Abstract
Backgrounds/Aims
Laparoscopic cholecystectomy (LC) has become a standard procedure for treatment of benign gallbladder diseases. There has been a small proportion of gallbladder cancer (GBC) which was incidentally found in the gallbladder specimen, and LC has been tried in some patients with faintly suspected GBC. This study intended to analyze the prognosis of patients with pT1b/T2 GBC who have undergone LC and the outcome of extended re-operation.
Methods
After analyzing the institutional profiles of 500 GBC patients who have undergone surgical resection, we selected 64 patients who underwent LC initially from January 1996 to December 2008 and whose gallbladder pathology was confined to pT1b or pT2 lesions. Of them, 34 patients (53.1%) underwent extended reoperation. Their medical records were reviewed retrospectively.
Results
In the LC only group (n=30), mean age of the 16 pT1 patients was 65.7±12.5 years and mean age of the 14 pT2 patients was 66.7±10.1 years. In the reoperation group (n=34), mean age of the 8 pT1b patients was 52.6±9.9 years and in 26 pT2 patients, mean age was 59.2±7.9 years. The reoperation group showed a younger patient age pattern than the LC only group (p=0.001). The types of reoperation were liver resection with lymph node (LN) dissection in 17, bile duct resection with LN dissection in 2, and hepatectomy and bile duct resection with LN dissection in 15. In the LC only group, the 5-year survival rate (5-YSR) was 70.3% in pT1b and 43.2% in pT2. In the reoperation group, 5-YSR was 62.5% in pT1b (n=8) and 59.5% in pT2 (n=26). A survival comparison between the two groups showed no significant survival gain in pT1 patients (p=0.69) and in pT2 patients (p=0.14). In our whole database analysis, 5-YSR of pT1bNx lesions was 70% after cholecystectomy and 78% after extended cholecystectomy. Lymph node metastasis was identified in 11% of pT1b lesions. For pT2N0 lesions, overall 5-YSR was 62% after R0 resection, showing no survival difference between primary extended surgery and LC-redo operation (p=0.45).
Conclusions
The survival gain of reoperation was not evident in pT1b lesions. In contrast, some noticeable but not statistically significant survival difference was observed in pT2 lesions. Thus, reoperation for pT1b/T2 GBC following LC is indicated for individualized reasons, especially in patients with pT1b lesions. Old age was one of the important factors in deciding not to reoperate.
Keywords: Gallbladder carcinoma, Laparoscopic cholecystectomy, Extended cholecystectomy, Recurrence, Reoperation
INTRODUCTION
Gallbladder carcinoma (GBC) is a relatively rare tumor; however, its prognosis has been poor over the past few decades. There is no effective therapy for GBC except for curative surgical resection. The prognosis for patients with early GBC shows a 5-year survival rate of 82-100%.1-3 Due to the anatomical proximity to important organs, surgery for advanced GBC requires an aggressive approach. For T2 or more advanced tumors, it is advocated to perform radical resection with lymph node dissection.
Laparoscopic cholecystectomy (LC) has become a standard procedure for the treatment of benign gallbladder diseases. There is a small proportion of GBC cases which were incidentally found in the gallbladder specimens, and LC has been tried in some patients with faintly suspected GBC.
When GBC is pathologically diagnosed after LC in the gallbladder specimens, extended reoperation has been suggested according to the customized guidelines as well as on an individual basis.4 When confined to T1b GBC lesions, the incidence of lymph node metastasis is 16%; thus, extended re-do surgery is recommended if the patient's condition permits.5
It was reported that a second radical resection was associated with significantly better survival than simple cholecystectomy alone in T2 GBC patients whose cancers were incidentally found after cholecystectomy, whereas, it was reported that 40.5% of patients with inapparent pT2 tumors survived for more than 5 years after cholecystectomy alone.6
It is difficult to propose reliable treatment guidelines for histologically confirmed T1b and T2 GBC after LC. This study intended to analyze the prognosis of patients with pT1b/T2 GBC who had undergone LC and the outcome of extended re-operation.
METHODS
Patient selection
From our institutional database of GBC which contained more than 600 cases of patients who had undergone surgical resection, we selected 64 patients who underwent LC initially from January 1996 to December 2008 and whose gallbladder pathology was confined to pT1b or pT2 lesions. They had a follow-up period for more than 4 years. Their medical records were reviewed retrospectively after approval by the Institutional Review Board of our institution.
In addition to these GBC patients who underwent LC first, we also investigated the survival outcomes of GBC patients with pT1bNx lesions and pT2Nx lesions who underwent open surgery as the first operation.
Reoperation
Of overall 64 patients, 34 patients (53.1%) did not undergo repeat surgery, with 16 cases of pT1b and 14 cases of pT2. In contrast, 30 patients (46.9%) underwent extended reoperation, with 8 cases of pT1b and 26 cases of pT2. The suggested types of extended reoperation were extended cholecystectomy with lymph node sampling for pT1b lesions and extended cholecystectomy (or more extended hepatectomy) with lymph node dissection±bile duct resection for pT2 lesions. The laparoscopic port site delivering the gallbladder specimen was also suggested to be removed.
Statistics
Numerical variables were presented as the means with standard deviations or as medians with ranges. Survival and recurrence rates were determined by the Kaplan-Meier method and compared by the log-rank test. Incidences were compared with the chi-square test. A value of p<0.05 was considered to be statistically significant.
RESULTS
In this series, 8 of 24 pT1b patients (33.3%) and 26 of 40 pT2 patients (65%) underwent extended reoperation (p=0.02).
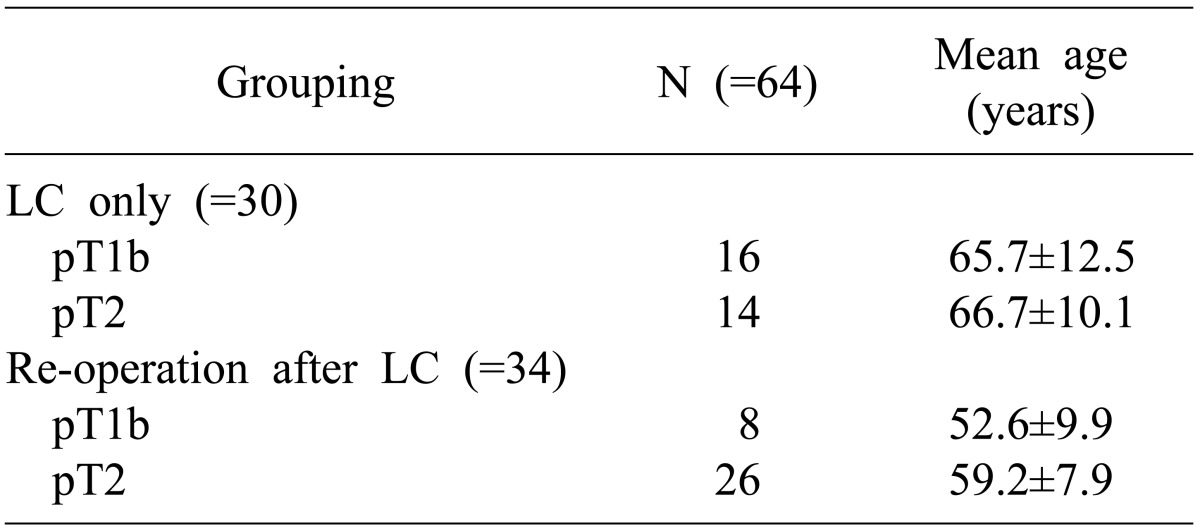
In the LC only group (n=30), the mean age was 65.7±12.5 years in 16 cases of pT1 patients and 66.7±10.1 years in 14 cases of pT2 patients. In the reoperation group (n=34), the mean age was 52.6±9.9 years in 8 cases pT1b patients and 59.2±7.9 years in 26 cases of pT2 patients. Thus, the reoperation group showed a younger patient age pattern than the LC only group (p=0.001) (Table 1).
Table 1.
Comparison of patient age between the laparoscopic cholecystectomy (LC) only group and re-operation. Reoperation group showed a younger patient age pattern than LC only group (p=0.001)
Types of reoperation were liver resection (hepatic wedge resection in 14 and S4aS5 resection in 3) with lymph node dissection in 17, bile duct resection with lymph node dissection in 2, and hepatectomy (hepatic wedge resection in 10 and S4aS5 resection in 5) and bile duct resection with lymph node dissection in 15.
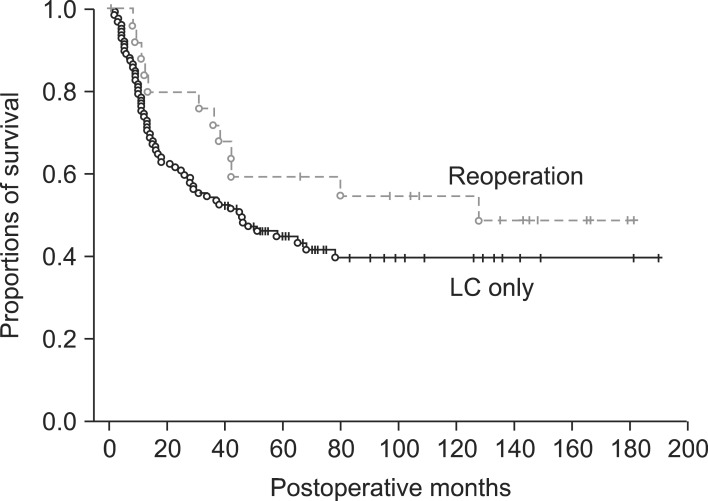
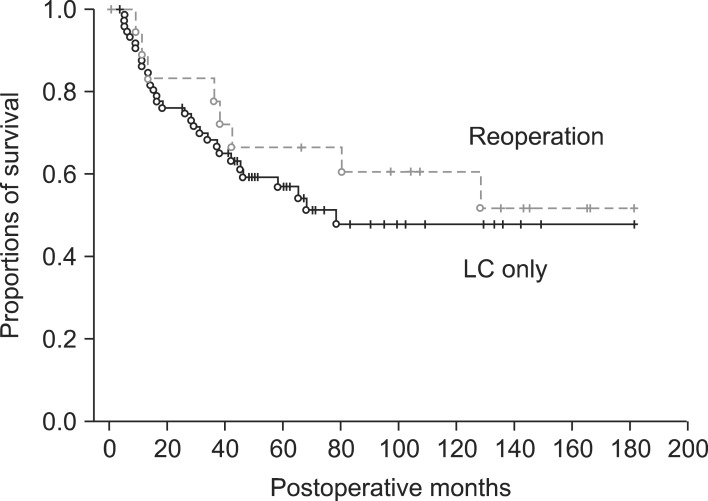
In the LC only group, the 5-year survival rate (5-YSR) was 70.3% in pT1b and 43.2% in pT2. In the reoperation group, the 5-YSR was 62.5% in pT1b (n=8) and 59.5% in pT2 (n=26). A survival comparison between the two groups showed no significant survival gain in pT1b patients (p=0.69) (Fig. 1) and in pT2 patients (p=0.14) (Fig. 2).
Fig. 1.
Comparison of overall patient survival in pT1b gallbladder patients who underwent laparoscopic cholecystectomy (LC) as the first operation.
Fig. 2.
Comparison of overall patient survival in pT2 gallbladder patients who underwent laparoscopic cholecystectomy (LC) as the first operation.
In an additional analysis of our whole GBC database, the 5-YSR of pT1bNx lesions was 70% after cholecystectomy and 78% after extended cholecystectomy. Lymph node metastasis was identified in 11% of pT1b lesions. For pT2N0 lesions, overall 5-YSR was 62% after R0 resection, showing no survival difference between primary extended surgery and LC-redo operation (p=0.45).
DISCUSSION
The treatment of choice for patients with Tis and T1a disease is a simple cholecystectomy with a 5-YSR of 100% in most series. But, there is controversy regarding the optimum management of T1b lesions.5
Shirai et al.7 had 11 patients with T1b with no evidence of loco-regional recurrences on follow up following a simple cholecystectomy. Wakai et al.8 compared the survival outcomes in patients of T1b undergoing simple cholecystectomy or extended cholecystectomy, in which the overall 10-YSR was 87% with no difference between the two surgical methods. On the other hand, in patients with pT1b, consequently simple cholecystectomy may have a higher incidence of loco-regional failure. The pT1b diseases are known to have potential of lymph node metastasis. De Aretxabala et al.9 reported a 20% incidence of nodal metastasis, which would warrant an extended cholecystectomy, in T1b lesions. Other series have reported a 28% incidence of lymphatic or venous involvement and 15.6% nodal metastasis in patients with T1b lesions.10,11 Ouchi et al.5,10 encountered recurrences in 60% of patients with T1b treated by a simple cholecystectomy, and similar experience is reported by other authors, suggesting that simple cholecystectomy may not be an adequate procedure for T1b disease. Wagholikar et al.12 reported that the cumulative survival in the whole group was 68% at 5 years. They concluded that T1b disease is a locally aggressive disease and the treatment of choice was extended resection to improve long-term survival. Since T1b disease is associated with node metastasis and does have loco-regional failure, an extended cholecystectomy rather than simple cholecystectomy is the procedure of choice.
In this study, the 5-YSR was 70.3% in the pT1b in LC only group, and 62.5% in pT1b (n=8) in the reoperation group. The 5-YSR of pT1bNx lesions was 70% after cholecystectomy and 78% after extended cholecystectomy. Lymph node metastasis was identified in 11% of pT1b lesions. Considering that a majority of patients were old-aged, survival outcome with meticulous age adjustment did not show statistical significance in survival outcomes regarding the extent of resection.
The management of pT2 disease is less controversial. Lymph node metastasis occurs in 20-62% of patients with pT2 GBC.5,13-21 The majority of authors recommend that these patients merit re-operative surgery and if there is no evidence of dissemination, then extended cholecystectomy is the procedure of choice (Table 2). Suzuki et al.15 presented their results of pT2 GBC undergoing extended resection as a primary procedure (n=12) or a re-operation following cholecystectomy (n=8), which showed a 5-year survival of 77%. There was no difference in the outcome of patients undergoing primary surgery versus re-operative extended surgery. Grade of tumor differentiation and the presence of perineural invasion were significant factors related to long-term survival. Shirai et al.18 retrospectively analyzed 98 patients of inapparent GBC. 39 patients with pT1 (pT1b=4) had a 5-YSR of 100% after cholecystectomy. Of the 48 patients with pT2, the 5-YSR was 40% after simple cholecystectomy compared to 90% in the patients undergoing re-operation, indicating the survival advantage of extended resections in T2 disease.
Table 2.
Literature review of 5-year survival in patients with pt2 disease after simple cholecystectomy and following re-operative surgery and extended resection
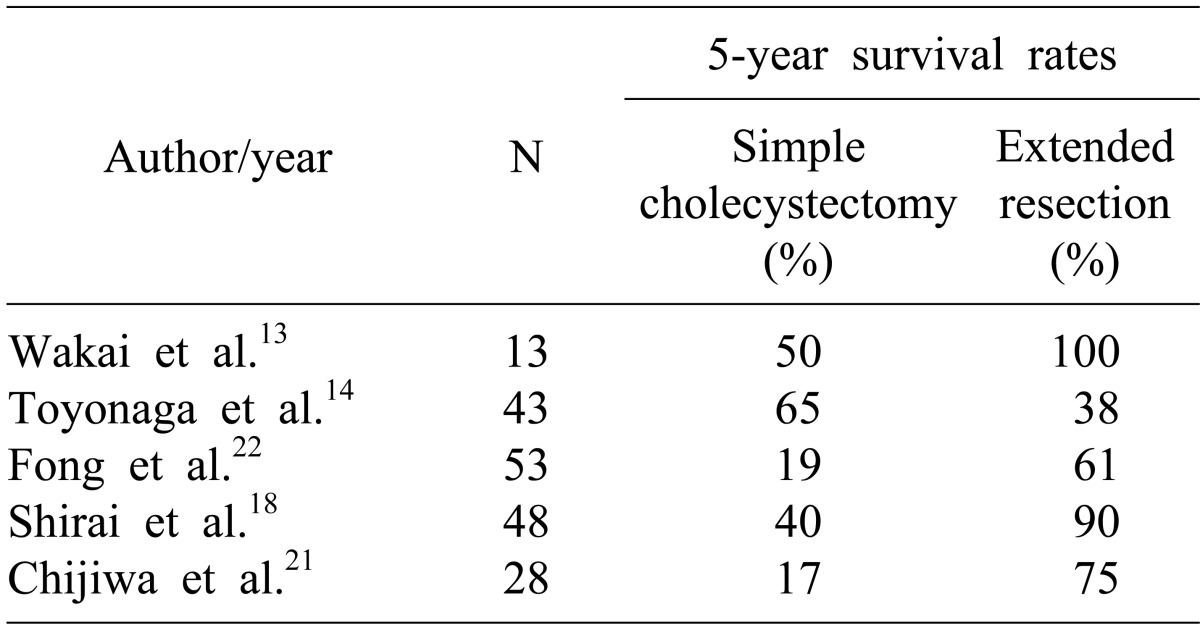
Wakai et al.13 analyzed 28 patients with early GBC detected after LC. 10 patients (3 cases of pT1 and 7 cases of pT2) underwent re-operation with extended resections following LC. A significant survival benefit at 5 years was observed in patients with pT2 undergoing extended resections compared to those with only LC (50% vs. 100%; p=0.039). In this study, the 5-YSR was 43.2% in pT2 with LC only. In the reoperation group, 5-YSR was 59.5% in pT2 (n=26).
In this study, a survival comparison between the two groups showed no significant survival gain in pT1b patients (p=0.69) and in pT2 patients (p=0.14). In our whole database analysis, the 5-YSR of pT1bNx lesions was 70% after cholecystectomy and 78% after extended cholecystectomy. For pT2N0 lesions, overall 5-YSR was 62% after R0 resection, showing no survival difference between primary extended surgery and LC-redo operation (p=0.45). We strongly presume that old age was one of the important factors for determining whether to reoperate or not. The reoperation group showed a younger patient age pattern than the LC only group (p=0.001) (Table 1).
In conclusion, the survival gain of reoperation was not evident in pT1b lesions of our study series. In contrast, some noticeable but not statistically significant survival difference was observed in pT2 lesions. Thus, reoperation for pT1b/T2 GBC following LC is indicated for individual reasons, especially in patients with pT1b lesions. Old age was one of the important factors in the decision to not perform a reoperation.
References
- 1.Kohya N, Miyazaki K. Hepatectomy of segment 4a and 5 combined with extra-hepatic bile duct resection for T2 and T3 gallbladder carcinoma. J Surg Oncol. 2008;97:498–502. doi: 10.1002/jso.20982. [DOI] [PubMed] [Google Scholar]
- 2.Nimura Y. Extended surgery in bilio-pancreatic cancer: the Japanese experience. Semin Oncol. 2002;29(6 Suppl 20):17–22. doi: 10.1053/sonc.2002.37371. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada K, Hatakeyama K, Kurosaki I, et al. Outcome of radical surgery for carcinoma of the gallbladder according to the TNM stage. Surgery. 1996;120:816–821. doi: 10.1016/s0039-6060(96)80089-4. [DOI] [PubMed] [Google Scholar]
- 4.Ogura Y, Mizumoto R, Isaji S, et al. Radical operations for carcinoma of the gallbladder: present status in Japan. World J Surg. 1991;15:337–343. doi: 10.1007/BF01658725. [DOI] [PubMed] [Google Scholar]
- 5.Sikora SS, Singh RK. Surgical strategies in patients with gallbladder cancer: nihilism to optimism. J Surg Oncol. 2006;93:670–681. doi: 10.1002/jso.20535. [DOI] [PubMed] [Google Scholar]
- 6.Wakai T, Shirai Y, Yokoyama N, et al. Early gallbladder carcinoma does not warrant radical resection. Br J Surg. 2001;88:675–678. doi: 10.1046/j.1365-2168.2001.01749.x. [DOI] [PubMed] [Google Scholar]
- 7.Shirai Y, Yoshida K, Tsukada K, et al. Early carcinoma of the gallbladder. Eur J Surg. 1992;158:545–548. [PubMed] [Google Scholar]
- 8.Wakai T, Shirai Y, Yokoyama N, et al. Early gallbladder carcinoma does not warrant radical resection. Br J Surg. 2001;88:675–678. doi: 10.1046/j.1365-2168.2001.01749.x. [DOI] [PubMed] [Google Scholar]
- 9.de Aretxabala X, Roa I, Burgos L, et al. Gallbladder cancer in Chile. A report on 54 potentially resectable tumors. Cancer. 1992;69:60–65. doi: 10.1002/1097-0142(19920101)69:1<60::aid-cncr2820690112>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Ouchi K, Owada Y, Matsuno S, et al. Prognostic factors in the surgical treatment of gallbladder carcinoma. Surgery. 1987;101:731–737. [PubMed] [Google Scholar]
- 11.Ogura Y, Mizumoto R, Isaji S, et al. Radical operations for carcinoma of the gallbladder: present status in Japan. World J Surg. 1991;15:337–343. doi: 10.1007/BF01658725. [DOI] [PubMed] [Google Scholar]
- 12.Wagholikar GD, Behari A, Krishnani N, et al. Early gallbladder cancer. J Am Coll Surg. 2002;194:137–141. doi: 10.1016/s1072-7515(01)01136-x. [DOI] [PubMed] [Google Scholar]
- 13.Wakai T, Shirai Y, Hatakeyama K. Radical second resection provides survival benefit for patients with T2 gallbladder carcinoma first discovered after laparoscopic cholecystectomy. World J Surg. 2002;26:867–871. doi: 10.1007/s00268-002-6274-z. [DOI] [PubMed] [Google Scholar]
- 14.Toyonaga T, Chijiiwa K, Nakano K, et al. Completion radical surgery after cholecystectomy for accidentally undiagnosed gallbladder carcinoma. World J Surg. 2003;27:266–271. doi: 10.1007/s00268-002-6609-9. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki S, Yokoi Y, Kurachi K, et al. Appraisal of surgical treatment for pT2 gallbladder carcinomas. World J Surg. 2004;28:160–165. doi: 10.1007/s00268-003-7080-y. [DOI] [PubMed] [Google Scholar]
- 16.Muratore A, Polastri R, Bouzari H, et al. Radical surgery for gallbladder cancer: a worthwhile operation? Eur J Surg Oncol. 2000;26:160–163. doi: 10.1053/ejso.1999.0762. [DOI] [PubMed] [Google Scholar]
- 17.Wakai T, Shirai Y, Yokoyama N, et al. Depth of subserosal invasion predicts long-term survival after resection in patients with T2 gallbladder carcinoma. Ann Surg Oncol. 2003;10:447–454. doi: 10.1245/aso.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Shirai Y, Yoshida K, Tsukada K, et al. Inapparent carcinoma of the gallbladder. An appraisal ofa radical second operation after simple cholecystectomy. Ann Surg. 1992;215:326–331. doi: 10.1097/00000658-199204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi K, Tsuneyoshi M. Subclinical gallbladder carcinoma. Am J Surg. 1992;163:382–386. doi: 10.1016/0002-9610(92)90038-s. [DOI] [PubMed] [Google Scholar]
- 20.Kosuge T, Sano K, Shimada K, et al. Should the bile duct be preserved orremoved in radical surgery for gallbladder cancer? Hepatogastroenterology. 1999;46:2133–2137. [PubMed] [Google Scholar]
- 21.Chijiiwa K, Nakano K, Ueda J, et al. Surgical treatment of patients with T2 gallbladder carcinoma invading the subserosal layer. J Am Coll Surg. 2001;192:600–607. doi: 10.1016/s1072-7515(01)00814-6. [DOI] [PubMed] [Google Scholar]
- 22.Fong Y, Heffernan N, Blumgart LH. Gallbladder carcinoma discovered during laparoscopic cholecystectomy: aggressive reresection is beneficial. Cancer. 1998;83:423–427. doi: 10.1002/(sici)1097-0142(19980801)83:3<423::aid-cncr9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]