Abstract
Backgrounds/Aims
Since most transplantation studies for alcoholic liver disease (ALD) were performed on deceased donor liver transplantation, little was known following living donor liver transplantation (LDLT).
Methods
The clinical outcome of 18 ALD patients who underwent LDLT from Febraury 1997 to December 2004 in a large-volume liver transplantation center was assessed retrospectively.
Results
The model for end-stage liver disease score was 23±11, and mean pretransplant abstinence period was 16±13 months, with 14 (77.8%) patients being abstinent for at least 6 months. Graft types were right lobe grafts in 11, left lobe grafts in 2 and dual grafts in 5. Graft to recipient body weight ratio was 0.94±0.16. The relapse rates in patients who did and did not maintain 6 months of abstinence were 7.1% and 50%, respectively (p=0.097). Younger recipient age was a significant risk factor for alcohol relapse (p=0.027). Five recipients with antibody to hepatitis B surface antigen (HBsAg) received core antibody-positive liver graft, but two of them showed positive HBsAg seroconversion. Overall 5-year patient survival rate following LDLT was 87.8%, with a 5-year relapse rate of 16.7%.
Conclusions
Pretransplant abstinence for 6 months appears to be benefical for preventing posttransplant relapse. Life-long prophylactic measure should be followed after use of anti-HBc-positive liver grafts regardless of hepatitis B viral marker status of the recipient.
Keywords: Living donor liver transplantation, Alcoholic liver disease, Relapse
INTRODUCTION
Alcoholic liver disease (ALD) has been one of the most common causes of end-stage liver disease in Western countries, resulting in very high mortality rate in patients with advanced alcoholic cirrhosis and 7.9 deaths per 100,000 individuals in the United States.1 As a result, alcoholic cirrhosis is one of the most common indications of liver transplantation in Western countries.
Since high prevalence of ALD and high volume of liver transplantation in Western countries, most clinical studies on liver transplantation for ALD have involved deceased donor liver transplantation (DDLT). Due to the shortage of deceased donor organs in Eastern countries, adult-to-adult living donor liver transplantation (LDLT) has been replacing DDLT since the late 1990s. However, ALD had not been regarded as an optimal indication of LDLT because of the self-inflicted nature of ALD and the risk of posttransplant relapse of alcohol abuse. On the other hand, special relationship between the recipient and living donor was considered to make some clinical latitude toward permitting LDLT in selected patients with pretransplant abstinence.
We have presented the importance of pretransplant abstinence before.2 In this study, we evaluated the long-term outcome of LDLT for ALD patients in a large-volume LDLT center.
METHODS
From February 1997 to December 2004, 789 adult-to-adult LDLTs were performed in our institution. Of these, 27 recipients were primarily diagnosed with ALD, but 9 were excluded due to concurrent association with viral hepatitis or other diseases. All 18 patients were confirmed as having ALD, by both a history of chronic alcohol intake and a profile of biochemical parameters. Alcoholic cirrhosis was later confirmed by histological examination of the explanted liver. The surviving patients were monitored until September 2007 or their death, and their medical records were retrospectively reviewed with special attention to the relapse incidents of alcohol abuse.
Indication criteria for liver transplantation consisted primarily of Child-Pugh class B or C and concurrent complications of portal hypertension, such as esophageal variceal bleeding, hepatic encephalopathy, ascites or hepatorenal syndrome.
Pretransplant workup for hepatocellular carcinoma included multidetector dynamic liver computed tomography (CT) positron emission tomography, radioisotope bone scan, and chest CT. Multidetector CT scan was repeated 1 week before LDLT to confirm the suitability of the donor liver with regard to HCC extent and hepatic vasculature.
An independence from alcohol was confirmed by psychiatric assessment. Six months of alcohol abstinence was requested before LDLT.
Surgical procedures for LDLT were described in detail elsewhere.3,4 The immunosuppression regimen was the same as for other indications of adult LDLT, including anti-interleukin 2 induction, calcineurin inhibitor, mycophenolate mofetil and corticosteroid.
Since it was not practical to define alcoholic relapse as consumption of more than a given amount of alcohol, alcohol relapse was arbitrarily defined as any consumption of alcohol. Surveillance of alcohol drinking has been performed routinely at follow-up during every outpatient visit.
Mean values with standard deviation and median values with ranges were used for numeric data. Independent t-test and Fisher's exact test were used for comparisons. Survival curves were estimated by the Kaplan-Meier method. A difference of p-value<0.05 was considered statistically significant.
RESULTS
Patient and donor demographics
The clinical profiles of the 18 patients are summarized in Table 1. Fifteen patients (83.3%) manifested complications associated with portal hypertension. All patients underwent LDLT within 3 months of enrollment for LDLT. Of the 18 patients, 4 (22.2%) did not meet the 6-month abstinence rule, but none of these underwent transplantation in urgent or emergency situations.
Table 1.
Demographic characteristics of the 18 alcoholic liver disease patients undergoing living donor liver transplantation
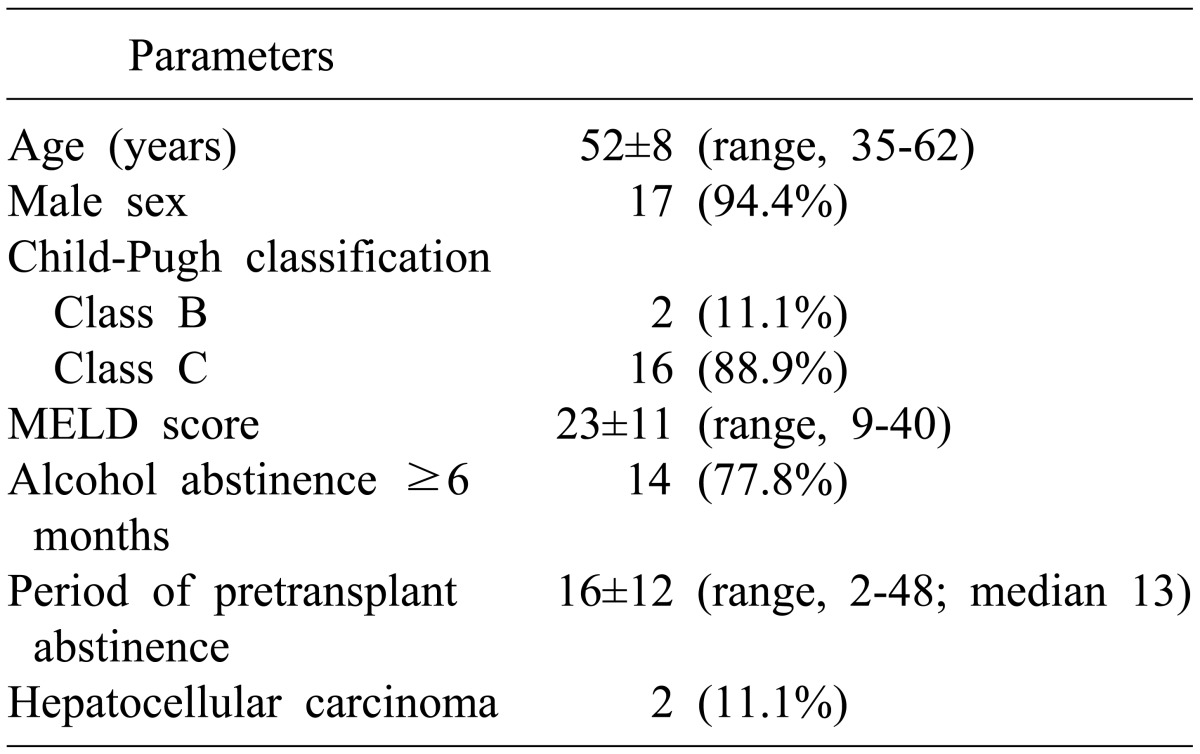
MELD, model for end-stage liver disease
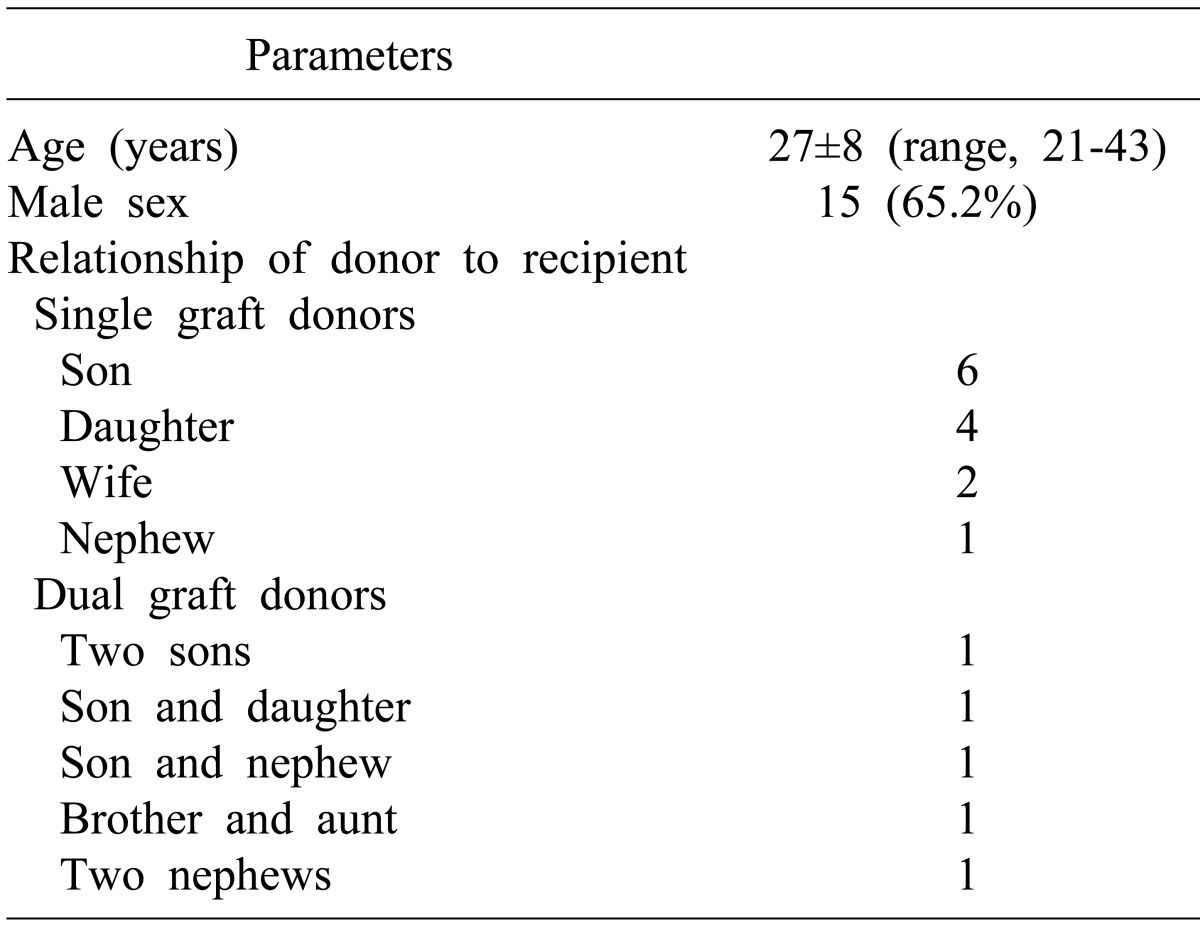
The demographic profiles of donors are summarized in Table 2. All living donors were consanguineously or maritally related to the recipients. There was no ABO blood group-incompatible donor. All of these donors recovered uneventfully after liver donation operations.
Table 2.
Demographic characteristics of living donors
Operation and surgical complications
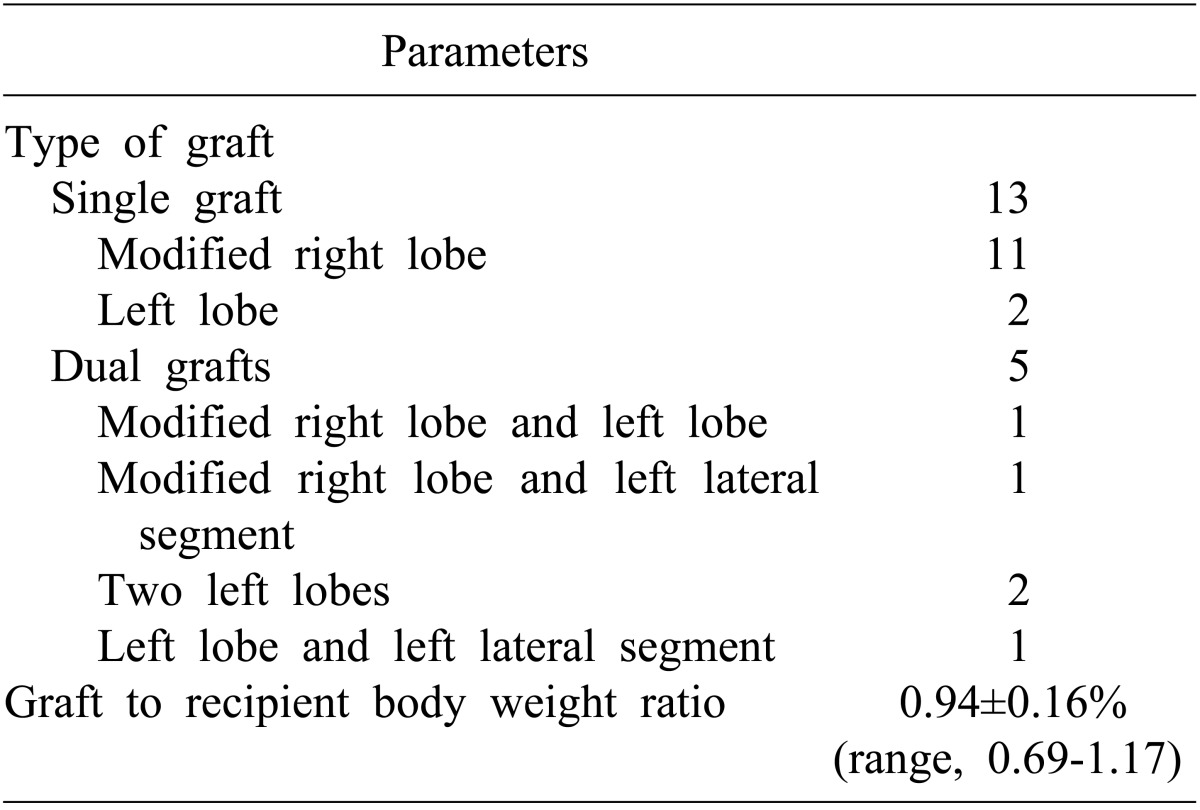
Thirteen patients (72.2%) received single grafts, and 5 (27.8%) received dual grafts (Table 3). Total graft weight to recipient body weight ratio was 0.94±0.16 (range, 0.69-1.17). All recipients recovered graft function uneventfully without perioperative mortality. During mean follow-up period of 57 months, biliary complications occurred in 6 patients (33.3%), all of whom were successfully treated by radiologic or endoscopic intervention. Within the first year, 3 patients experienced acute cellular rejection, which was successfully managed with steroid pulse therapy. Three years later, another patient suffered from an episode of acute cellular rejection. One patient who underwent dual-grafts implantation underwent hepatic vein embolization to facilitate unilateral graft atrophy due to intractable biliary complication.
Table 3.
Surgical profiles for living donor liver transplantation
Survival outcome
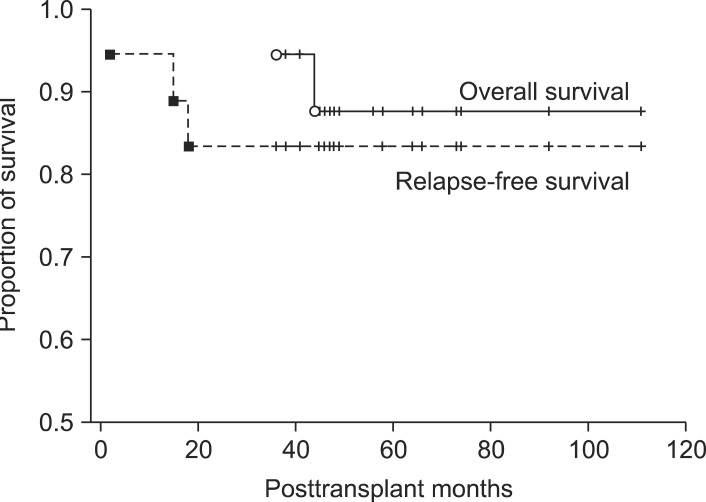
Patients were followed-up for 36-111 months (mean, 57±20 months; median, 49 months). One patient, who had experienced clinical complications requiring intensive care after alcohol relapse, died from aspergillus pneumonia 36 months after transplantation. Another patient who also showed alcohol relapse died from suspected intractable relapse. The other 16 patients remain alive to date, resulting in overall 5-year survival rate of 87.8% (Fig. 1).
Fig. 1.
Cumulative overall and alcohol relapse-free survival curves of 18 alcoholic liver disease patients who underwent living donor liver transplantation. Overall 1-year, 3-year and 5-year patient survival rates were 100%, 100% and 87.8%, respectively (solid line). Relapse-free 1-year, 3-year and 5-year survival rates were 95.4%, 83.3% and 83.3%, respectively (dotted line).
Two recipients who had concomitant hepatocellular carcinoma meeting the Milan criteria had no evidence of tumor recurrence over each 48 and 36 months. One patient was diagnosed of early gastric cancer 45 months after LDLT. He underwent resection and is doing well to date.
Alcohol relapse
Three recipients (16.7%) relapsed to alcohol abuse, after a mean of 9±8 months and a median of 15 months (Table 4). One out of the 14 patients (7.1%) who had been abstinent for at least 6 months relapsed, but 2 out of the 4 patients (50%) who had been abstinent for less than 6 months relapsed (p=0.097). In contrast, younger age of recipients was a significant risk factor for alcohol relapse (41±8 years versus 53±6 years; p=0.027).
Table 4.
Characteristics of the living donor liver recipients who showed relapse of alcohol abuse

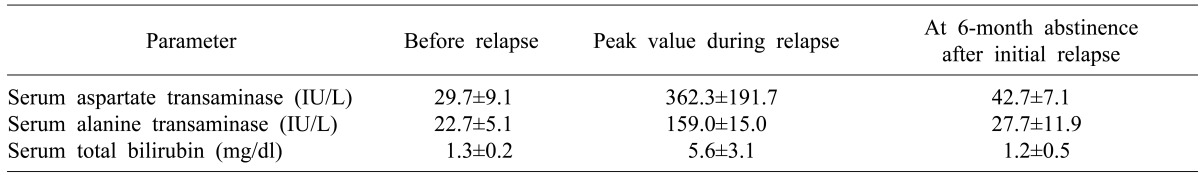
One-year and 3-year rates of alcohol relapse were 5.6% and 16.7%, respectively (Fig. 1). Two patients drank moderate amounts of alcohol for a while, but their liver function became unstable on occasion. Changes in their liver function around the time of alcohol relapse are summarized in Table 5.
Table 5.
Changes of graft liver function around the relapse timing of alcohol abuse in 3 relapsed recipients
After these periods of resumed drinking, one of these 3 patients did not become abstinent, leading to graft failure 44 months after LDLT. Another recipient start to drink at 2 months after LDLT, but he concealed it from us during monthly checkups. Five months after LDLT, this patient was admitted to hospital due to hepatorenal syndrome. After intensive care for 1 month, his liver function recovered progressively. However, he died from aspergillus pneumonia 36 months after LDLT (Fig. 2).
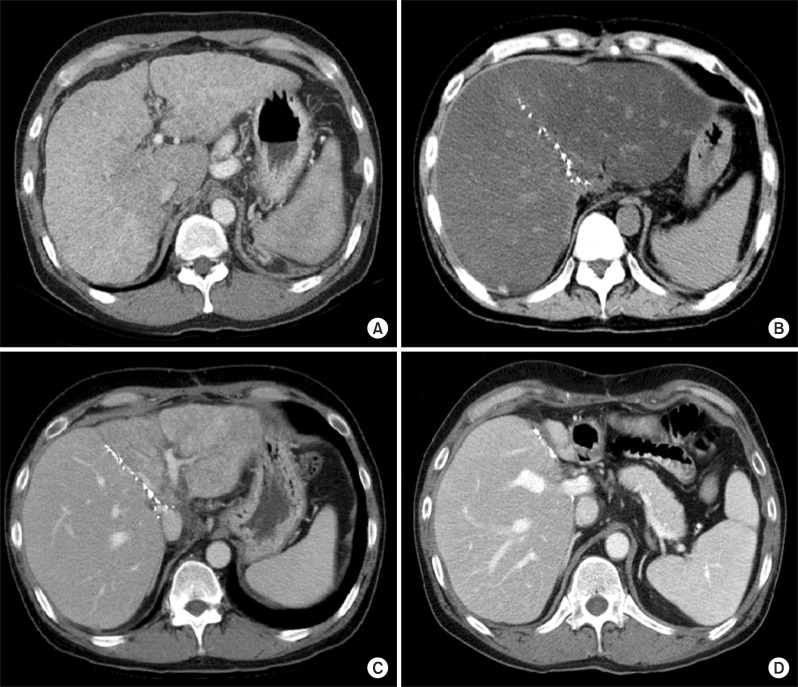
Fig. 2.
Computed tomography (CT) follow-up of a 35-year-old male patient who showed severe alcohol intake after living donor liver transplantation using dual liver grafts. (A) Preoperative CT scan showing cirrhotic changes. Model for end-stage liver disease score was 30. (B) 3 month CT scan showing marked steatosis at both of the dual grafts. Liver biopsy confirmed severe fatty change, which was a result of posttransplant alcohol abuse for 2-3 months. Hepatorenal syndrome occurred at 5 months posttransplant. Serum total bilirubin concentration exceeded 40 mg/dl. Acute renal failure was managed with continuous venovenous hemodiafiltration. (C) After 1 month of supportive care in the intensive care unit, the patient's liver function recovered progressively. This CT scan was taken at posttransplant 8 months. The right-sided graft looked normal, but the left-sided graft revealed marked atrophic change. (D) At 2 years after liver transplantation, the left-sided graft was completely atrophied, whereas the right-sided graft became hypertrophied. This patient died from aspergillus pneumonia 36 months after transplantation.
Prevention of de novo hepatitis B
Nine recipients received one or two liver grafts positive for hepatitis B core antibody (anti-HBc). Patients who received liver grafts from anti-HBc-positive donors were administered short-term prophylaxis for hepatitis B infection soon after LDLT, during the time of treatment with potent immunosuppressive drugs. Recipients positive for hepatitis B surface antibody (anti-HBs) were not administered long-term prophylactic regimens because we thought that the anti-HBs positivity in recipient per se would have a protective effect against de novo occurrence of hepatitis B infection. However, two out of the five anti-HBs-positive recipients who received anti-HBc-positive grafts showed seroconversion to hepatitis B surface antigen (HBsAg) positivity 6 and 8 months after LDLT respectively. These patients became HBsAg-seronegative soon after treatment with antiviral drugs. Afterwards, recipients of anti-HBc-positive liver grafts received high-dose immunoglobulin monotherapy to maintain serum anti-HBs titers greater than 500 IU/L, regardless of recipient's anti-HBs status. No subsequent instances of de novo hepatitis were observed in this series.
DISCUSSION
Although little is known about the optimal timing of liver transplantation in ALD patients, prognostic models such as MELD (model for end-stage liver disease) and Maddrey discriminant function are important tools for predicting short-term mortality of patients with severe ALD.5-10 MELD is especially useful for assessing mortality and guiding treatment decisions, particularly in ALD patients complicated by ascites and/or encephalopathy.5,9
Alcohol relapse is a potential problem in liver transplantation to ALD patients, in which it can induce liver damage and deteriorate patient compliance to medications and clinic visits.11 In practice, many liver transplant programs require 6 months of pretransplant abstinence prior to transplanation. However, many of these patients relapse to alcohol drinking, with some even returning to heavy alcohol consumption. However, there is little evidence showing that resumption of drinking has a significant detrimental effect on graft or patient survival.12 Posttransplant alcohol relapse in ALD patients does not have a significant effect on liver function or morphology, and the survival rate of these patients does not differ from that of patients who received DDLT for primary diseases other than ALD.13 Furthermore, the long-term survival rates of relapsed and non-relapsed ADL patients were similar, suggesting that alcohol relapse did not decrease compliance with immunosuppressant medications.14
It was shown that the liver function of patients who were abstinent after a period of alcohol relapse did not show additional deterioration. Fatty changes and pericellular fibrosis are the most relevant histological signs of heavy alcohol intake.13 It was seen that alcoholic hepatic steatosis became reversed in this series. Although the 10-year survival rate of ALD recipients who relapsed has been reported to be significantly lower than that of individuals who remained abstinent, this decrease was not related to graft evolution, rejection, infection, or metabolic disturbance, but rather to a higher incidence of malignancies and cardiovascular events.14
There are few reliable risk factors for alcohol relapse in ALD patients after liver transplantation. It has been suggested that the characteristics of pretransplant alcohol consumption, such as length of abstinence, alcohol dependence, alcohol abuse in first degree relatives and younger age, are useful for detecting patients at high risk of severe posttransplant alcohol relapse.1,15,16 The results of this study show a close relation between younger age and risk of relapse.
Abstinence for less than 6 months prior to liver transplantation may predict relapse and is widely employed as a criterion for listing for DDLT.17 The result of this study did not show that abstinence for 6 months before LDLT was a statistically significant risk factor. However, the relaspe rate of 50% in the recipients who did not fulfill the 6-month abstistinence rule was definitely high comparing with 7.1% of recipients who met this rule. If the sample number had been a little larger than that in this study, there would be a statistically significant difference. We think that it is reasonable to emphasize the need to observe the 6-month abstistinence rule in ALD patients undergoing LDLT.18-20
Early recognition of alcohol relapse during clinic visits is important for its effective management. Since relapse is considered shameful, relapsed patients are not willing to admit to it during clinic visits. In one patient with severe steatosis, we initially failed to suspect alcohol relapse after LDLT due to a preconceived notion regarding the recipient-donor relationship. Reliable objective tests for monitoring abstinence are required. Standard liver function tests are not helpful as they are non-specific, but definite deterioration of liver profiles may suggest that the patient has resumed drinking more than a moderate amount. Assays of carbohydrate-deficient transferrin may be useful, because its concentration remains constant over weeks, comparable to that of hemoglobin A1c in diabetic patients.21 This parameter, however, is not regarded as reliable for pretransplant screening of ALD patient candidates. Measurements of alcohol in the breath, blood or urine have a limited role at best, because relapsed patients avoid any alcohol consumption in the few days before routine checkups.
When considering the ethical aspects of live organ donation, it is clearly reasonable to exclude recipient candidates who have a considerable risk for alcohol relapse. In contrast to DDLT, LDLT has unique aspects regarding donor-recipient relationships. A very close relationship between the living donor and the recipient may be crucial in preventing alcohol relapse or at least a return to heavy drinking. Our 5-year relapse rate of 16.7% is lower than in most studies with DDLT.
The high prevalence of viral hepatitis B in the general population in Korea has resulted in a high prevalence of anti-HBc positivity in living donors. This anti-HBc positivity can induce de novo hepatitis B in recipients positive for anti-HBs-positive and in hepatitis B-naive recipients.22 Thus, a prophylactic regimen is mandatory to prevent de novo hepatitis in recipients.2,23,24 In our institution, high-dose hepatitis B immunoglobulin has been used prophylactically in these patients.
In conclusion, it was shown that 6 months of abstinence prior to LDLT appear to be benefical for prevention of posttransplant alcohol relapse. Overall 5-year patient survival rate following LDLT was 87.8% in this series, with a 5-year relapse rate of 16.7%. Life-long prophylactic measure should be followed after use of anti-HBc-positive liver grafts regardless of hepatitis B viral marker status of the recipient.
References
- 1.Pageaux GP, Michel J, Coste V, et al. Alcoholic cirrhosis is a good indication for liver transplantation, even for cases of recidivism. Gut. 1999;45:421–426. doi: 10.1136/gut.45.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang S, Lee SG, Kim KK, et al. Efficacy of 6-month pretransplant abstinence for patients with alcoholic liver disease undergoing living donor liver transplantation. Transplant Proc. 2006;38:2937–2940. doi: 10.1016/j.transproceed.2006.08.139. [DOI] [PubMed] [Google Scholar]
- 3.Lee SG, Park KM, Hwang S, et al. Adult-to-adult living donor liver transplantation at the Asan Medical Center, Korea. Asian J Surg. 2002;25:277–284. doi: 10.1016/S1015-9584(09)60192-5. [DOI] [PubMed] [Google Scholar]
- 4.Hwang S, Lee SG, Lee YJ, et al. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl. 2006;12:920–927. doi: 10.1002/lt.20734. [DOI] [PubMed] [Google Scholar]
- 5.Mathurin P. Is alcoholic hepatitis an indication for transplantation? Current management and outcomes. Liver Transpl. 2005;(11 Suppl 2):S21–S24. doi: 10.1002/lt.20601. [DOI] [PubMed] [Google Scholar]
- 6.Veldt BJ, Lainé F, Guillygomarc'h A, et al. Indication of liver transplantation in severe alcoholic liver cirrhosis: quantitative evaluation and optimal timing. J Hepatol. 2002;36:93–98. doi: 10.1016/s0168-8278(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 7.Maddrey WC, Boitnott JK, Bedine MS, et al. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- 8.Mathurin P, Mendenhall CL, Carithers RL, Jr, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36:480–487. doi: 10.1016/s0168-8278(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 9.Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353–358. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 10.Burra P, Lucey MR. Liver transplantation in alcoholic patients. Transpl Int. 2005;18:491–498. doi: 10.1111/j.1432-2277.2005.00079.x. [DOI] [PubMed] [Google Scholar]
- 11.Mackie J, Groves K, Hoyle A, et al. Orthotopic liver transplantation for alcoholic liver disease: a retrospective analysis of survival, recidivism, and risk factors predisposing to recidivism. Liver Transpl. 2001;7:418–427. doi: 10.1053/jlts.2001.23789. [DOI] [PubMed] [Google Scholar]
- 12.Lim JK, Keeffe EB. Liver transplantation for alcoholic liver disease: current concepts and length of sobriety. Liver Transpl. 2004;10(10 Suppl 2):S31–S38. doi: 10.1002/lt.20267. [DOI] [PubMed] [Google Scholar]
- 13.Burra P, Mioni D, Cecchetto A, et al. Histological features after liver transplantation in alcoholic cirrhotics. J Hepatol. 2001;34:716–722. doi: 10.1016/s0168-8278(01)00002-2. [DOI] [PubMed] [Google Scholar]
- 14.Cuadrado A, Fábrega E, Casafont F, et al. Alcohol recidivism impairs long-term patient survival after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl. 2005;11:420–426. doi: 10.1002/lt.20386. [DOI] [PubMed] [Google Scholar]
- 15.Jauhar S, Talwalkar JA, Schneekloth T, et al. Analysis of factors that predict alcohol relapse following liver transplantation. Liver Transpl. 2004;10:408–411. doi: 10.1002/lt.20086. [DOI] [PubMed] [Google Scholar]
- 16.Perney P, Bismuth M, Sigaud H, et al. Are preoperative patterns of alcohol consumption predictive of relapse after liver transplantation for alcoholic liver disease? Transpl Int. 2005;18:1292–1297. doi: 10.1111/j.1432-2277.2005.00208.x. [DOI] [PubMed] [Google Scholar]
- 17.Miguet M, Monnet E, Vanlemmens C, et al. Predictive factors of alcohol relapse after orthotopic liver transplantation for alcoholic liver disease. Gastroenterol Clin Biol. 2004;28:845–851. doi: 10.1016/s0399-8320(04)95146-9. [DOI] [PubMed] [Google Scholar]
- 18.Williams RS, Alisa AA, Karani JB, et al. Adult-to-adult living donor liver transplant: UK experience. Eur J Gastroenterol Hepatol. 2003;15:7–14. doi: 10.1097/00042737-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Testa G, Malagó M, Nadalin S, et al. Right-liver living donor transplantation for decompensated end-stage liver disease. Liver Transpl. 2002;8:340–346. doi: 10.1053/jlts.2002.32941. [DOI] [PubMed] [Google Scholar]
- 20.Kasahara M, Takada Y, Fujimoto Y, et al. Impact of right lobe with middle hepatic vein graft in living-donor liver transplantation. Am J Transplant. 2005;5:1339–1346. doi: 10.1111/j.1600-6143.2005.00817.x. [DOI] [PubMed] [Google Scholar]
- 21.Berlakovich GA, Windhager T, Freundorfer E, et al. Carbohydrate deficient transferrin for detection of alcohol relapse after orthotopic liver transplantation for alcoholic cirrhosis. Transplantation. 1999;67:1231–1235. doi: 10.1097/00007890-199905150-00006. [DOI] [PubMed] [Google Scholar]
- 22.Markowitz JS, Martin P, Conrad AJ, et al. Prophylaxis against hepatitis B recurrence following liver transplantation using combination lamivudine and hepatitis B immune globulin. Hepatology. 1998;28:585–589. doi: 10.1002/hep.510280241. [DOI] [PubMed] [Google Scholar]
- 23.Takemura N, Sugawara Y, Tamura S, et al. Liver transplantation using hepatitis B core antibody-positive grafts: review and university of Tokyo experience. Dig Dis Sci. 2007;52:2472–2477. doi: 10.1007/s10620-006-9656-5. [DOI] [PubMed] [Google Scholar]
- 24.Prakoso E, Strasser SI, Koorey DJ, et al. Long-term lamivudine monotherapy prevents development of hepatitis B virus infection in hepatitis B surface-antigen negative liver transplant recipients from hepatitis B core-antibody-positive donors. Clin Transplant. 2006;20:369–373. doi: 10.1111/j.1399-0012.2006.00495.x. [DOI] [PubMed] [Google Scholar]