Abstract
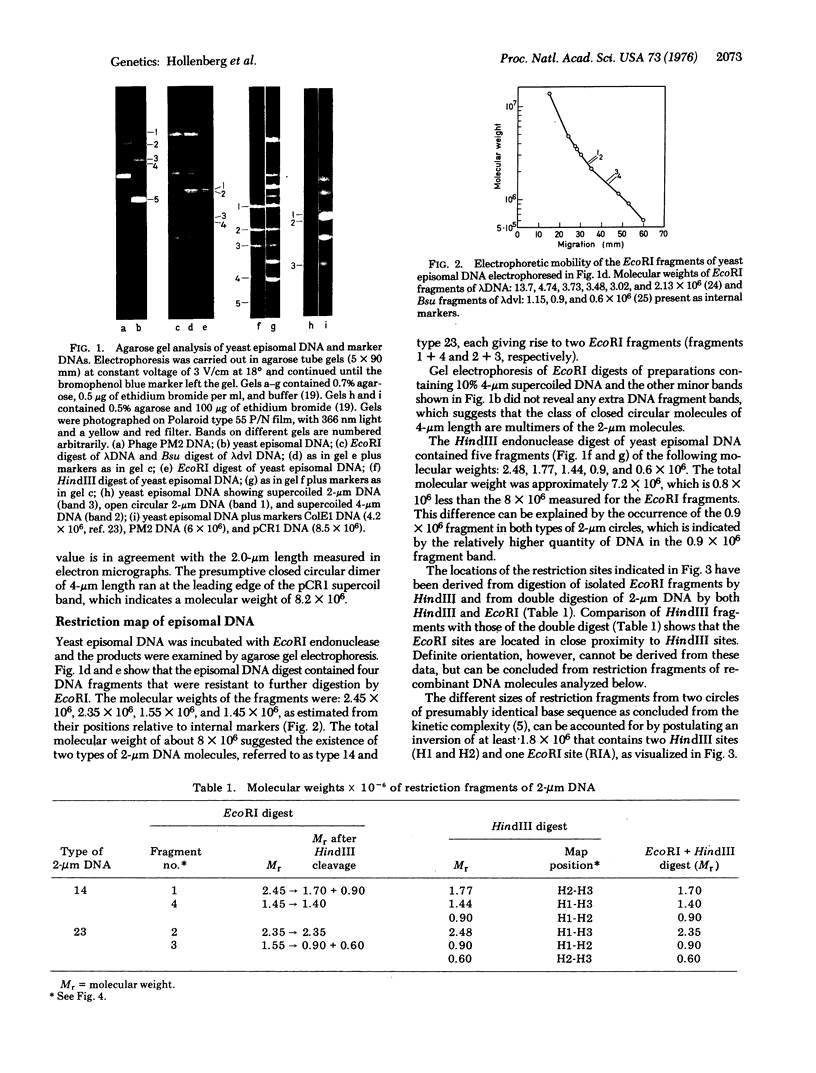
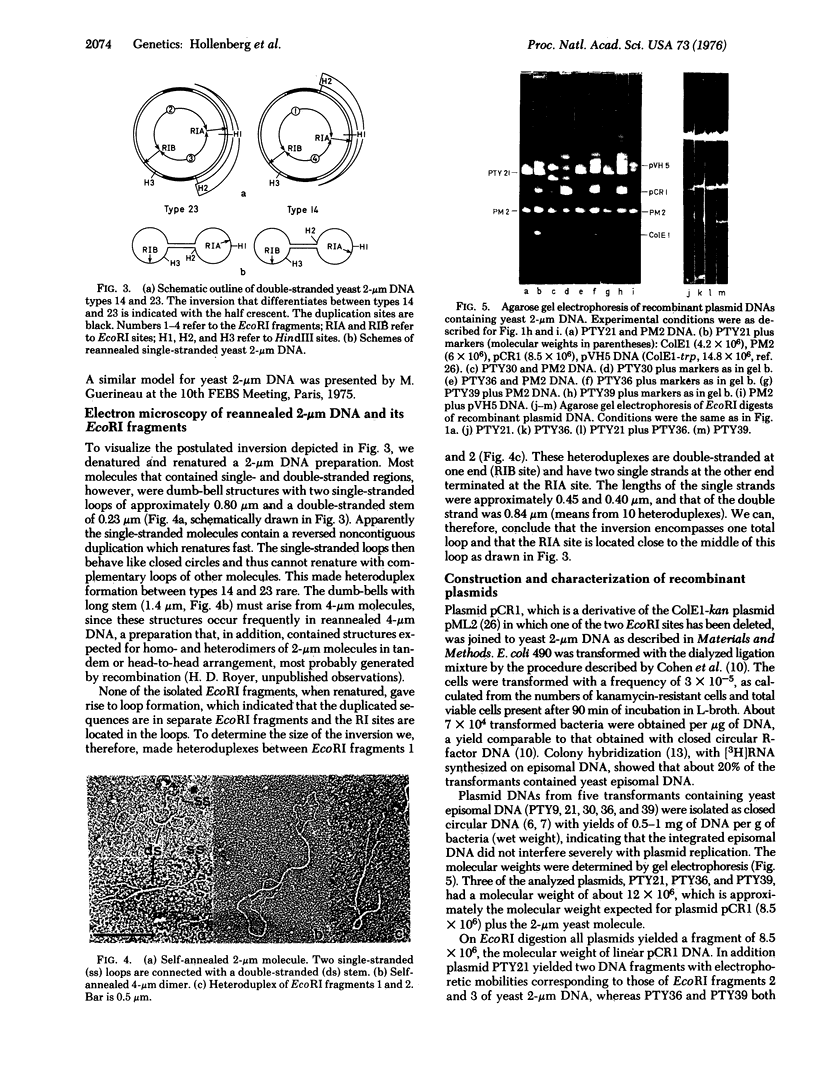
Electrophoretic analysis of EcoRI and HindIII restriction fragments of 2-mum supercoiled DNA of Saccharomyces cerevisiae indicated that this class of DNA is heterogeneous and probably consists of two types of molecules. Integration of the 2-mum yeast DNA in E. coli plasmid pCR1 directly showed that existence of two types of molecules as each of these could be individually inserted into separate bacterial plasmids. The difference between the two types of 2-mum circles is due to an inversion of about 1.6 X 10(6) daltons. The inversion is flanked by a reversed duplicated sequence of 0.45 X 10(6) daltons. Possible implications of this structure are dicussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaij C., Borst P. The gel electrophoresis of DNA. Biochim Biophys Acta. 1972 May 10;269(2):192–200. doi: 10.1016/0005-2787(72)90426-1. [DOI] [PubMed] [Google Scholar]
- Bak A. L., Christiansen C., Christiansen G. Circular, repetitive DNA in yeast. Biochim Biophys Acta. 1972 May 29;269(3):527–530. doi: 10.1016/0005-2787(72)90144-x. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Bernardi G., Faures M., Piperno G., Slonimski P. P. Mitochondrial DNA's from respiratory-sufficient and cytoplasmic respiratory-deficient mutant yeast. J Mol Biol. 1970 Feb 28;48(1):23–42. doi: 10.1016/0022-2836(70)90216-0. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Clark-Walker G. D., Miklos G. L. Localization and quantification of circular DNA in yeast. Eur J Biochem. 1974 Jan 16;41(2):359–365. doi: 10.1111/j.1432-1033.1974.tb03278.x. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D. Size distribution of circular DNA from petite-mutant yeast lacking rho DNA. Eur J Biochem. 1973 Jan 15;32(2):263–267. doi: 10.1111/j.1432-1033.1973.tb02606.x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. Replication of bacteriophage PM2 deoxyribonucleic acid: a closed circular double-stranded molecule. J Mol Biol. 1971 Mar 28;56(3):597–621. doi: 10.1016/0022-2836(71)90404-9. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerineau M., Grandchamp C., Paoletti C., Slonimski P. Characterization of a new class of circular DNA molecules in yeast. Biochem Biophys Res Commun. 1971 Feb 5;42(3):550–557. doi: 10.1016/0006-291x(71)90406-2. [DOI] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg C. P., Borst P., van Bruggen E. F. Mitochondrial DNA. V. A 25 micron closed circular duplex DNA molecule in wild-type yeast mitochondria. Stucture and genetic complexity. Biochim Biophys Acta. 1970 May 21;209(1):1–15. [PubMed] [Google Scholar]
- Inman R. B., Schnös M. Partial denaturation of thymine- and 5-bromouracil-containing lambda DNA in alkali. J Mol Biol. 1970 Apr 14;49(1):93–98. doi: 10.1016/0022-2836(70)90378-5. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Chan R. K., Tye B. K., Botstein D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J Mol Biol. 1975 Oct 5;97(4):561–575. doi: 10.1016/s0022-2836(75)80059-3. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Petes T. D., Williamson D. H. Replicating circular DNA molecules in yeast. Cell. 1975 Mar;4(3):249–253. doi: 10.1016/0092-8674(75)90172-5. [DOI] [PubMed] [Google Scholar]
- RADDING C. M., KAISER A. D. GENE TRANSFER BY BROKEN MOLECULES OF LAMBDA-DNA: ACTIVITY OF THE LEFT HALF-MOLECULE. J Mol Biol. 1963 Sep;7:225–233. doi: 10.1016/s0022-2836(63)80002-9. [DOI] [PubMed] [Google Scholar]
- Randerath K. An evaluation of film detection methods for weak beta-emitters, particularly tritium. Anal Biochem. 1970 Mar;34:188–205. doi: 10.1016/0003-2697(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Sinclair J. H., Stevens B. J., Sanghavi P., Rabinowitz M. Mitochondrial-satellite and circular DNA filaments in yeast. Science. 1967 Jun 2;156(3779):1234–1237. doi: 10.1126/science.156.3779.1234. [DOI] [PubMed] [Google Scholar]
- Streeck R. E., Hobom G. Mapping of cleavage sites for restriction endonucleases in lambdadv plasmids. Eur J Biochem. 1975 Sep 15;57(2):595–606. doi: 10.1111/j.1432-1033.1975.tb02335.x. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B., Schnös M., Inman R. Construction and characterization of a chimeric plasmid composed of DNA Pfrom Escherichia coli and Drosophila melanogaster. Biochemistry. 1975 May 20;14(10):2064–2072. doi: 10.1021/bi00681a005. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]