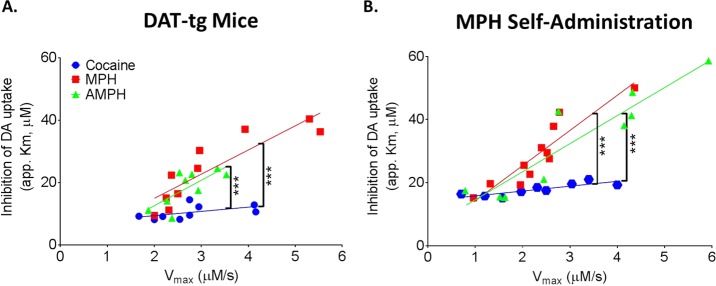
Maximal rates of dopamine (DA) uptake (Vmax) are positively correlated with the app. Km of psychostimulants at the dopamine transporter (DAT). DAT transgenic overexpressing mice (A, DAT-tg) and rats that had undergone MPH self-administration (B) were used to determine the effects of increased DAT on the app. Km of psychostimulants. Correlational analysis was run to compare the predrug measure of Vmax with the app. Km (app. Km) of dopamine uptake inhibition for methylphenidate (MPH; red; 30 μM; MPH, n = 11; DAT-tg, n = 10), amphetamine (AMPH; green; 10 μM; MPH, n = 9; DAT-tg, n = 9) and cocaine (blue; 30 μM; MPH, n = 9; DAT-tg, n = 9). Regression lines of AMPH, MPH, and cocaine were compared to determine if the range over which increases in Vmax increased app. Km was similar. The extent to which increased Vmax augmented app. Km was different between MPH and AMPH as compared to cocaine. ***p < 0.001.
Table 1. Regression Coefficients for Each Psychostimulant in MPH Self-Administration and DAT-tg Groupsa.
| drug | MPH self-administration | DAT-tg |
|---|---|---|
| cocaine | 1.54 ± 0.37 | 1.37 ± 0.74 |
| amphetamine | 8.90 ± 1.38 | 8.17 ± 2.86 |
| methylphenidate | 11.14 ± 1.59 | 7.76 ± 1.47 |
Values are reported as ± SEM.
1.2. Dopamine Release Is Not a Strong Predictor of Drug Effects at the DAT
In addition to increased uptake, both DAT-tg mice and animals that have a history of MPH self-administration also display increased stimulated dopamine release; therefore, we aimed to determine if Vmax or release was a better predictor of changes in the potency of each of the psychostimulants tested. Vmax and release are frequently correlated and emerging evidence suggests that DAT function directly influences recycling of DA into vesicles. Although we do not have tissue content information on the DAT-tg animals, dopamine tissue content is greatly reduced (95%) in DAT KO mice, suggesting that DATs are instrumental in maintaining intracellular dopamine levels.23
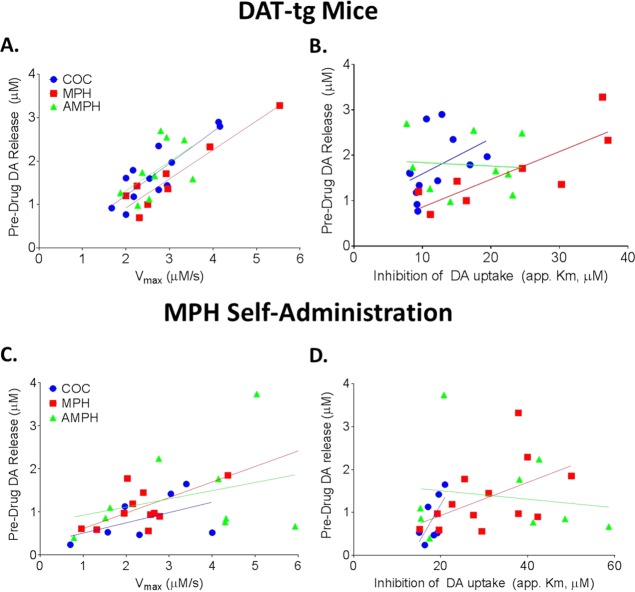
Predrug stimulated dopamine release and Vmax were positively correlated in all groups (DAT-tg, r = 0.71, p < 0.0001; MPH self-administration, r = 0.77, p < 0.001) and there was no difference in the strength of this correlation within each group (p > 0.05; Figure 2A, C). Although Vmax and stimulated release were correlated at baseline, and Vmax was correlated with drug-induced uptake inhibition, there was only a significant correlation between stimulated release and drug-induced uptake inhibition for MPH (DAT-tg, r = 0.50, p < 0.05; MPH SA, r = 0.82, p < 0.01) (Figure 2B, D). The correlation of stimulated release with the app. Km of MPH, but not the app. Km of other drugs, could be due to the ability of MPH to promote shifts in vesicles from membrane-bound to releasable pools, thus increasing MPH’s effects.24−26 It is possible that increased stimulated release at baseline may indicate the presence of more available vesicles or higher dopamine concentrations per each vesicle, as discussed previously. Thus, because MPH mobilizes vesicles, the increased availability leads to greater MPH-induced increases in synaptic dopamine levels. This is particularly important as it further suggests that MPH is unique from other traditional psychostimulants in its mechanism of action.
Figure 2.
Predrug evoked dopamine (DA) release is not correlated with the app. Km for cocaine or amphetamine. (A) Correlational analysis of the relationship between Vmax and predrug evoked dopamine release in the methylphenidate (MPH; red; MPH, n = 11; DAT-tg, n = 10), amphetamine (AMPH, green; MPH, n = 9; DAT-tg, n = 9), and cocaine (COC, blue; MPH, n = 9; DAT-tg, n = 9) groups. (B) Predrug evoked dopamine release did not correlate with cocaine or AMPH app. Km, but did correlate with app. Km of MPH. (C) Relationship between Vmax and predrug evoked dopamine release in DAT-tg mice. (D) Predrug evoked dopamine release did not correlate with the app. Km of cocaine or AMPH, but did correlate with the app. Km of MPH.
Although the potency of MPH, but not cocaine or AMPH, did correlate with stimulated release, it seems that changes in dopamine uptake are more likely to have a causal relationship with potency as direct genetic overexpression of the DAT (DAT-tg) was able to change uptake inhibition for all three compounds tested. This is supported by the fact that the relationship between release and uptake inhibition was far less robust and more variable than the relationship between Vmax and uptake inhibition. Additionally, stimulated release after drug application did not correlate with uptake inhibition for any of the drugs tested (Figure 3), indicating that differences in release are not always predictive of changes in uptake inhibition.
Figure 3.
No relationship between drug effects on dopamine release and drug effects on dopamine uptake. Correlational analysis was run in order to confirm that evoked dopamine release was not influencing drug induced uptake inhibition. Evoked dopamine release in the presence of drug was correlated with the app. Km of the psychostimulants methylphenidate (MPH; 30 μM; MPH, n = 11; DAT-tg, n = 10), cocaine (30 μM; MPH, n = 9; DAT-tg, n = 9), and amphetamine (AMPH; 10 μM; MPH, n = 9; DAT-tg, n = 9) (A) in transgenic DAT overexpressing mice (DAT-tg) or (B) following MPH self-administration.
1.3. A New Hypothesis: DAT Levels Alter Releaser, But Not Blocker, Effects at the DAT
Here we propose a basic neurochemical mechanism by which fluctuations in DAT levels drive changes in AMPH-like drug effects. Namely, increases in DAT levels are responsible for the increases in AMPH, MPH, and other dopamine releaser, but not DAT blocker, effects at the dopamine terminal. This is supported by the current results showing significant correlations between Vmax and the potency of MPH and AMPH. While the potency of cocaine was significantly correlated with uptake rates, it is clear that the magnitude of potency shifts for AMPH and MPH across shifts in Vmax is substantially greater than the small to almost nonexistent shifts in cocaine potency following similar shifts in Vmax. In fact, our range of app. Km values for cocaine across the entire spectrum of Vmax is quite restricted, which may artificially exaggerate the correlation, something that is highlighted by the slope of the regression line, which does not significantly deviate from zero.
Because prior behavioral studies have shown that, following DAT overexpression, the reinforcing efficacy and locomotor activating effects of AMPH and MPH were increased while cocaine effects remained unchanged,2,18 we would suggest that this model is a good predictor of the consequences of elevated DAT levels on both a neurochemical and behavioral level. The positive correlation between DAT levels and psychostimulant-induced neurochemical and behavioral changes may help to explain drug abuse vulnerability, especially in regard to releaser compounds, in certain subsets of the human population with increased DAT levels.
1.4. MPH: Uptake Profile of Releaser, Release Profile of Blocker
In this study and others, MPH, although characterized as a DAT blocker, seems to be more similar to releasers in its effects at the DAT.17−19 MPH is traditionally categorized as a blocker because it is not actively transported into cells, and thus, cannot interact with vesicles and release dopamine through reverse transport.12 Because of this, much of the MPH literature has considered it to be similar to cocaine in both acute and chronic pharmacological effects. Although it is unclear exactly how transporter levels affect the potency of MPH, the fact that this phenomenon occurs suggests that care should be taken when administering this drug to individuals that may have elevated DAT levels, such as ADHD sufferers,27 as its potency/efficacy may be increased in these individuals.
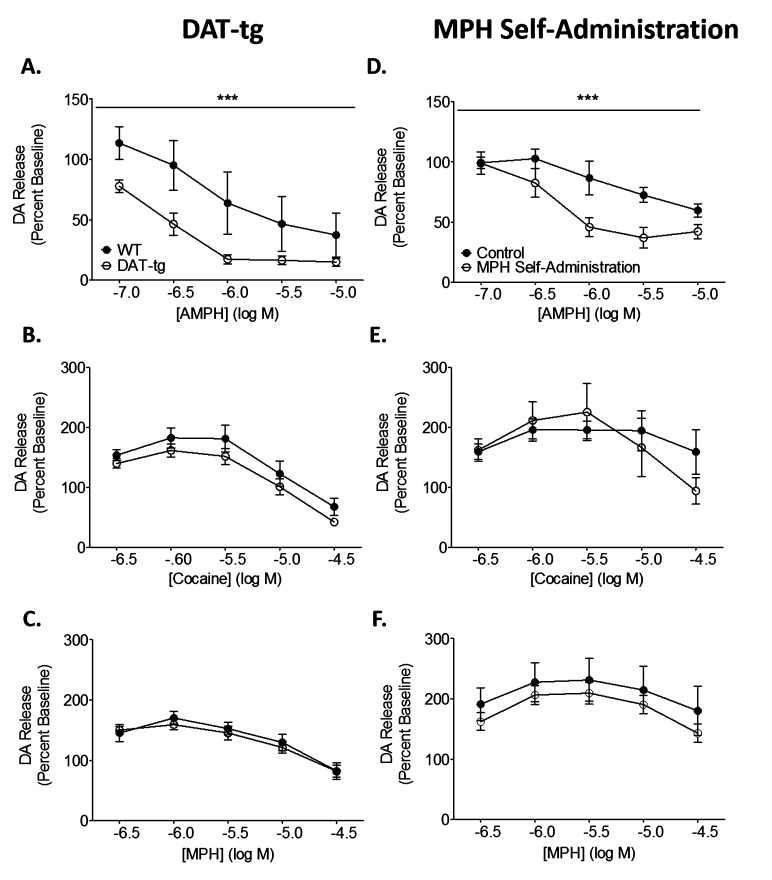
To assess how MPH effects at the dopamine terminal relate to those of traditional DA releasers we assessed the release profile of MPH. In voltammetric studies of electrically evoked dopamine dynamics, releasers have a distinct effect on dopamine release by incrementally decreasing the magnitude of stimulated release over increasing concentrations. Conversely, blockers produce an inverted “U” shaped curve, where lower concentrations increase stimulated release, but at higher concentrations release returns to predrug levels and then often fall below predrug levels. The release profile of MPH more closely resembled that of cocaine, another transporter blocker (Figure 4).
Figure 4.
Methylphenidate (MPH) has the release profile of a blocker. Full concentration–response curves for MPH (MPH, n = 11; DAT-tg, n = 10), amphetamine (AMPH; MPH, n = 9; DAT-tg, n = 9), and cocaine (MPH, n = 9; DAT-tg, n = 9) were run in DAT overexpressing mice (DAT-tg; left) and following MPH self-administration (right). (A) AMPH, a prototypical dopamine releaser, attenuated evoked release to a greater extent in DAT-tg mice. (B) Cocaine, a prototypical DAT blocker, resulted in an inverted “U” shape release curve. Release was not different between wild type and DAT-tg mice. (C) Evoked release in the presence of MPH was unchanged between groups, and also resulted in an inverted “U” shaped curve. (D) Following MPH self-administration, AMPH-induced reductions in release were exacerbated. (E) Evoked dopamine release in the presence of cocaine was unchanged between MPH self-administration and control groups. (F) The release profile of MPH was unchanged following MPH self-administration. ***p < 0.001.
Although DAT levels affected the potency of MPH in a similar manner to releasers, its release profile demonstrated that it was not a pure releaser (Figure 4). In the groups that had increased Vmax, the releaser AMPH was more efficacious at reducing evoked dopamine release (two-way ANOVA; DAT-tg, F1,40 = 14.79, p < 0.001; MPH self-administration, F1,39 = 16.32, p < 0.001). The increase in the ability of AMPH to reduce dopamine release is due to disruption of vesicular storage, and is most likely increased in animals with high DAT levels because AMPH is taken up through the DATs at a faster rate, accumulates to higher concentrations in the cytoplasm, and has increased access to intracellular compartments.15,16 This is supported by studies which have demonstrated that releasers, like AMPH, are more neurotoxic in scenarios where DAT function/levels are elevated, suggesting that these compounds are likely more potent in their neurotoxic activities due to increased intracellular accumulation.28,29 Because MPH is not transported into cells, it cannot interact with dopamine vesicles directly and deplete dopamine from terminals, an effect that is highlighted by the increase, not decrease, in dopamine release when MPH is applied to the brain slice. The effects of MPH are congruent with cocaine effects on stimulated dopamine release, whereby alterations in DAT levels in either the DAT-tg (Figure 4C, E) or MPH self-administration (Figure 4D, F) groups did not alter its release profile. This indicates that, although potency of MPH at the DAT responds similarly to a releaser in regard to changes in DAT levels, it is not functioning as a releaser. Presently, it is unclear as to how MPH can be affected by DAT levels in a manner similar to releasers in regard to uptake inhibition, without being a substrate for the DAT. One possible explanation is that the effects are related to its binding site on the DAT, where MPH and AMPH have been shown to exhibit some similarities10,11 and it is possible that MPH alters DAT conformation in the same manner or direction that releasers do, but not to the same extent. Another, related explanation might be that MPH’s releaser-like effects are highly dose-dependent, and it has the ability to release dopamine at high concentrations, which has been seen in cell culture studies, thus making it a blocker at lower concentrations and a releaser at higher concentrations.13,14
1.5. MPH Self-Administration Produces Unique Effects on the Dopamine System
One important finding of the current study is that drug-induced alterations in stimulated release (Figure 4) following MPH self-administration are similar to findings from previous work utilizing different self-administration paradigms.30 The distinction between previous work, utilizing intermittent access self-administration of MPH,30 and the current study, utilizing extended-access, is critical, as the temporal profile of brain drug levels can influence the neurochemical changes associated with cocaine self-administration. In fact, previous work from our group has shown that the intermittent access (used in previous studies) and extended-access (used in the current manuscript) self-administration can have opposite effects on the dopamine system, when cocaine is contingently administered.22
The fact that intermittent and extended access MPH self-administration causes similar dopaminergic adaptations is particularly relevant as it further delineates MPH as a unique psychostimulant that has effects that are divergent from other compounds of the same pharmacological class. Taken together, this work highlights the idea that MPH is a unique compound, both in its acute effects as well as the compensatory effects that occur following self-administration.
2. Conclusions
Here we show that Vmax for dopamine uptake positively affected the potency of MPH and AMPH, but not cocaine. These data are supported by previous studies showing enhanced effects of MPH and AMPH, but not cocaine, on locomotor activity and reinforcement.2,18 Although MPH is traditionally characterized as a DAT blocker, its mechanism of action is more complicated, with characteristics of both blockers and releasers. Changes in Vmax result in large increases in the potency of MPH, similar to releasers. Conversely, the release profile, as measured by stimulated dopamine release across a concentration–response curve for the compound, more closely mimics that of the blocker cocaine, indicating that MPH shares aspects of blockers and releasers. In addition, although Vmax and stimulated dopamine release are correlated, dopamine release was only correlated with MPH potency, indicating that the factor that is actually driving the differences in drug potency is the predrug uptake rate, which is controlled by DAT levels. These data indicate that MPH, a drug commonly used in the clinic, is a unique compound that has characteristics of both blockers and releasers and its effects at the DAT can be affected purely by variability in DAT levels.
The dopamine system is responsible for mediating the reward- and reinforcement-related effects of drugs, thus it is important to define the mechanisms by which the behavioral and neurochemical effects of psychostimulants are altered by differences in dopamine system functioning. These data demonstrate (1) a positive relationship between DAT levels and psychostimulant releaser, but not blocker effects on the dopamine system, and (2) because MPH is uniquely affected by dopamine changes that affect releasers, but still has the release profile of a blocker, it is difficult to make generalizations about potential effects of MPH use and abuse based on work performed on other drugs.
Further, this work defines the role of the DAT in the addictive potential of AMPHs by demonstrating that increased DAT expression robustly augments the effects of AMPH-like drugs on dopamine neurotransmission. Previous work has shown that these changes are also associated with drug seeking and reward/reinforcement.18 Thus, DAT levels may serve as a biomarker for addiction vulnerability. This could be clinically important as it identifies a subset of individuals who may be most sensitive to the positive reinforcing and addictive effects of stimulant drugs of abuse. Variability in human DAT expression has been observed in disorders such as attention deficit/hyperactivity disorder (ADHD), post-traumatic stress disorder (PTSD), and early life stress. For example, individuals suffering from ADHD, who are treated with AMPH and MPH, have elevated DAT levels that range from 17 to 70% higher than normal.28 Taken together, DAT levels are highly and positively correlated with the effects of psychostimulant releasers on the dopamine system and may be a novel predictor of increased substance use vulnerability in humans.
3. Methods
3.1. Animals
Male Sprague–Dawley rats (375–400 g; Harlan Laboratories, Frederick, MD) were used for all self-administration experiments. Rats were maintained on a 12:12 h reverse light-dark cycle (3:00 am lights off; 3:00 pm lights on) with food and water ad libitum. Eight to twelve animals were included per group. Transgenic DAT overexpressing mice were maintained on a 12:12 h light cycle with food and water ad libitum. Nine to ten animals were included per group. All animals were maintained according to the National Institutes of Health guidelines in Association for Assessment and Accreditation of Laboratory Animal Care accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Wake Forest University School of Medicine.
3.2. Self-Administration
The behavioral data from these animals was published previously.18,19 Rats were anesthetized and implanted with chronic indwelling jugular catheters and trained for i.v. self-administration as previously described.31,32 Following surgery, animals were singly housed. Self-administration sessions took place in the home cage during the active/dark cycle (9:00 am to 3:00 pm). Sessions were 6 h in length and were terminated after the animal responded for 40 injections of drug or at the end of the 6 h session. Animals self-administered MPH (0.56 mg/kg/injection over 4 s) on a fixed-ratio 1 schedule of administration.18 For self-administering animals, acquisition (day 1) was defined to be when the animal reached 35 or more responses, and animals normally reached acquisition criteria in 0–3 days. Following establishment of stable responding, the animals were allowed to self-administer 40 injections per day for a period of 5 consecutive days.
3.3. In Vitro Voltammetry
Fast scan cyclic voltammetry was used to characterize baseline dopamine system kinetics and the ability of psychostimulants to inhibit dopamine uptake in the NAc core, as previously described.33,34 Voltammetry experiments were conducted during the dark phase of the light cycle 24 h after commencement of the final self-administration session in rats. Voltammetry in mice were conducted at the same time point, however, mice were not exposed to any procedures. A vibrating tissue slicer was used to prepare 400 μm thick coronal brain sections containing the NAc core. The tissue was immersed in oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl (126), KCl (2.5), NaH2PO4 (1.2), CaCl2 (2.4), MgCl2 (1.2), NaHCO3,25 glucose,11 and l-ascorbic acid (0.4) and pH adjusted to 7.4. Once sliced, the tissue was transferred to the testing chambers containing bath aCSF (32 °C), which flowed at 1 mL/min. After a 30 min equilibration period, a cylindrical carbon fiber microelectrode (100–200 μM length, 7 μM radius) and a bipolar stimulating electrode were placed into the core of the NAc. Endogenous dopamine release was evoked by a single electrical pulse (350 μA, 4 ms, monophasic) applied to the tissue every 5 min. Extracellular dopamine was recorded by applying a triangular waveform (−0.4 to +1.2 to −0.4 V vs Ag/AgCl, 400 V/s). Once the extracellular dopamine response was stable for three consecutive stimulations (within 10% variability), cocaine (0.03–30 μmol/L), MPH (0.03–30 μmol/L), or AMPH (0.1–10 μmol/L) was applied cumulatively to the brain slice to determine the relationship between DAT levels and drug-induced uptake inhibition.
3.4. Data Analysis
Demon Voltammetry and Analysis Software was used for data acquisition and analysis.35 To evaluate the effects of drugs, evoked levels of dopamine were modeled using Michaelis–Menten kinetics as a balance between release and uptake.36 Michaelis–Menten modeling defines parameters that describe the amount of dopamine released following stimulation, the maximal rate of dopamine uptake (Vmax), and alterations in the ability of dopamine to bind to the DAT, or app. Km. For predrug modeling, we followed standard voltammetric modeling procedures by setting the baseline Km parameter to 160 nM based on the affinity of dopamine for the DAT,37 whereas Vmax values were allowed to vary as the predrug measure of the rate of dopamine uptake. Following drug application, app. Km was allowed to vary to account for changes in drug-induced dopamine uptake inhibition while the respective Vmax value determined for that subject at baseline was held constant. The app. Km parameter models the amount of dopamine uptake inhibition following a particular concentration of drug.
3.5. Statistics
Graph Pad Prism (version 5, La Jolla, CA) was used to statistically analyze data sets and create graphs. Data are presented as mean ± standard deviation and percentage unless otherwise stated. Correlational analyses were used to assess the association uptake rates (Vmax) and release with cocaine, AMPH, and MPH induced dopamine uptake inhibition. Pearson’s correlation coefficients were used to measure the strength of correlation between Vmax and release, Vmax and app. Km, and release and app. Km. Regression analysis was used to compare the extent to which Vmax altered app. Km between MPH, cocaine, and AMPH. All p values of <0.05 were considered to be statistically significant.
Glossary
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
- NAc
nucleus accumbens
- FSCV
fast scan cyclic voltammetry
- CPU
caudate putamen
- Vmax
maximal rate of uptake
- MPH
methylphenidate
- AMPH
amphetamine
Author Contributions
E.S.C. and M.J.F. conceptualized the experiments. E.S.C., M.J.F. collected the data. E.S.C., M.J.F., and C.A.S. analyzed the data and made figures. E.S.C., M.J.F., C.A.S., S.R.J. interpreted the data and prepared the manuscript.
This work was funded by National Institutes of Health Grants R01 DA021325, R01 DA030161, R01 DA014030, P50 DA006634 (S.R.J.), T32 DA007246 and F31 DA031533 (E.S.C.), K99 DA031791 (M.J.F.), and T32 AA007565 (C.A.S.).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Chen N.; Reith M. E. (2007) Substrates and inhibitors display different sensitivity to expression level of the dopamine transporter in heterologously expressing cells. J. Neurochem. 101(2), 377–388. [DOI] [PubMed] [Google Scholar]
- Salahpour A.; Ramsey A. J.; Medvedev I. O.; Kile B.; Sotnikova T. D.; Holmstrand E.; Ghisi V.; Nicholls P. J.; Wong L.; Murphy K.; Sesack S. R.; Wightman R. M.; Gainetdinov R. R.; Caron M. G. (2008) Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc. Natl. Acad. Sci. U. S. A. 105(11), 4405–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D. J.; Nelson A. M.; Mandt B. H.; Larson G. A.; Rorabaugh J. M.; Ng C. M.; Barcomb K. M.; Richards T. L.; Allen R. M.; Zahniser N. R. (2013) Rats classified as low or high cocaine locomotor responders: A unique model involving striatal dopamine transporters that predicts cocaine addiction-like behaviors. Neurosci. Biobehav. Rev. 37(8), 1738–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A.; Sorkin A.; Zahniser N. R. (2013) Mice expressing markedly reduced striatal dopamine transporters exhibit increased locomotor activity, dopamine uptake turnover rate, and cocaine responsiveness. Synapse 67(10), 668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A. M.; Larson G. A.; Zahniser N. R. (2009) Low or high cocaine responding rats differ in striatal extracellular dopamine levels and dopamine transporter number. J. Pharmacol. Exp. Ther. 331(3), 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.; Han D. D.; Gu H. H. (2005) A triple mutation in the second transmembrane domain of mouse dopamine transporter markedly decreases sensitivity to cocaine and methylphenidate. J. Neurochem. 94(2), 352–359. [DOI] [PubMed] [Google Scholar]
- Chen R.; Tilley M. R.; Wei H.; Zhou F.; Zhou F. M.; Ching S.; Quan N.; Stephens R. L.; Hill E. R.; Nottoli T.; Han D. D.; Gu H. H. (2006) Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc. Natl. Acad. Sci. U. S. A. 103(24), 9333–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley M. R.; Gu H. H. (2008) The effects of methylphenidate on knockin mice with a methylphenidate-resistant dopamine transporter. J. Pharmacol. Exp. Ther. 327(2), 554–560. [DOI] [PubMed] [Google Scholar]
- Ritz M. C.; Lamb R. J.; Goldberg S. R.; Kuhar M. J. (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237, 1219–1223. [DOI] [PubMed] [Google Scholar]
- Wayment H. K.; Deutsch H.; Schweri M. M.; Schenk J. O. (1999) Effects of methylphenidate analogues on phenethylamine substrates for the striatal dopamine transporter: Potential as amphetamine antagonists?. J. Neurochem. 72, 1266–1274. [DOI] [PubMed] [Google Scholar]
- Dar D. E.; Mayo C.; Uhl G. R. (2005) The interaction of methylphenidate and benztropine with the dopamine transporter is different than other substrates and ligands. Biochem. Pharmacol. 70, 461–469. [DOI] [PubMed] [Google Scholar]
- Sonders M. S.; Zhu S. J.; Zahniser N. R.; Kavanaugh M. P.; Amara S. G. (1997) Multiple ionic conductances of the human dopamine transporter: The actions of dopamine and psychostimulants. J. Neurosci. 17(3), 960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell V.; de Villiers A.; Sagvolden T.; Lamm M.; Taljaard J. (1998) Differences between electrically-, ritalin- and D-amphetamine-stimulated release of [3H]dopamine from brain slices suggest impaired vesicular storage of dopamine in an animal model of Attention-Deficit Hyperactivity Disorder. Behav. Brain Res. 94(1), 163–171. [DOI] [PubMed] [Google Scholar]
- Dyck L. E.; Boulton A. A.; Jones R. S. (1980) A comparison of the effects of methylphenidate and amphetamine on the simultaneous release of radiolabelled dopamine and p- or m-tyramine from rat striatal slices. Eur. J. Pharmacol. 68(1), 33–40. [DOI] [PubMed] [Google Scholar]
- Sulzer D. (2011) How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69(4), 628–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein A. E.; Volz T. J.; Riddle E. L.; Gibb J. W.; Hanson G. R. (2007) New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol. 47, 681–698. [DOI] [PubMed] [Google Scholar]
- Ferris M. J.; Calipari E. S.; Mateo Y.; Melchior J. R.; Roberts D. C.; Jones S. R. (2012) Cocaine self-administration produces pharmacodynamic tolerance: Differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate. Neuropsychopharmacology 37, 1708–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari E. S.; Ferris M. J.; Salahpour A.; Caron M. G.; Roberts D. C. S.; Jones S. R. (2013) Methylphenidate amplifies the potency and reinforcing effects of amphetamines by increasing dopamine transporter expression. Nat. Commun. 4, 2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari E. S.; Ferris M. J.; Melchior J. R.; Bermejo K.; Salahpour A.; Roberts D. C. (2012d) Methylphenidate and cocaine self-administration produce distinct dopamine terminal alterations. Addict. Biol. 19(2), 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris M. J.; Mateo Y.; Roberts D. C.; Jones S. R. (2011) Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration. Biol. Psychiatry 69(3), 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris M. J.; Calipari E. S.; Melchior J. R.; Roberts D. C.; España R. A.; Jones S. R. (2013) Paradoxical tolerance to cocaine after initial supersensitivity in drug-use-prone animals. Eur. J. Neurosci 38(4), 2628–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari E. S.; Siciliano C. A.; Zimmer B. A.; Jones S. R. (2014) Brief Intermittent Cocaine Self-Administration and Abstinence Sensitizes Cocaine Effects on the Dopamine Transporter and Increases Drug Seeking. Neuropsychopharmacology 10.1038/npp.2014.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov R. R.; Jones S. R.; Fumagalli F.; Wightman R. M.; Caron M. G. (1998) Re-evaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain Res. Rev. 26(2–3), 148–153. [DOI] [PubMed] [Google Scholar]
- Chadchankar H.; Ihalainen J.; Tanila H.; Yavich L. (2012) Methylphenidate modifies overflow and presynaptic compartmentalization of dopamine via an α-synuclein-dependent mechanism. J. Pharmacol. Exp. Ther. 341(2), 484–492. [DOI] [PubMed] [Google Scholar]
- Volz T. J.; Farnsworth S. J.; Hanson G. R.; Fleckenstein A. E. (2009) Methylphenidate-induced alterations in synaptic vesicle trafficking and activity: Functional consequences and therapeutic implications. Ann. N.Y. Acad. Sci. 1139, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz T. J.; Farnsworth S. J.; King J. L.; Riddle E. L.; Hanson G. R.; Fleckenstein A. E. (2007) Methylphenidate administration alters vesicular monoamine transporter-2 function in cytoplasmic and membrane-associated vesicles. J. Pharmacol Exp. Ther. 323(2), 738–745. [DOI] [PubMed] [Google Scholar]
- aMadras B. K.; Miller G. M.; Fischman A. J. (2005) The dopamine transporter and attention-deficit/hyperactivity disorder. Biol. Psychiatry 57, 1397–1409. [DOI] [PubMed] [Google Scholar]
- Xie T.; McCann U. D.; Kim S.; Yuan J.; Ricaurte G. A. (2000) Effect of temperature on dopamine transporter function and intracellular accumulation of methamphetamine: Implications for methamphetamine-induced dopaminergic neurotoxicity. J. Neurosci. 20(20), 7838–7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F.; Gainetdinov R. R.; Valenzano K. J.; Caron M. G. (1998) Role of dopamine transporter in methamphetamine-induced neurotoxicity: Evidence from mice lacking the transporter. J. Neurosci. 18(13), 4861–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari E. S.; Jones S. R. (2014) Sensitized nucleus accumbens dopamine terminal responses to methylphenidate and dopamine transporter releasers after intermittent-access self-administration. Neuropharmacology 82, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari E. S.; Beveridge T. J.; Jones S. R.; Porrino L. J. (2013) Withdrawal from extended access cocaine self-administration results in dysregulated functional activity and altered locomotor activity in rats. Eur. J. Neurosci. 38(12), 3749–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari E. S.; Ferris M. J.; Jones S. R. (2013) Extended Access Cocaine Self-Administration Results in Tolerance to the Dopamine-Elevating and Locomotor-Stimulating Effects of Cocaine. J. Neurochem. 128(2), 224–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano C. A.; Calipari E. S.; Ferris M. J.; Jones S. R. (2014) Biphasic mechanisms of amphetamine action at the dopamine terminal. J. Neurosci. 34(16), 5575–5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano C. A.; Calipari E. S.; Jones S. R. (2014) Amphetamine potency varies with dopamine uptake rate across striatal subregions. J. Neurochem. 131(3), 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason J. T.; España R. A.; Jones S. R. (2011) Demon voltammetry and analysis software: Analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J. Neurosci. Methods 202(2), 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R. M.; Amatore C.; Engstrom R. C.; Hale P. D.; Kristensen E. W.; Kuhr W. G. (1988) Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience 25, 513–523. [DOI] [PubMed] [Google Scholar]
- Wu Q.; Reith M. E. A.; Wightman R. M.; Kawagoe K. T.; Garris P. A. (2001) Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J. Neurosci. Methods 112, 119–133. [DOI] [PubMed] [Google Scholar]