Abstract
Herein, we present a case of coexisting neuroendocrine carcinoma and conventional adenocarcinoma (collision tumor) in the ampulla of Vater, which has seldom been reported in the literature. A 51-year-old man presented with a month history of jaundice. MRCP disclosed about 1.9×1.8 cm sized heterogeneously enhancing mass in ampulla of Vater, causing obstructions of distal common bile duct. He underwent pylorus-preserving pancreaticoduodenectomy under the diagnosis on ampulla of Vater cancer. Pathologically, sections on the ampulla of Vater showed conventional ductal adenocarcinoma extended and collided with poorly differentiated neuroendocrine carcinoma. In conclusion, we hereby presented a case of coexisting neuroendocrine carcinoma and conventional adenocarcinoma in the ampulla of Vater.
Keywords: Collision tumor, Ampulla of Vater, Neuroendocrine carcinoma, Adenocarcinoma
INTRODUCTION
Collision cancers are malignancies in the same organ or anatomical site that comprises at least two different tumor components, with no mixed or transitional area between the two components. Collision cancers are very rare in the ampulla of Vater. And, it is known that the prognosis of collision cancer is very poor even after radical resection. Herein, we present a case of coexisting neuroendocrine carcinoma and conventional adenocarcinoma (collision tumor) in the ampulla of Vater, which has seldom been reported in literature.
CASE
A 51-year-old man was presented with a month history of jaundice, pruritis and choluria. He had no history on medical problems, but was paraplegic due to his car accident. Physical examination showed acutely ill appearances without fever, with generalized skin and mucosal jaundice also being observed, but otherwise, his overall symptoms were unremarkable.
In laboratory findings, we found elevated serum levels of bilirubin (3.38 mg/dl), alkaline phosphatase (229 U/L), gamma-glutamyl transpeptidase (843 U/L), alanine aminotransferase (389 U/L), and aspartate aminotransferase (602 U/L). There were no leukocytosis, and viral hepatitis markers were normal. All tumor markers, including serum carcino-embryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) were within normal limits.
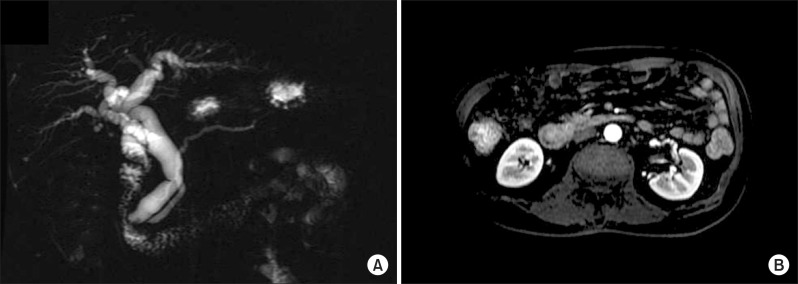
Abdominal computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP) disclosed about 1.9×1.8 cm sized heterogeneously enhancing mass in ampulla of Vater, causing obstruction of distal common bile duct, and dilatation of biliary tree and Wirsung duct. No remarkable enlarged lymph nodes were seen in the abdomen (Fig. 1). With the clinical diagnosis on tumor of the ampulla of Vater, endoscopic retrograde cholangiopancreatography (ERCP) was performed, which revealed huge prominent papilla and dilated common bile duct with asymmetrically irregular structures (Fig. 2). Biopsy was also performed under ERCP, and poorly differentiated adenocarcinoma was noted.
Fig. 1.
Radiologic findings. (A) Magnetic resonance cholangiogram indicated about 1.9×1.8 cm sized heterogeneously enhancing mass in ampulla of Vater, that led to obstructions of distal common bile duct, and dilatation of biliary tree and Wirsung duct. (B) There were no remarkable enlarged lymph nodes on scanned abdomen.
Fig. 2.
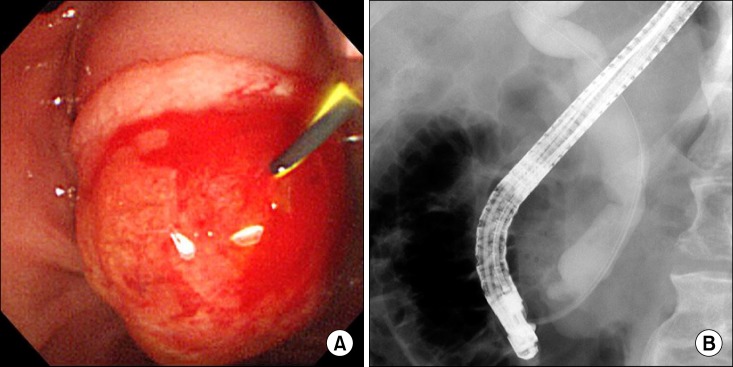
ERCP findings. (A) Huge and prominent papilla was noted on ERCP. (B) ERCP cholangiogram also revealed dilated common bile ducts with asymmetrical irregular strictures.
Subsequently, the patient was referred to our surgery department. He underwent pylorus-preserving pancreaticoduodenectomy under the diagnosis of ampulla of Vater cancer. In operation finding, there was about 2×1.5 cm sized hard mass on ampulla of Vater. Several enlarged lymph nodes were found around periampullary region, and all of them were cleared.
The patient suffered from delayed gastric emptying during his recovery, probably because he was paraplegic and bed-ridden. He recovered from delayed gastric emptying in 50 days after surgery, and was discharged on postoperative day 59. After discharge, he was re-admitted due to ongoing nausea, and was later expired due to tumor lysis syndrome on postoperative day 90.
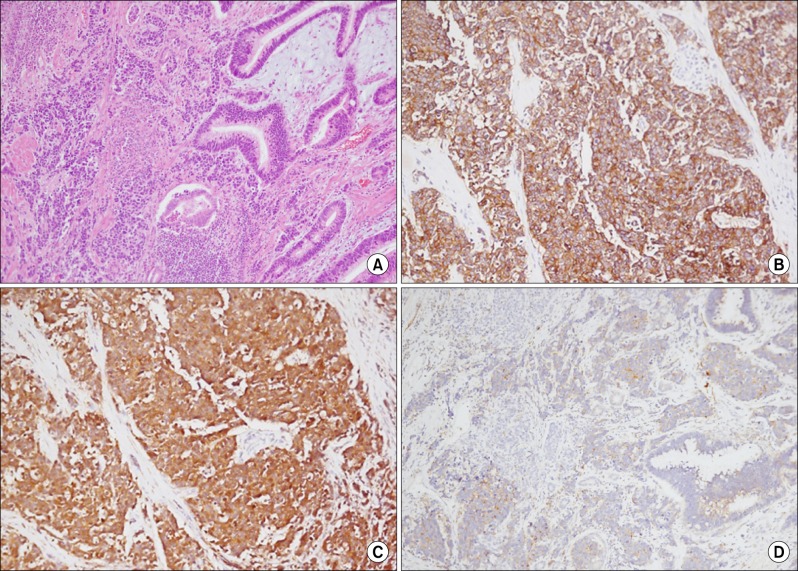
Pathologically, routine haematoxylin and eosin stains through the sections on the ampulla of Vater showed multiple nests of tumor cells displaying a prominent organoid pattern of growth and forming rosettes. The tumor cells were large and had a moderate amount of eosinophilic cytoplasm, a low nuclear to cytoplasmic ratio, and round or oval nuclei of various sizes. The nuclei were vesicular and contained coarsely granular chromatin and prominent nucleoli. The tumor cells were immunoreactive for CD56, neuron specific enolase (NSE) and synaptophysin. These findings led to diagnosis of poorly differentiated neuroendocrine carcinoma. In addition, conventional ductal adenocarcinoma composed of infiltrating glands was seen. In this area, the immunochemical stain with CD56, NSE and synaptophysin was negative, and this area extended and collided with poorly differentiated neuroendocrine carcinoma (Fig. 3). Thus, we finally diagnosed the condition as 'collision tumor' of adenocarcinoma and neuroendocrine carcinoma in ampulla of Vater.
Fig. 3.
Microscopic findings. (A) Collision site shows abrupt morphologic alterations between usual adenocarcinoma and poorly differentiated endocrine carcinoma (hematoxylin & eosin, ×100). (B, C and D) In immunohistochemical staining, tumor cells of the poorly differentiated neuroendocrine carcinoma are immunoreactive for CD56, neuron specific enolase (NSE), and synaptophysin, respectively.
DISCUSSION
The majority of neuroendocrine tumor (NET) occurs in the GI tract (67.5%) and in the bronchopulmonary system (25.3%). Within the GI tract, most NETs occur in the small intestine (41.8%), rectum (27.4%), and stomach (8.7%).1 Less than 1% of NETs occur in the ampulla of Vater.2
The WHO classification published in 2010 fundamentally divides NET into 2 clinically distinct pathologic classes: well- and poorly differentiated.3 Well-differentiated NETs can be classified as either low or intermediate grade. Well-differentiated grade 1 and grade 2 NETs traditionally have been referred to as carcinoids, regardless of grade or site of origin. Poorly differentiated NETs are high-grade, large cell and small cell carcinomas are characterized by rapid dissemination, resistance to treatment, and have a highly fatal course.
NETs are rarely diagnosed before they metastasize. At diagnosis, disease extent varies according to location of the primary tumor.4 Patients with distant metastases (well- and moderately differentiated) have a median survival of 33 months.4 The median survival of patients with distant metastases who have anaplastic NETs is only 5 months.4 Across all types of NETs, prognosis seems to be dependent on both the histology and extent of the disease. Well-differentiated grade 1 NETs are generally associated with less-aggressive behaviors, grade 2 NETs have a more variable clinical course, and poorly differentiated grade 3 NETs are characterized by extremely aggressive tumor biology and poor prognosis.5
Simultaneous coexistence of different tumor is assorted into two characteristics: collision tumor and composite tumor.6 A collision tumor is defined as the simultaneous coexistence of at least two independent tumors located in close proximity with no mixed or transitional areas in between. Nevertheless, composite tumor is formed by two or more neoplastic tissues with different histological characteristics developed from the same histological origin, as shown by transition area.7,8 Collision tumors have occasionally been reported in the past, but recently, there has been an increasing number of emerging reports. The overall prognosis of collision tumor around the periampullary region varies from its differentiation, but malignant collision tumor has dismal prognosis even after the radical resection. This means that the malignant collision tumor is more malignant than the adenocarcinoma of periampullary region.
Collision tumors can be found in any parts of the human body. Stomach and esophagus are the main sites of origin for collision tumor in the gastrointestinal tract. Adenocarcinomas and other malignancies such as squamous cell carcinomas, carcinoid and neuroendocrine tumors are usually situated in these areas.9-11 However, occurrences of collision tumors in pancreas and periampullary regions are very rare. Only few reports on occurrences of collision tumors in periampullary regions have been published in English literature.2,8,12-18
Preoperative diagnosis of collision tumors is difficult due to its lack of specific symptoms and radiologic features. In addition, serum levels of tumor markers such as CEA and CA 19-9 are not significantly different from the adenocarcinoma patients. Although it is difficult to diagnose collision tumors preoperatively, radical resection of tumor is still the reasonable treatment choice for resectable tumors. Nevertheless, some studies insisted that it is debatable whether surgical resection is a proper treatment, because of its dismal prognosis.19 Chemotherapy using gemcitabine has been widely used for periampullary malignancies clinically, but there is no proven efficacy for collision tumors in periampullary region.19 This is probably due to the highly malignant nature of collision tumors and some unknown oncological features of collision tumor.
The prognosis of the patient in our report was poor. Generally, neuroendocrine tumor has higher invasiveness and poorer prognosis than adenocarcinoma in collision tumor for ampulla of Vater.20 Therefore, it is reasonable to treat more aggressive tumors in collision tumors, such as the neuroendocrine tumor in this case. According to the WHO complementary notes on TNM staging of malignant tumors, if more than one tumor co-exists in one organ, the staging of the tumors should be referred to lesions of a relatively later stage.
In conclusion, we presented a case of coexisting neuroendocrine carcinoma and conventional adenocarcinoma in the ampulla of Vater. Such cases are seldom reported in literature. Thus, principle therapeutic modalities are not available, such as the extent of resection, off-the-shelf chemo-regimen, and adjuvant therapeutic tools. Further investigations are required to determine the tumorigenesis of collision tumor, and to generate principle therapeutic modalities of collision tumor in ampulla of Vater.
References
- 1.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 2.Sato K, Waseda R, Tatsuzawa Y, et al. Composite large cell neuroendocrine carcinoma and adenocarcinoma of the common bile duct. J Clin Pathol. 2006;59:105–107. doi: 10.1136/jcp.2005.028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosman FT. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer; 2010. [Google Scholar]
- 4.Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 5.Klimstra DS, Modlin IR, Adsay NV, et al. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol. 2010;34:300–313. doi: 10.1097/PAS.0b013e3181ce1447. [DOI] [PubMed] [Google Scholar]
- 6.Cubilla AL, Fitzgerald PJ. Surgical pathology aspects of cancer of the ampulla-head-of-pancreas region. Monogr Pathol. 1980;21:67–81. [PubMed] [Google Scholar]
- 7.Kim HS, Kim HJ, Kim HM, et al. Concurrent occurrence of adenocarcinoma and neuroendocrine type small cell carcinoma in the ampulla of Vater. Korean J Med. 2009;76:70–73. [Google Scholar]
- 8.Williams IM, Williams NW, Stock D, et al. Collision tumour of the ampulla of Vater: carcinoid and adenocarcinoma. HPB Surg. 1997;10:241–244. doi: 10.1155/1997/46751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Leval L, Hardy N, Deprez M, et al. Gastric collision between a papillotubular adenocarcinoma and a gastrinoma in a patient with Zollinger-Ellison syndrome. Virchows Arch. 2002;441:462–465. doi: 10.1007/s00428-002-0707-9. [DOI] [PubMed] [Google Scholar]
- 10.Komatsu D, Sakurai M, Nakafuji H, et al. Granulocyte colony stimulating factor-producing collision tumor of the gastric cardia. J Gastroenterol. 2003;38:1013–1015. doi: 10.1007/s00535-003-1188-6. [DOI] [PubMed] [Google Scholar]
- 11.Nishino N, Konno H, Baba S, et al. Synchronous lymphoma and adenocarcinoma occurring as a collision tumor in the stomach: report of a case. Surg Today. 1996;26:508–512. doi: 10.1007/BF00311557. [DOI] [PubMed] [Google Scholar]
- 12.Shah IA, Schlageter MO, Boehm N. Composite carcinoid-adenocarcinoma of ampulla of Vater. Hum Pathol. 1990;21:1188–1190. doi: 10.1016/0046-8177(90)90158-2. [DOI] [PubMed] [Google Scholar]
- 13.Wenig BM, Albores-Saavedra J, Buetow PC, et al. Pancreatic mucinous cystic neoplasm with sarcomatous stroma: a report of three cases. Am J Surg Pathol. 1997;21:70–80. doi: 10.1097/00000478-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Alex WR, Auerbach HE, Pezzi CM. Adenocarcinoid tumor of the ampulla of Vater. Am Surg. 1998;64:355–359. [PubMed] [Google Scholar]
- 15.del Vecchio MT, Pergola L, Tripodi SA, et al. Microcystic adenoma associated with a mucinous cystadenocarcinoma: a "collision tumor" of the pancreas. Pancreas. 2002;24:106–108. doi: 10.1097/00006676-200201000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Moncur JT, Lacy BE, Longnecker DS. Mixed acinar-endocrine carcinoma arising in the ampulla of Vater. Hum Pathol. 2002;33:449–451. doi: 10.1053/hupa.2002.124040. [DOI] [PubMed] [Google Scholar]
- 17.Cheng SP, Yang TL, Chang KM, et al. Large cell neuroendocrine carcinoma of the ampulla of Vater with glandular differentiation. J Clin Pathol. 2004;57:1098–1100. doi: 10.1136/jcp.2004.019414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilardell F, Velasco A, Cuevas D, et al. Composite papillary intestinal-type adenocarcinoma/poorly differentiated neuroendocrine carcinoma of the ampulla of Vater. J Clin Pathol. 2011;64:174–177. doi: 10.1136/jcp.2010.084954. [DOI] [PubMed] [Google Scholar]
- 19.Niu GM, Jin da Y, Ji Y, et al. Survival analysis of pancreatic and periampullary collision cancers. J Dig Dis. 2010;11:231–236. doi: 10.1111/j.1751-2980.2010.00443.x. [DOI] [PubMed] [Google Scholar]
- 20.Jiang SX, Mikami T, Umezawa A, et al. Gastric large cell neuroendocrine carcinomas: a distinct clinicopathologic entity. Am J Surg Pathol. 2006;30:945–953. doi: 10.1097/00000478-200608000-00003. [DOI] [PubMed] [Google Scholar]