Abstract
This study was to investigate the absorption and antioxidant effect of a mangosteen-based functional beverage in humans. The beverage contained mangosteen, aloe vera, green tea, and multivitamins. A randomized, double-blind, placebo-controlled clinical trial was conducted with generally healthy male and female subjects between 18 and 60 years of age. Ten men and 10 women participated in this study. Participants were randomly divided into two groups, treatment and placebo group. Participants received either a daily single dose (245 mL) of the beverage or a placebo. Blood samples were collected from each participant at time points 0, 1, 2, 4, and 6 h. The plasma samples were analyzed by LC/MS for α-mangostin and vitamins B2 and B5. Results indicated that the three analytes were bioavailable, with observed Cmax at around 1 h. The antioxidant capacity measured with the oxygen radical absorbance capacity (ORAC) assay was increased with a maximum effect of 60% after 1 h, and the elevated antioxidant level lasted at least 6 h. This study demonstrated the bioavailability of α-mangostin and B vitamins from a xanthone-rich beverage and the mechanisms of the increase in plasma antioxidant may be direct effects from antioxidants, enhancement of endogenous antioxidant activity through activation of Nrf2 pathway, and synergism of the antioxidants.
Keywords: Aloe vera, bioavailability, green tea, LC/MS, mangosteen, ORAC
Introduction
There is overwhelming evidence of the importance of antioxidants to scavenge the reactive oxygen/nitrogen species (ROS/RNS) which are known to be involved in the pathogenesis of aging and many common diseases (Halliwell 1991, 1996; Ames et al. 1993). Based on the review conducted by the Institute of Medicine, a dietary antioxidant is defined as “a substance in foods that significantly decreases the adverse effects of reactive species, such as ROS and RNS, on normal physiological function in humans” (Monsen 2000). It is believed that consumption of tropical fruits can reduce the incidence of certain degenerative diseases, the protective effect is considered mainly to be due to the presence of various phyto-bioactives in fruits such as antioxidants (Lim et al. 2007; Alothman et al. 2009; Rufino et al. 2010). Garcinia mangostana L. (Clusiaceae), commonly known as mangosteen, is a slow-growing tropical evergreen tree mainly found in India, Myanmar, Sri Lanka, and Thailand. Mangosteen has dark purple to red-purple fruits. The edible fruit aril is white, soft, and juicy with a sweet taste. Recently, mangosteen extract has been used as a botanical dietary supplement in the United States, as mangosteen peel has been reported to contain a variety of bioactive compounds with potential applications as therapeutic agents or as functional food additives such as phenolic acids, tannins, xanthones, anthocyanins, and other bioactive compounds (Mahabusarakam et al. 1987). Nowadays, there is a worldwide pursuit of designing new functional beverages and healthy food products based on tropical fruits (Sabbe et al. 2009; Granato et al. 2010; Schauss et al. 2010). In this sense, a design of new beverages combining mangosteen with green tea, aloe vera, and antioxidant vitamins was favorable on the basis of the antioxidant synergistic effects among these bioactives. The aim of this work was to determine the bioavailability of α-mangostin and vitamins B2 and B5 found in a mangosteen-based functional beverage and their antioxidant capacity on plasma antioxidant status in the human body.
Materials
α-Mangostin was purchased from Chromadex (Irvine, CA), Vitamin B2 (riboflavin), Vitamin B5 (pantothenic acid), Trolox (6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid), and Fluorescein sodium were obtained from Sigma-Aldrich (St. Louis, MO). 2,2′-Azobis (2-amidinopropane) dihydrochloride was purchased from Wako Chemicals USA (Richmond, VA). Verve® and placebo (fructose liquid) were provided by Vemma Nutrition Co. (Tempe, AZ). Verve® is a multivitamin/antioxidant liquid nutrition beverage containing a full spectrum of vitamins, green tea, aloe vera and mangosteen, and a caffeinated energy blend.
Subjects and study protocol
A randomized, double-blind, placebo-controlled clinical trial was conducted with generally healthy male and female subjects between 18 and 60 years of age. All subjects were screened by evaluation of a medical history and assessment of diet history and supplement history using a self-developed semiquantitative questionnaire. Written informed consent was obtained from each volunteer participating in this study. All procedures of the protocol were approved by the Institutional Review Board of HummingbirdIRB (Cambridge, MA). Ten men and 10 women participated in this study. Participants were randomly divided into two groups, treatment and placebo group, with the same number of male and female participants in each group, and the trial duration was 6 h. After the baseline tests were completed in the morning, participants received either a daily single dose (245 mL) of the Verve formula or a placebo (a liquid that looks and tastes like Verve, but with no active ingredients). The use of fructose was to mimic the taste of Verve with minimal interference with absorption and availability of vitamins and phytonutrients. Blood samples were collected from each participant after consumption of the Verve formula or the placebo at time points 1, 2, 4, and 6 h. Plasma was obtained by centrifugation and stored at −80°C until further analysis.
LC-MS/MS analysis of plasma samples
LC-MS/MS analyses were performed using a system consisting of an Agilent 1100 system comprising a binary pump with a vacuum degasser, a thermostatted column compartment, a C18 column, an autosampler, and a diode array detector (Agilent Technologies, Inc., Santa Clara, CA), coupled with an API-3000 mass spectrometer with a turbo ion spray source (Applied Biosystem, Foster City, CA). Sample preparations and conditions for LC-MS analyses were as given below. The chemical structures of test analytes are illustrated in Figure1.
Figure 1.
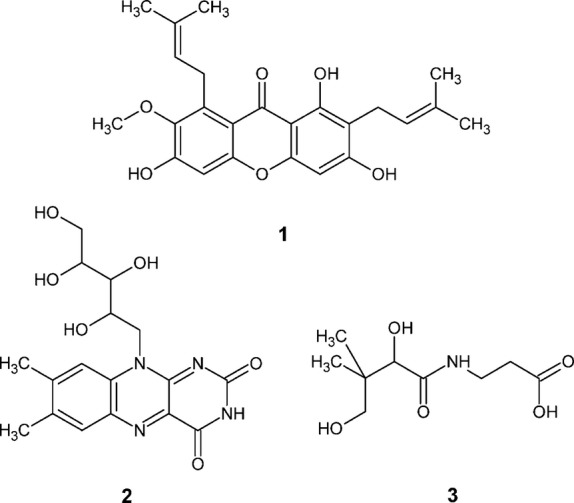
Structures of test analytes: 1, α-mangostin; 2, vitamin B2; 3, vitamin B5.
α-Mangostin
One hundred microliters of reconstituted human plasma was extracted using 300 μL of methanol. The supernatant was collected after centrifugation at 14,000 rpm for 10 min (4°C). Ten microliters was injected onto the LC-MS/MS for mangostin analysis. Chromatography was carried out on a 2.1 × 50 mm, 3 μm, MacMod HyroBond PS-C18 column (MAC-MOD Analytical, Inc., Chadds Ford, PA), and the solvent system consisted of a gradient system with water (0.4% formic acid, v/v) (A) and acetonitrile (B). Gradient elution was performed at 0.6 mL/min with the following conditions: 1 min hold at 100% A, 0.5 min linear gradient from 100% to 60% A, 4.5 min linear gradient from 60% to 2% A, 1.1 min hold at 2% A, 0.1 min linear gradient from 2% to 100% A, and 2.9 min hold at 100% A. The mass detector was equipped with a turbo ion spray (electrospray ionization, ESI) source and operated in multiple reaction monitoring mode (MRM) under negative ion mode. The heated capillary and voltage were maintained at 550°C and −4500 V, respectively. The optimized instrument setting is listed in Table1. Representative chromatograms of MRM scans are shown in Figure2. The lower limit of quantification (LLOQ) for α-mangostin was 1.0 ng/mL.
Table 1.
Mass spectrometry parameter for α-mangostin, vitamin B2, and vitamin B5
| Transition (m/z) |
||||||
|---|---|---|---|---|---|---|
| Analyte | MW | Parent ion | Product ion | DP (V) | CE (V) | CXP (V) |
| α-Mangostin | 410.46 | 411.3 | 355.3 | 61 | 23 | 4 |
| Vitamin B2 | 376.36 | 377 | 243.2 | 96 | 33 | 14 |
| Vitamin B5 | 219.23 | 220.3 | 90.1 | 41 | 21 | 6 |
DP: De-clustering potential;
CE: Collision Energy;
CXP:Collision Cell Exit Potential;
MW: Molecular Weight.
Figure 2.
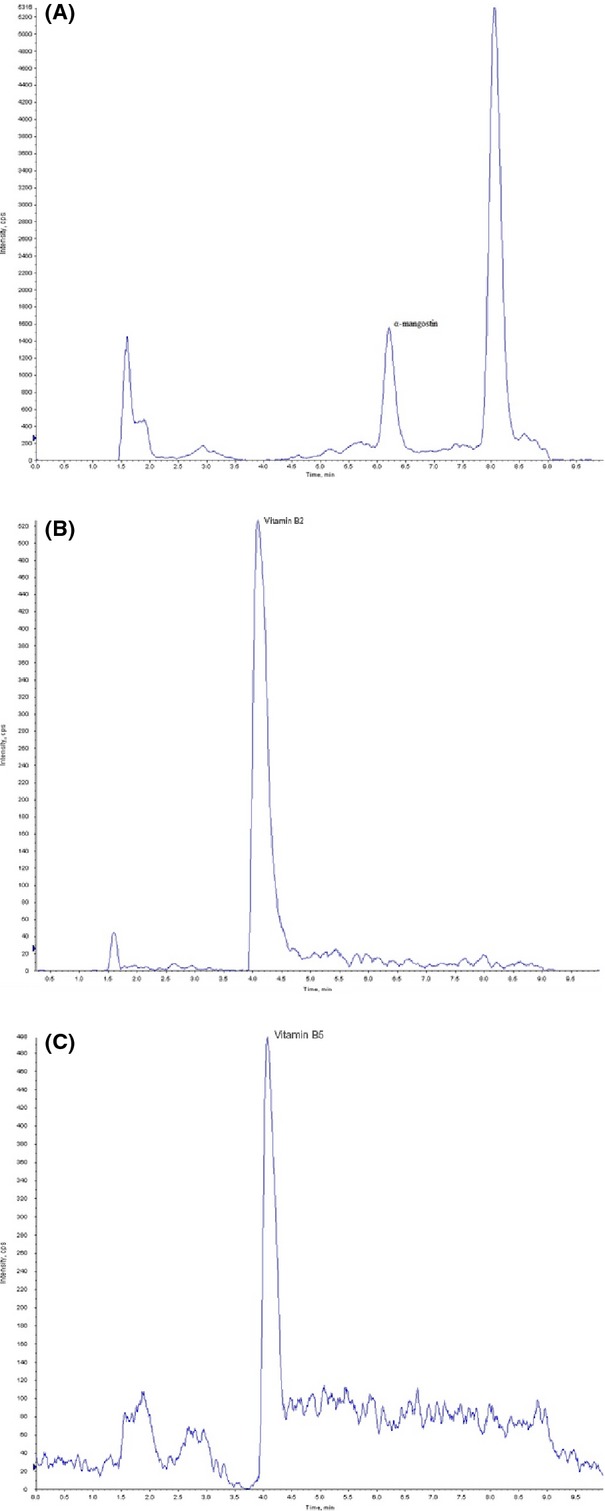
Representative chromatograms of MRM scans for (A) a-Mangostin, (B) vitamin B2, and (C) vitamin B5. MRM, multiple reaction monitoring mode.
Vitamins B2 and B5
Two hundred microliters of reconstituted human plasma was mixed with 280 μL of methanol and 20 μL of trifluoroacetic acid. The supernatant was collected after vortexing and centrifugation at 14,000 rpm for 10 min (4°C). Five microliters was injected onto the LC-MS/MS for vitamin B analysis. Chromatography was carried out on a 50 × 4.6 mm, 3 μm, Ascentis RP-AMIDE column (Sigma-Aldrich), and the solvent system consisted of a gradient system with water (0.4% formic acid, v/v) (A) and acetonitrile (B). Gradient elution was performed at 0.6 mL/min with the following conditions: 0.9 min hold at 100% A, 0.1 min linear gradient from 100% to 90% A, 3.0 min linear gradient from 90% to 40% A, 1 min linear gradient from 40% to 2% A, 2.0 min hold at 2% A, 0.1 min linear gradient from 2% to 100% A, and 3.0 min hold at 100% A. The mass detector was equipped with a turbo ion spray (ESI) source and operated in MRM under positive ion mode. The heated capillary and voltage were maintained at 550°C and 5500 V, respectively. The optimized instrument settings for vitamins B2 and B5 analysis are listed in Table1. Representative chromatograms of MRM scans are shown in Figure2. The LLOQs for vitamins B2 and B5 were 2 and 13 ng/mL, respectively.
Antioxidant capacity (ORAC Assay)
Plasma antioxidant capacity was determined by the ORAC assay as previously described using a Synergy H4 microplate florescence reader (Bio-Tek Instruments, Inc., Winooski, VT) with Gen 5 V 2 software (excitation wavelength 485 ± 20 nm, emission wavelength 530 ± 25 nm) (Ou et al. 2001; Prior et al. 2003). Plasma samples were thawed, vortexed, and centrifuged at 16,000g at 4°C for 3 min, 240 μL of supernatant was deproteinized using 720 μL of methanol. The mixture was vortexed for 30 sec, and then centrifuged at 16,000 g for 5 min at 4°C. Peroxyl radicals were generated by the spontaneous decomposition of AAPH at 37°C. Fluorescein was used as a fluorescent probe, with loss of fluorescence indicating fluorescein damage from its reaction with peroxyl radicals. The protective effects of the plasma tested were determined by comparing fluorescence time/intensity area under the curve (AUC) of the sample with a control.
Results and Discussion
Bioavailability
A variety of health-promoting attributes have been associated with the mangosteen, which include antiinflammatory, antibacterial activity, cardioprotective, and antioxidant activity. A class of compounds known as xanthones have been isolated from mangosteen which are thought to be responsible for mangosteen's bioactivity. Of all the xanthones, α-mangostin (1,3,6-Trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)-9H-xanthen-9-one) has been identified as the most abundant xanthone and as a result has received the most attention for its health-promoting properties. The plasma level of α-mangostin was analyzed in this study. As demonstrated in Figure3, an increase of α-mangostin was observed during the course in the treatment group, whereas no α-mangostin was found in the placebo group within the detection range. The α-mangostin plasma concentration reached its maximum (Cmax), 4.16 ± 2.85 ng/mL at tmax of 1 h after Verva formula consumption, the elevated level of α-mangostin was sustained through the rest of course of the trial.
Figure 3.
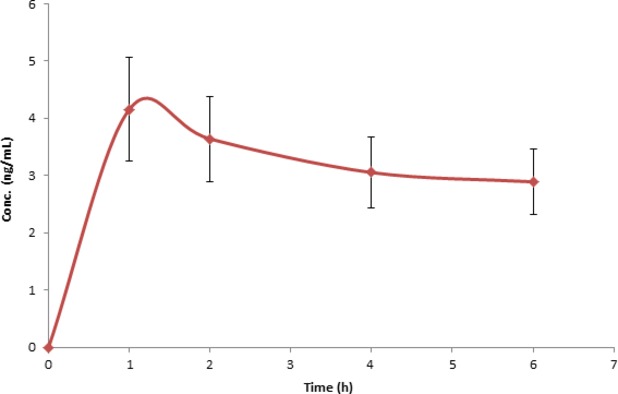
Concentrations of α-mangostin in human plasma vs. time curve after a single oral administration of Verve® (245 mL). Values were expressed as means ± SEM.
Verva formula is enriched with vitamins B2 and B5. B vitamins are essential nutrients and important for cell metabolism, therefore, they are vital as part of one's health promotion. In this study, we also evaluated the bioavailability of B vitamins. Figures4 and 5 illustrate the mean plasma concentration–time curves of tested analytes. As shown, both vitamin B2 and B5 were found bioavailable, and after 1 h their concentrations in plasma reached the highest level at 5.61 ± 2.59 ng/mL and 64.41 ± 26.89 ng/mL, respectively, this trend was aligned with the absorption of α-mangostin.
Figure 4.
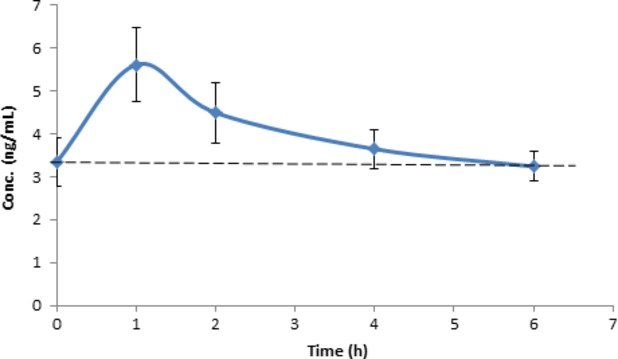
Concentrations of vitamin B2 in human plasma vs. time curve after a single oral administration of Verve® (245 mL). Values were expressed as means ± SEM. Dashed line represents baseline level.
Figure 5.
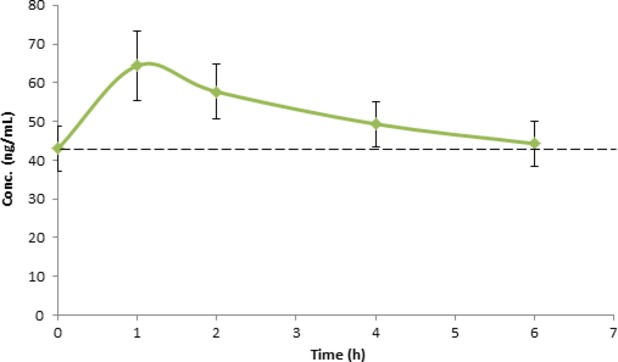
Concentrations of vitamin B5 in human plasma vs. time curve after a single oral administration of Verve® (245 mL). Values were expressed as means ± SEM. Dashed line represents baseline level.
Antioxidant capacity
The plasma antioxidant capacity (ORAC) in the Verva formula group increased as much as 60% at 1 h following consumption of the drink (Fig.6). The ORAC values then declined gradually and became stable between 4 and 6 h with 10% increase when compared to the zero hour. While in the placebo group, the antioxidant activity in plasma was found slightly decreased over the trial period.
Figure 6.
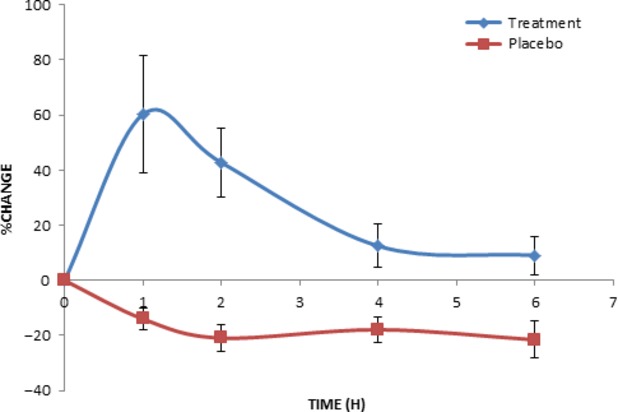
Increase of human plasma antioxidant capacity after a single oral administration of Verve® (245 mL). Values were expressed as means ± SEM.
Mechanism of plasma ORAC increase
The increases in plasma B vitamins and α-mangostin following Verve consumption indicate that at least a small part of the increase in ORAC may be accounted for by absorbed B vitamins and α-mangostin. Additionally, Verva also contains vitamin E, vitamin C, green tea extract, and aloe vera. Vitamins C and E are well-known antioxidants, and green tea catechins belong to flavonoid family and are known to have fairly strong antioxidant activity with ORAC values of 8100−22,000 μmol of Trolox equiv/g (Ou et al. 2002). Polysaccharides from aloe vera scavenge hydroxyl radicals, the most harmful reactive oxygen species found in the body (Hu et al. 2003; Kardošová and Machová 2006; Miranda et al. 2009). Hence, it is reasonable to propose that vitamins C and E, α-mangostin, green tea catechins, and aloe vera polysaccharides might account for the increase in plasma ORAC.
On the basis of the nanogram levels of absorbed B vitamins and α-mangostin, the direct effects from the antioxidants on the increase in ORAC are therefore limited. The human body's endogenous antioxidant network consists of several enzymatic antioxidants, such as glutathione, thioredoxin, and NADPH, etc (Halliwell 1996). Recently, antioxidants present in fruit and vegetables are found to act together to produce an additive increase in electrophilic signaling that results in the induction of endogenous antioxidant responses to oxidative stress (Su et al. 2013). The upregulation of endogenous antioxidant defense provides the more profound cellular protection than that of antioxidant supplementation. This is because some phytochemicals can activate the transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a “master regulator” of antioxidant defense (Jung and Kwak 2010; Zhang et al. 2010; Lee et al. 2013). Shen et al. (2014) reported that α-mangostin-mediated antioxidant response via Nrf2 is a mechanism preventing adipogenesis and inflammation in adipocytes. Molecular mechanisms underlying antioxidant activity exerted by green tea and its components have been extensively investigated as well. (−)-Epigallocatechin-3-gallate (EGCG), a major green tea catechins, has been shown to induce expression of endogenous enzymatic antioxidants such as glutathione S-transferase, glutathione peroxidase, glutamate cysteine ligase, hemeoxygenase-1, etc. (Na and Surh 2008). Vitamin E is a well-known antioxidant compound that inhibits lipid peroxidation and other free radical-mediated reactions in biological systems (Niki 2014). In an in vivo study Cardozo et al. (2013) observed that Nrf2 was significantly upregulated following consumption of a vitamin E- rich diet. Furthermore Yang et al. (2014) found that wolfberry polysaccharide showed the antioxidative and anti-inflammatory effects on experimental models of insulin resistance in vivo, one of the mechanisms was determined to be induction of Nrf2 Pathway. Taken together, these studies suggest that phytochemicals have the ability to activate the Nrf2 system, which in turn, increases the activity of antioxidant enzymes and inhibits oxidative stress. Although the molecular mechanism of the observed antioxidant effect was not studied, we would expect that the increase in enzymatic antioxidant activity through activation of Nrf2 might have been taken in place.
The additive and synergetic effects of phytochemicals in antioxidant activity have been well studied (Liu 2003; Seeram et al. 2004; Chen et al. 2005; Milde et al. 2007; Mikstacka et al. 2010; Wang et al. 2011). Liu et al. (2000) found that the antioxidant activity of vitamin E could be enhanced by green tea extract, as catechins in green tea can effectively reduce α-tocopheroxyl radicals to α-tocopherol, prolonging the antioxidant-free radical chain breaking reaction. In addition, the combination of vitamin C and vitamin E results in more effective in inhibiting oxidation in vivo, this is because coexisting vitamin C reduces α-tocopheroxyl radicals rapidly in membranes and LDL to regenerate α-tocopherol and possibly inhibits α-tocopheroxyl radicals initiated propagation (Sato et al. 1990). As demonstrated above, the direct effects from individual antioxidants contributed to the increase in plasma ORAC; however, the significant plasma ORAC after consumption of Verve in a single dose cannot be interpreted by single antioxidant. It is logical to propose that the observed ORAC increase may be partially due to the synergism of the combination of mangosteen, green tea, antioxidant vitamins, and aloe vera polysaccharides. It is widely believed that the health benefit of a diet rich in fruits and vegetables is attributed to the complex mixture of plant antioxidants present in these and other whole foods, no single antioxidant can replace the combination of phytochemicals in achieving the optimal health benefits (Liu 2003). In plant extracts and in various foods, antioxidants are present as mixtures and therefore there is currently a great interest in synergistic interaction between antioxidants and between mechanisms of such interaction (Almeida et al. 2011). It appears that Verve is formulated based on the balanced antioxidants combining water-soluble antioxidants (vitamin C, catechins, and polysaccharides) and fat soluble antioxidants (vitamin E and α-mangostin). Therefore, the synergy between water soluble and fat soluble antioxidants may provide at least one of the explanations to Verve's antioxidant health benefit.
In summary, we determined the physiological availability of α-mangostin and B vitamins present in a xanthone-rich functional beverage, and their effects on the degree of antioxidant potency in the human body. Noticeable bioavailability was seen in α-mangostin and vitamins B2 and B5 along with the increase in antioxidant capacity. In the experiment group within 1 h after consumption of Verva, significant increases of α-mangostin, vitamins B2 and B5, and ORAC were observed in these subjects. In the placebo group, no significant change was observed in vitamins B2 and B5, and no α-mangostin was detected. Based on the observation of the result and literature, we proposed that the increase in plasma ORAC may be due to three mechanisms: First, bioavailable antioxidants directly scavenged free radicals. Second, phytochemicals upregulated Nrf2 signaling pathway, resulting in increase in endogenous antioxidant activity. Third, the synergistic and additive effects among the antioxidants may account for plasma ORAC. Our results suggest that Verve is an excellent source to obtain balanced natural antioxidants to maintain well-being and possibly against chronic diseases caused by the aging process.
Conflict of Interest
None declared.
References
- Almeida IMC, Barreira JCM, Oliveira MBPP. Ferreira ICFR. Dietary antioxidant supplements: benefits of their combined use. Food Chem. Toxicol. 2011;49:3232–3237. doi: 10.1016/j.fct.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Alothman M, Bhat R. Karim AA. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009;115:785–788. [Google Scholar]
- Ames BN, Shigenaga MK. Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo LFMF, Pedruzzi LM, Stenvinkel P, Stockler-Pinto MB, Daleprane JB, Leiter M, Jr, et al. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie. 2013;95:1525–1533. doi: 10.1016/j.biochi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Milbury PE, Lapsley K. Blumberg JB. Flavonoids from almond skins are bioavailable and act synergistically with Vitamins C and E to enhance hamster and human LDL resistance to oxidation. J. Nutr. 2005;135:1366–1373. doi: 10.1093/jn/135.6.1366. [DOI] [PubMed] [Google Scholar]
- Granato D, Branco GF, Nazzaro F, Cruz AG. Faria JAF. Functional foods and nondairy probiotic food development: trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010;9:292–302. doi: 10.1111/j.1541-4337.2010.00110.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am. J. Med. 1991;91:S14–S22. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- Hu Y, Xu J. Hu Q. Evaluation of antioxidant potential of Aloe vera (Aloe barbadensis Miller) extracts. J. Agric. Food Chem. 2003;51:7788–7791. doi: 10.1021/jf034255i. [DOI] [PubMed] [Google Scholar]
- Jung K-A. Kwak M-K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15:7266–7291. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardošová A. Machová E. Antioxidant activity of medicinal plant polysaccharides. Fitoterapia. 2006;77:367–373. doi: 10.1016/j.fitote.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Lee JH, Khor TO, Shu L, Su Z-Y, Fuentes F, Kong A-NT. Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacol. Ther. 2013;137:153–171. doi: 10.1016/j.pharmthera.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Lim TT. Tee JJ. Antioxidant properties of several tropical fruits: a comparative study. Food Chem. 2007;103:1003–1008. [Google Scholar]
- Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003;78:517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- Liu Z-Q, Ma L-P, Zhou B, Yang L. Liu Z-L. Antioxidative effects of green tea polyphenols on free radical initiated and photosensitized peroxidation of human low density lipoprotein. Chem. Phys. Lipids. 2000;106:53–63. doi: 10.1016/s0009-3084(00)00133-x. [DOI] [PubMed] [Google Scholar]
- Mahabusarakam W, Wiriyachitra P. Taylor WC. Chemical constituents of Garcinia mangostana. J. Nat. Prod. 1987;50:474–478. [Google Scholar]
- Mikstacka R, Rimando A. Ignatowicz E. Antioxidant effect of trans-resveratrol, pterostilbene, quercetin and their combinations in human erythrocytes in vitro. Plant Foods Hum. Nutr. 2010;65:57–63. doi: 10.1007/s11130-010-0154-8. [DOI] [PubMed] [Google Scholar]
- Milde J, Elstner EF. Graßmann J. Synergistic effects of phenolics and carotenoids on human low-density lipoprotein oxidation. Mol. Nutr. Food Res. 2007;51:956–961. doi: 10.1002/mnfr.200600271. [DOI] [PubMed] [Google Scholar]
- Miranda M, Maureira H, Rodríguez K. Vega-Gálvez A. Influence of temperature on the drying kinetics, physicochemical properties, and antioxidant capacity of Aloe Vera (Aloe Barbadensis Miller) gel. J. Food Eng. 2009;91:297–304. [Google Scholar]
- Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J. Am. Diet. Assoc. 2000;100:637–640. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- Na H-K. Surh Y-J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008;46:1271–1278. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Niki E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic. Biol. Med. 2014;66:3–12. doi: 10.1016/j.freeradbiomed.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Ou B, Hampsch-Woodill M. Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- Ou B, Hampsch-Woodill M, Flanagan J, Deemer EK, Prior RL, Huang D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002;50:2772–2777. doi: 10.1021/jf011480w. [DOI] [PubMed] [Google Scholar]
- Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, Howard L, et al. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003;51:3273–3279. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- Rufino MDSM, Alves RE, de Brito ES, Pérez-Jiménez J, et al. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010;121:996–1002. [Google Scholar]
- Sabbe S, Verbeke W, Deliza R, Matta VM. Van Damme P. Consumer liking of fruit juices with different Açaí (Euterpe oleracea Mart.) concentrations. J. Food Sci. 2009;74:S171–S176. doi: 10.1111/j.1750-3841.2009.01146.x. [DOI] [PubMed] [Google Scholar]
- Sato K, Niki E. Shimasaki H. Free radical-mediated chain oxidation of low density lipoprotein and its synergistic inhibition by vitamin E and vitamin C. Arch. Biochem. Biophys. 1990;279:402–405. doi: 10.1016/0003-9861(90)90508-v. [DOI] [PubMed] [Google Scholar]
- Schauss AG, Clewell A, Balogh L, Szakonyi IP, et al. Safety evaluation of an açai-fortified fruit and berry functional juice beverage (MonaVie Active®. Toxicology. 2010;278:46–54. doi: 10.1016/j.tox.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Adams LS, Hardy ML. Heber D. Total cranberry extract versus its phytochemical constituents: antiproliferative and synergistic effects against human tumor cell lines. J. Agric. Food Chem. 2004;52:2512–2517. doi: 10.1021/jf0352778. [DOI] [PubMed] [Google Scholar]
- Shen Q, Chitchumroonchokchai C, Thomas JL, Gushchina L V, DiSilvestro D, Failla ML, et al. Adipocyte reporter assays: application for identification of anti-inflammatory and antioxidant properties of mangosteen xanthones. Mol. Nutr. Food Res. 2014;58:239–247. doi: 10.1002/mnfr.201300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z-Y, Shu L, Khor T, Lee J, Fuentes F, Kong A-NA. A perspective on dietary phytochemicals and cancer chemoprevention: oxidative stress, Nrf2, and epigenomics. In: Pezzuto JM, Suh N, et al., editors. Natural products in cancer prevention and therapy. Berlin, Heidelberg: Springer; 2013. pp. 133–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Meckling KA, Marcone MF, Kakuda Y. Tsao R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J. Agric. Food Chem. 2011;59:960–968. doi: 10.1021/jf1040977. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li W, Li Y, Wang Q, Gao L, Zhao J. Dietary lycium barbarum polysaccharide induces Nrf2/ARE pathway and ameliorates insulin resistance induced by high-fat via activation of PI3K/AKT signaling. Oxid. Med. Cell. Longev. 2014;2014:10. doi: 10.1155/2014/145641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Pi J, Woods CG. Andersen ME. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol. Appl. Pharmacol. 2010;244:84–97. doi: 10.1016/j.taap.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


