Abstract
Differences in opinion regarding the development of the infrahepatic inferior caval and azygos venous systems in mammals centre on the contributions of ‘caudal cardinal’, ‘subcardinal’, ‘supracardinal’, ‘medial and lateral sympathetic line’ and ‘sacrocardinal’ veins. The disagreements appear to arise from the use of topographical position rather than developmental origin as criterion to define separate venous systems. We reinvestigated the issue in a closely spaced series of human embryos between 4 and 10 weeks of development. Structures were visualized with the Amira® reconstruction and Cinema4D® remodelling software. The vertebral level and neighbouring structures were used as topographic landmarks. The main results were that the caudal cardinal veins extended caudally from the common cardinal vein between CS11 and CS15, followed by the development of the subcardinal veins as a plexus sprouting ventrally from the caudal cardinal veins. The caudal cardinal veins adapted their course from lateral to medial relative to the laterally expanding lungs, adrenal glands, definitive kidneys, sympathetic trunk and umbilical arteries between CS15 and CS18, and then became interrupted in the part overlaying the regressing mesonephroi (Th12-L3). The caudal part of the left caudal cardinal vein then also regressed. The infrarenal part of the inferior caval vein originated from the right caudal cardinal vein, while the renal part originated from subcardinal veins. The azygos veins developed from the remaining cranial part of the caudal cardinal veins. Our data show that all parts of the inferior caval and azygos venous systems developed directly from the caudal cardinal veins or from a plexus sprouting from these veins.
Keywords: azygos vein, caudal cardinal veins, human development, inferior caval vein, subcardinal veins, topographic embryology
Introduction
The veins in the cranial part of the body all develop from the cranial cardinal system, whereas at least three systems – the caudal cardinal, umbilical and vitelline vein pairs – and possibly as many as eight – additionally the subcardinal, supracardinal, medial and lateral sympathetic line, and sacrocardinal venous systems – are involved in the development of the veins in the caudal part of the body (Cornillie & Simoens, 2005). Of these, two systems – the umbilical and vitelline veins – return blood from extraembryonic tissues and only participate in the formation of veins that drain the gut and the liver. The embryonic origins of the infrahepatic part of the inferior caval and azygos venous systems remain elusive. The infrahepatic part is divided into a renal and an infrarenal part, and especially the origin of the latter is controversial.
Hochstetter (1893) claimed that both the infrahepatic inferior caval and azygos venous systems originated from the caudal cardinal veins only (Fig.1A). In Hochstetter's view, the renal part of the inferior caval vein arose from a medial branch of the caudal cardinal veins, which was named the subcardinal vein by Lewis (1902) and most authors since (Fig.1B–D). Following the persuasive studies of Butler and colleagues (McClure & Butler, 1925; Butler, 1927), most current textbooks (Patten, Arey, Larsen, Moore and Carlson) cite contributions of supracardinal veins to the development of the infrarenal inferior caval and azygos veins (Fig.1B), whereas other textbooks (Langman and Hamilton & Mossman) follow Grünwald (1938) and invoke the involvement of sacrocardinal veins for the development of the infrarenal part of the inferior caval and iliac veins (Fig.1C). Furthermore, Gladstone (1929) claimed that the main venous system above the diaphragm was not found lateral to the sympathetic trunk, as the supracardinal or ‘lateral sympathetic line’ veins below the diaphragm were, but medial. Therefore, the azygos venous system developed, in Gladstone's view, from yet another venous system, the ‘medial sympathetic line’ veins (Fig.1D).
Figure 1.
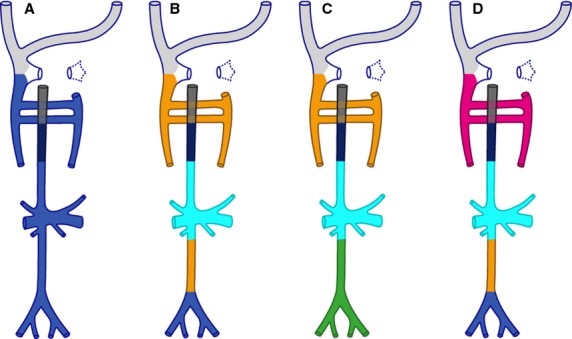
Different theories explaining inferior caval and azygos vein development. The scheme represents the adult configuration. The colour codes indicate the developmental origin of the veins according to the different theories on caudal vein development: light grey – superior caval and jugular veins; blue – derived from caudal cardinal veins; cyan – subcardinal veins; orange – supracardinal or lateral sympathetic line veins; pink – medial sympathetic line veins; green – sacrocardinal veins. The intrahepatic part of the inferior caval vein is shown in very dark blue and the hepatocardiac vein in grey. In (A), the entire caudal venous system originates from the caudal cardinal veins. In (B) the infrarenal part of the inferior caval and azygos venous systems originate from supracardinal veins, while the renal and associated veins are derive from the subcardinal system. (C) differs from (B) in that the infrarenal caval and iliac veins derive from the sacrocardinal veins. (D) is identical to (B) except that the azygos veins originate from the medial sympathetic line veins. In this last model, the infrarenal part originates from the supracardinal veins, which are now called lateral sympathetic line veins.
The reason why the development of the caudal venous system is so contentious, whereas the cranial is not, appears to rest in the presence of mesonephroi and the criteria used to define or distinguish separate venous systems. The mesonephros differs from the definitive kidney in that its renal tubules are perfused by a venous portal system that originates from the caudal cardinal veins rather than the arterial system that perfuses the renal tubules of the definitive kidney (Bremer, 1916; Butler, 1927; Tiedemann & Egerer, 1984; Carretero et al. 1997; Vollmerhaus et al. 2004). The efferent subcardinal veins drain mesonephric blood towards the liver.
The degree of mesonephros development varies between different mammalian species, from rudimentary in rodents [their mesonephroi have no glomeruli (Bremer, 1916; Butler, 1927)] to extremely large in pigs [its mesonephros is called the ‘Wolffian body’ and contains 54 glomeruli in a 10-mm (CS15) embryo (Bremer, 1916; Butler, 1927; Butler & Juurlink, 1987)]. Rabbits, cats and humans take an intermediate position, with 34 glomeruli in the human mesonephros at CS15 (Bremer, 1916; Huntington & McClure, 1920; Reagan & Ronbinson, 1927; Butler, 1927). Butler based his model largely on findings in species with well-developed and probably functional mesonephroi (McClure & Butler, 1925; Butler, 1927) and encountered considerable difficulties in adapting his model to species with either a very large or a very small mesonephros (Butler, 1927). It is, therefore, conceivable that Butler's model reflects these difficulties rather than the basic caudal venous body plan in mammals.
Another reason for the persisting controversy is the use of the topographical position of a vein or a venous network as a criterion for its identification rather than its developmental origin. In fact, the venous systems shown in Fig.1 are defined by their position relative to a prominent neighbouring structure such as the sympathetic trunk. This approach often leads to the assignation of a different name to the same vein or venous plexus, when its topographical position changes during successive stages of development due, for example, growth of a neighbouring organ. We, instead, argue in favour of the developmental origin of veins or venous plexuses as the sole criterion to assign a name. Ideally, such an approach would use genetically labelled cells, but such markers are not yet available for the caudal venous system. Instead, our present study shows that visualizing the three-dimensional topography of embryos that are closely spaced in developmental age, also allows us to follow the development of a vein or venous plexus over time.
We applied this approach in mouse and rat embryos, that is, in species in which the mesonephros hardly develops. This pilot study suggested that only the caudal cardinal veins contributed to the infrahepatic inferior caval and azygos vein development. In the present study, we tested this assumption in human embryos and confirmed that the simple developmental pattern also applies in a species with a well-developed mesonephros. To underscore our findings, we provide stylized yet realistic 3D models in the form of interactive PDFs of the successive stages of inferior caval and azygos vein development that can serve as reference for various anomalies that are found on CT scans or during surgery (Minniti et al. 2002).
Materials and methods
Embryos
We studied human embryos between 4 and 10 weeks of development from historical collections of the Academic Medical Centre (AMC) of the University of Amsterdam and the Leiden University Medical Centre (LUMC), and the Carnegie Collection in Washington (DC). The criteria of O'Rahilly as modified in 2010 (O'Rahilly & Muller, 2010) were used to determine the Carnegie stage of development.
Image acquisition and processing
Serial sections of embryos from the AMC and LUMC (Supporting Information Table S1), which had been stained with either haematoxylin and eosin or azophloxine, were digitized with an Olympus BX51 microscope and the dotslide program (Olympus, Zoeterwoude, The Netherlands). This program allows fully automated, high-resolution scanning of all sections on a glass slide. Digital images of serial sections from the Carnegie collection (Table S1) were obtained via the Virtual Human Embryo project (http://virtualhumanembryo.lsuhsc.edu). Resized JPEG images were converted to grey-scale images with photoshop cs5 (http://www.adobe.com). The image resizing factor was kept to a minimum to preserve detail and was correlated with embryo size. Voxel size was calculated from the microscopic magnification (X- and Y-axes) and the section thickness (Z-axis).
3D-reconstruction
amira (version 5.5; base package; FEI Visualization Sciences Group Europe, Mérignac Cédex, France) was used to generate 3D reconstructions after image loading, alignment and segmentation. All sections were automatically aligned using the least square method and then manually adjusted to account for correct curvatures in the sagittal and transverse planes of the body axis of the embryo with the help of photographic and magnetic resonance images (MRI) of age-matched human embryos [http://embryo.soad.umich.edu, http://virtualhumanembryo.lsuhsc.edu, http://www.ehd.org/ virtual-human-embryo, (O'Rahilly & Muller, 1987; Yamada et al. 2010; Pooh et al. 2011)]. Aligned slices were resampled into the Amira mesh-file format. Segmentation of vessels and organs was usually tried out first on printed images of the sections and discussed with colleagues. Thereafter, all labels were delineated manually in amira.
Visualization of the 3D reconstructions
Polygon meshes were created from the segmented labels and exported via ‘vrml export’ into cinema 4d (MAXON Computer GmbH, Friedrichsdorf, Germany). cinema 4d allows remodelling of the original Amira output with a ‘spline’ function for vessel reconstruction and a ‘sculpting’ function for organs. The accuracy of the remodelled cinema 4d images was always compared with the reconstructed amira images [cinema software allows simultaneous visualization of the amira and cinema renderings (Supporting Information Fig. S1)]. Furthermore, cinema 4d enabled export of modelled structures via ‘wrl export’ into adobe portable device format (PDF) reader version 9 (http://www.adobe.com), which allows the generation of three-dimensional interactive PDF files (Supporting Information Figs S2–S6).
Nomenclature
The parts of the cardinal venous system lying cranial or caudal to the heart are commonly referred to as posterior and anterior cardinal vein pairs. To make the nomenclature consistent, we refer to these as cranial and caudal cardinal veins. Superior and inferior as identifiers for cranial and caudal parts, respectively, of the caval vein are too established to abandon at present. For the same reason we retained the terms subcardinal and azygos veins.
Landmarks
To describe the precise topographic position of structures, landmarks such as somites, vertebrae, spinal ganglia, and intersegmental arteries were identified. In embryos up to CS16 we preferred the use of intersegmental arteries over spinal ganglia, as they arise directly from the aorta and are positioned near the developing venous systems. Spinal ganglia, which are positioned more dorsally, were used as markers when we could not positively identify an intersegmental artery. The exact somite level was deduced from the position of the 7th intersegmental artery (future subclavian artery) located between somites 10 and 11 (Muller & O'Rahilly, 2003; O'Rahilly & Muller, 2003). Between CS13 and CS15, somites differentiated into loose and dense zones that were reorganized (‘resegmented’) into the developing vertebrae after CS16. The 6th vertebra developed from the dense zone of somite 10 and the loose zone of somite 11, whereas the 7th vertebra developed from the dense zone of somite 11 and the loose zone of somite 12 (O'Rahilly & Muller, 2003). Once vertebral development begins, the 7th intersegmental artery is found in the loose zone of somite 11 and no longer between somites. Vertebral level differs from somite level by half a segment, with the vertebrae being more caudal. Cervical vertebra 1 develops, for example, from the dense zone of somite 5 and the loose zone of somite 6. Topographic positions up to CS16 were therefore corrected by subtracting 0.5 from the somite number to facilitate the comparison of younger and older stages. Subsequently, the length or height of a particular structure/organ was expressed in vertebral levels defined by a transverse slice perpendicular to the local curvature of the body axis.
Results
The development of the infrahepatic part of the inferior caval and azygos venous systems was followed over time. The caudal cardinal veins were first seen as bilateral caudal extensions (explaining their name) of the common cardinal vein in some of the CS11 and all of the CS12 embryos, while the inferior caval and azygos venous systems had acquired their definitive topography at CS20. To follow the changes in shape and topography of the veins and their surrounding organs we invite the reader to examine Figs S2–S6, which allow an interactive inspection of our reconstructed three-dimensional models. Furthermore, Fig.2 shows a pictorial abstract of our view of the changes of the infrahepatic inferior caval and azygos veins over time.
Figure 2.
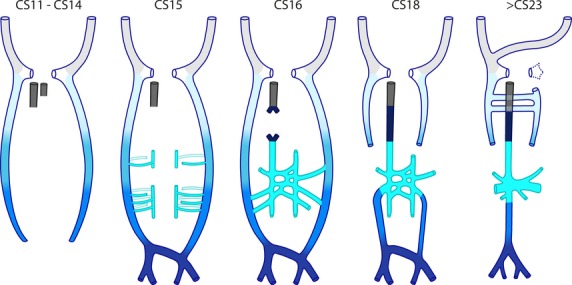
Schematic view of the development of the inferior caval and azygos venous systems in human. Colour code: light grey: cranial cardinal veins; gradient from light to dark blue – caudal extension of the caudal cardinal veins with time; cyan – subcardinal veins, with vessels dorsal (open) and ventral (filled) to the mesonephroi indicated separately; very dark blue –intrahepatic; grey – hepatocardiac vein. The essence of this ‘cardinal model’ is that the caudal cardinal veins first extend caudally between CS11 and CS15, and then form a ventral plexus around the mesonephroi at CS15. Between CS16 and CS20, the caudal extension transforms into the iliac veins and their confluence into the inferior caval vein, while the ventral extension transforms into the subcardinal veins and then the renal and associated veins. Major changes in the caudal cardinal veins are also their interruption at the level of the future diaphragm at CS18 and the disappearance of the left caudal part of the caudal cardinal vein at CS20. Between CS18 and CS23, the azygos venous system develops from the cranial part of the caudal cardinal veins.
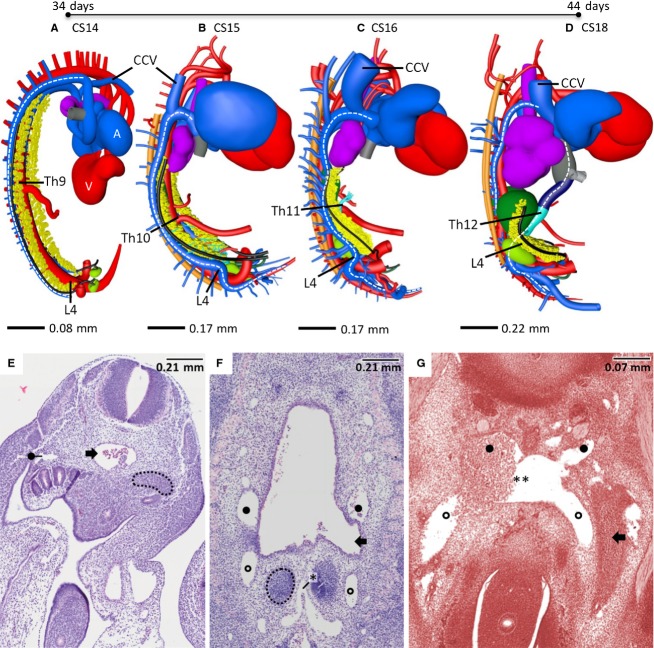
In the 3 weeks between CS11 and CS20, pronounced bends in the sagittal and transverse body axes developed as a result of rapid longitudinal growth between CS12 and CS14. These bends then resolved due to straightening of the body axes between CS14 and CS20 (Jackson, 1909; Auer, 1946; Pooh et al. 2011). Concurrent with the straightening of the body axes, structures ventral to the somites or vertebral column and cranial to the bifurcation of the aorta into the umbilical arteries were found to ‘descend’ relative to the somites or vertebral column (Fig.3).
Figure 3.
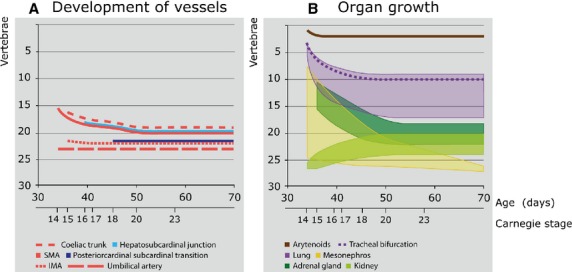
Growth, regression and migration of embryonic structures between 5 and 10 weeks of development. (A) Changes in position of vessels. At CS14, the superior mesenteric artery (SMA) branched from the aorta at the level of Th9 but gradually descended to an origin at the level L1 after CS20. The coeliac trunk, which could be identified from CS15 onward, showed a similar descent as the SMA, but one vertebral level higher. The entrance of the subcardinal vein into the liver, which is present from CS16 onwards, showed the same descent as the SMA. In contrast, the inferior mesenteric artery (IMA), which originated at the level of L2 at CS15, only descended one vertebra up to CS20, and the umbilical arteries and the caudal cardinal-subcardinal transition did not descend at all. (B) Changes in craniocaudal length of organs. At CS14, the mesonephroi (yellow) were identifiable between Th2 and L5. Their caudal pole gradually extended caudally between CS15 and CS23 to the level of S2, while their cranial pole regressed from Th3 at CS15 to L4 at CS23, with only a few nephrons left at 10 weeks of development. The caudal pole of the definitive kidneys (light green) was located at the level of S2 at CS14 and ascended to L5 at CS20, whereas their cranial pole gradually expanded from the level of S1 at CS14 to L1 at CS20. The adrenal glands (dark green) were first identifiable at CS15 between the level of Th4 and Th9, but gradually descended to between Th11 and L3 at CS20. The lungs (purple) expanded and descended from C3 to C6 at CS14 to the level of Th2 to Th10 at CS20. The tracheal bifurcation (interrupted purple line) followed the same course. The apex of the oesophagotracheal septum (subsequently the position of the developing arytenoid swellings; brown continuous line) descended only one vertebra between CS14-15. Note that on the Y-axis, vertebral levels 1–7 correspond to C1-7, levels 8–19 to Th1-12, and levels 20–24 to L1-5.
Organ growth in the urogenital ridge during caudal venous development
As many of the arguments about the fate of the caudal cardinal veins centre on the origin of the azygos veins and infrarenal part of the inferior caval vein, we hypothesized that the organs growing in the urogenital ridge [mesonephroi, adrenal glands, and definitive kidneys (metanephroi); Figs3B, 6] play a key role in caudal venous development.
Figure 6.
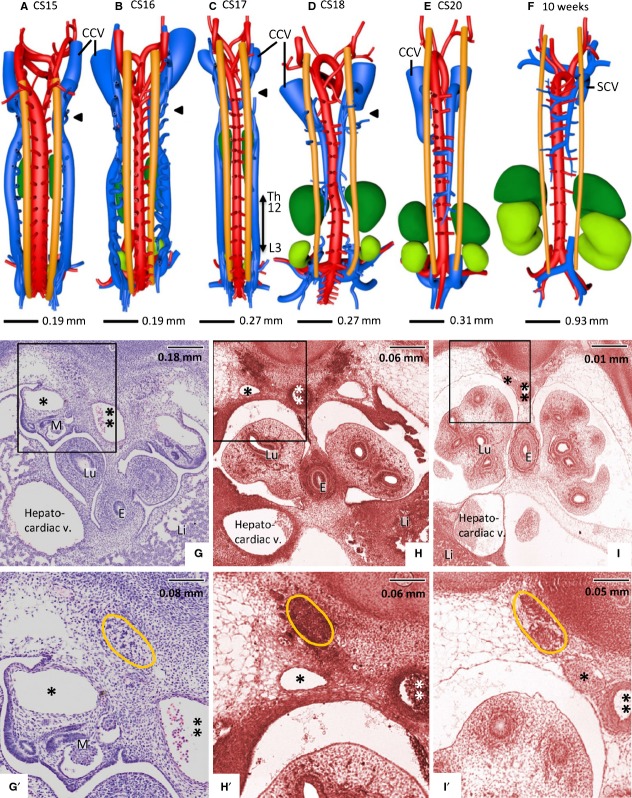
Development of the azygos venous system. (A–F) show dorsal views of veins and relevant structures from embryos of the indicated stages of development. (G–I) and (G'–I', magnifications of G–I), histological views of gradual rearrangement of the caudal cardinal veins relative to the sympathetic trunks at CS15 (G/G'), CS17 (H/H') and CS18 (I/I') taken from the level at which the hepatocardiac vein emerges from the liver. The caudal cardinal veins (blue in A–D and indicated with an asterisk in G–I and G'–I') change in a craniocaudal sequence (A–D; black arrowheads are positioned at level Th2) from a lateral to a medial position relative to the adrenal glands, definitive kidneys and sympathetic trunks (A–D and G–I, G'–I'). The intersegmental veins draining into the caudal cardinal veins disappear between vertebral levels Th12 and L3 (double-headed arrow in C) at CS17, followed by the interruption of the caudal cardinal veins in the same area at CS18 (D). The remaining caudal part of the right-sided caudal cardinal vein becomes the infrarenal part of the inferior caval vein, while the remaining cranial parts become the azygos and hemi-azygos veins (D–F). Note the disappearance of the cranial part of the hemi-azygos vein concurrent with the development of extensive anastomoses between the cranial cardinal veins (brachiocephalic vein) and between hemi-azygos and azygos veins (F). The color codes in (A–F) are identical to those in Fig.4. Note that the sympathetic trunks are only reconstructed between C6 and L5. CCV, cranial cardinal vein; SCV, superior caval vein. (G–I and G'–I') The aorta is indicated with a double asterisk; sympathetic trunk is encircled in orange; Li, liver; Lu, lungs; E, oesophagus; M, mesonephros.
Mesonephroi
In CS12 embryos, the bilateral mesonephroi extended from the level of C5 (just caudal to the heart) to the level of L3 (the end point of neural tube closure). In CS13 and CS14 embryos the mesonephroi spanned vertebral level C7 to L5, corresponding to the distance from the upper limb bud to the lower limb bud. After CS14, the mesonephroi gradually regressed at their cranial border, but still extended at their caudal border, with only a few nephrons left at the bifurcation of the umbilical (future internal) and external iliac arteries at 10 weeks (Figs3B and 5A–E). The mesonephroi were situated lateral to the aorta and ventral to the caudal cardinal veins. Their tubules drained into the mesonephric (Wolffian) duct, the position of which gradually changed in time from a dorsolateral to ventrolateral relative to the mesonephroi. About four small arteries branched bilaterally from the aorta to the mesonephric glomeruli. At CS14, these arteries originated cranial to the superior mesenteric artery (SMA). During mesonephric development, the same number of arterial branches continued to supply the mesonephric glomeruli, but with mesonephric regression, their origin from the aorta gradually moved to a more caudal position. Eventually all branches were situated caudal to the SMA. The mesonephric tubules are perfused by blood from the cardinal veins (so-called portal circulation). Blood from the mesonephric nephrons drained into the subcardinal veins, which were found on the ventromedial side of the mesonephroi.
Figure 5.
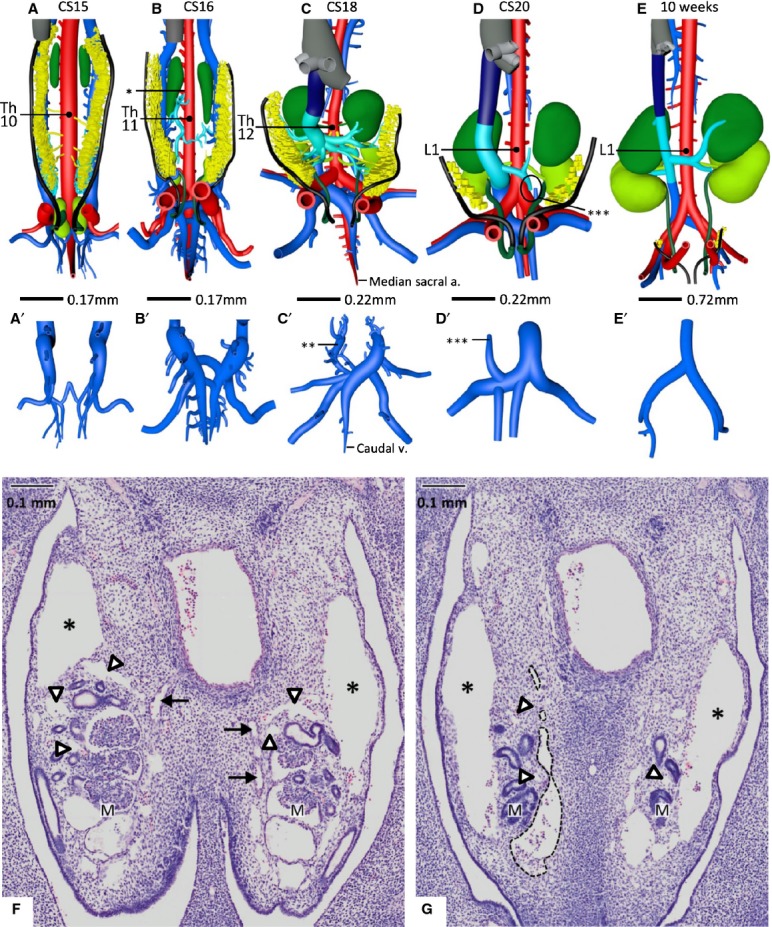
Development of the subcardinal veins as ventral outgrowths of the caudal cardinal veins. (A–E) Ventral views and (A'–E') corresponding dorsal views of the most caudal part of the caudal cardinal veins. (F,G) Histological views of subcardinal vein development. The subcardinal vascular network is developing in and around the mesonephroi from CS15 onwards (A,F,G), in particular in its caudal part, in continuity with the caudal cardinal veins that are positioned at the dorsolateral side of the mesonephroi (* in F and G; panels corresponding with vertebral levels L1 and L3, respectively). Some small vessels of the plexus inside the mesonephroi are indicated with arrowheads. The subcardinal system forms a bilateral longitudinal channel on the ventromedial side of the mesonephroi (one vessel lined with a black interrupted line in G). Just caudal to the SMA (the origin of the SMA from Th10 to L1 at different Carnegie stages is indicated in A–E), the left and right subcardinal vein formed intersubcardinal anastomoses at CS16 (B) and began to drain (* in B) into the capillary network of the liver. Furthermore, at CS15 the caudal cardinal veins had completed their caudal extension dorsally to the umbilical arteries and along the median sacral artery, and had started to form intercardinal anastomoses to form the confluence of the iliac veins (A'–E'). The caudal part of the left caudal cardinal vein began to regress at CS18 (** in C') and lost its continuity with the left subcardinal vein at CS20 (*** in D and D'). Between 7 (D) and 10 weeks (E), no new connections developed. The median sacral artery and caudal vein (indicated in C and C') were still prominent vessels at CS18, but began to regress thereafter. The colour code is identical to that in Fig.4. The arterial branches to the adrenal glands, mesonephroi and definitive kidneys are given in the colour of the supplied organ. (F) Mesonephric arteries are indicated with black arrows. M, mesonephros.
Definitive kidneys
Close to the outlet of the mesonephric duct into the cloaca and in between both umbilical arteries (corresponding to L4), the ureteric bud sprouted from the mesonephric duct at CS13 and began to grow in a dorsal direction to the metanephric blastema (note that ‘dorsal’ relates to the local topography; because of the pronounced bend of the body axis in this region, the dorsal side of the embryo changes ∼180°). The definitive kidneys were first identifiable at CS14 from vertebral level S1 to S2 and subsequently expanded cranially to occupy a position between vertebral level L1 and L5 by CS20 (Figs3B and 5A–E). At this stage (∼7 weeks of development), the renal arteries were first observed at the level of L3. The definitive kidneys occupy a key position between the renal and infrarenal parts of the inferior caval vein.
Adrenal glands
The adrenal glands were first identified at CS15 between vertebral level Th4 and Th9. Strikingly, there was, therefore, temporarily an ‘empty’ space of about seven vertebrae between the caudal pole of the developing adrenal glands and the cranial pole of developing definitive kidneys. This gap gradually disappeared during the next five Carnegie stages due to the descent of the adrenal glands relative to the vertebral column over seven vertebrae and a cranial expansion of the definitive kidneys by one vertebra. As a result, the cranial pole of the definitive kidneys came to overlap with the caudal pole of the adrenal glands between CS18 and CS20 (Figs3B and 6D–F). The craniocaudal length of the adrenal glands (expressed in vertebral levels) hardly changed over time, but their cylindrical shape at CS15 gradually transformed into a round shape at CS18, reflecting their pronounced mediolateral expansion (Figs5 and 6).
Lung growth during caudal venous development
The expansion of the lungs also determined craniocaudal and mediolateral changes in position of the caudal cardinal veins (Figs3B and 4A–D). The apex of the oesophagotracheal septum (subsequently the position of the developing arytenoid swellings) was a fixed point, as it descended only by one vertebra between CS14 and CS15, from just cranial to vertebral level C1 to the level of C2 (Fig.3B). The tracheal bifurcation, which corresponded to the level of C3 at CS14, descended seven vertebrae to the level of Th3 at CS20, which corresponds to the adult position. The lungs themselves were situated between vertebral level C3 and C5 at CS14. During the next 16 days, the lungs gradually expanded to a craniocaudal length of eight vertebrae and descended five vertebral levels, so that they occupied Th2–Th10 relative to the vertebral column at CS20 (Fig.3B).
Figure 4.
Development of the caudal cardinal veins. (A–D) Right-sided views of vessels and relevant organs in embryos between CS14 and CS18. (E–G) Histological views of the caudal extension of the caudal cardinal veins at CS14, CS15 and CS18. The caudal cardinal veins had not yet extended beyond the umbilical arteries at CS14 (A and E; black dot), but had done so at CS15 [B and F,G; black circle (future iliac veins)]. Adaptation of the course of the caudal cardinal veins to changes in size and/or position of the underlying organs (lungs, mesonephroi and umbilical arteries) resulted in a transition of a single ‘C’-shape (A; stippled white line in CS14) into a triple ‘C’-shape from CS15 onwards, with the adrenal glands and definitive kidneys developing between the caudal cardinal veins and aorta (A–C). Furthermore, intercardinal anastomoses started to develop at CS15 (* in F), from which the iliac veins (black circle in G) and their confluence into the inferior caval vein originated (CS18; ** in G). Note that the caudal cardinal veins became interrupted at CS18 (D). Th9–Th12 correspond to the level of the origin of the SMA in different Carnegie stages. Vertebral level L4 corresponds to the bifurcation of the aorta into the umbilical arteries. The dorsally oriented arterial and venous ‘spikes’ represent the intersegmental arteries and veins. (A–D) CCV, cranial cardinal vein; V, ventricles; A, atria. The colour codes in Figs 4, 5 and 6 are: yellow – mesonephroi; black – Wolffian duct; light green – definitive kidneys; green – adrenal glands; purple – lungs; orange – sympathetic trunk; red – arterial system; blue – caudal cardinal veins; cyan – subcardinal veins; very dark blue – intrahepatic part inferior caval vein; grey – hepatocardiac vein. (E–G) Black arrow, umbilical artery; stippled line, caudal pole of definitive kidney.
The early development of the caudal cardinal veins
The caudal cardinal veins were first observed at CS11/12, that is, in the first half of the 5th week of development, as bilateral caudal extensions of the common cardinal vein lying dorsolateral to the aorta and developing mesonephroi. At CS13/CS14, the caudal cardinal veins coursed along the mesonephroi but had not yet reached the caudal pole of the mesonephroi and did not yet extend beyond the umbilical arteries (corresponding to L4) (Figs4A,E and S2). The trajectory of these caudal cardinal veins was still complementary to the shape of the longitudinal body axis (‘C’-shaped) (Fig.4A). Intersegmental veins coursed between the somites towards the ipsilateral caudal cardinal veins over the entire length of the cardinal veins. During CS15, in the first half of the 6th week, the caudal cardinal veins extended caudally into the most caudal body region, that is, along the median sacral artery while passing the umbilical arteries dorsally (Figs4B,F, 5A and S3).
The position of the caudal cardinal veins relative to the aorta changed strikingly between CS15 and CS16 because the veins adapted their course to the changing contours of the underlying organs (Figs4B,C, S3 and S4). The adrenal glands and definitive kidneys expanded dorsolaterally into the space between the aorta and caudal cardinal veins (Figs4B,C, 5A,B and 6A,B), while the lungs also expanded dorsolaterally, but passed the caudal cardinal veins ventrolaterally (Fig.4B,C). Where the caudal cardinal veins passed the expanding lungs, mesonephroi and umbilical arteries, they retained dorsolateral position relative to the aorta, whereas in between these expanding organs, they gradually acquired a ventrolateral position. As a result, the caudal cardinal veins assumed a triple ‘C’-shape in a lateral view (Fig.4B,C).
The caudal-most part of the caudal cardinal veins along the median sacral artery changed position relative to this artery from lateral at CS15 to ventrolateral at CS16. Meanwhile, the left and right caudal cardinal veins began to form small intercardinal anastomoses ventral to the median sacral artery at CS15, which expanded in number and diameter at CS16 (Fig.5A,A',B,B'). These anastomoses will form parts of the iliac veins and the median caudal vein. The confluence of both common iliac veins into the inferior caval vein developed from these left–right connections (Figs4F,G and 5C,C'). The median caudal vein as well as the median sacral artery regressed at between CS18 and 10 weeks' development.
Starting at CS15 and continuing up to CS18, that is, during the sixth and the first part of the 7th week of development, the part of the caudal cardinal veins overlying the lungs and mesonephroi began to assume, in a craniocaudal sequence, a more medial position relative to these organs and the adrenal glands, definitive kidneys, and sympathetic trunks (Fig.6A–D,G,G'–I,I'). Furthermore, the intersegmental veins disappeared between the level of Th12 and L3 during CS17 (Fig.6C), that is, from the area overlying the mesonephroi. In the next stage (CS18), the continuity of the caudal cardinal veins was lost in the same area (Th12-L3), now corresponding to the position of the future diaphragm (Figs4D, 6D and S5).
The development of the subcardinal veins
At CS15, when the caudal expansion of the caudal cardinal veins was nearly complete, another venous plexus began to develop from the caudal cardinal veins that surrounded the mesonephroi medially and laterally (Figs5A,F,G and S3). Especially in the caudomedial region of the mesonephroi, this venous plexus was well developed. At CS16, a longitudinal channel began to form from this venous plexus ventromedial to both mesonephroi to establish what is generally known as the subcardinal venous system. The left and right subcardinal veins started forming anastomoses just caudal to the SMA (Fig.5B). The right-sided dominance of the subcardinal venous system was first observed at CS16 and complete at CS18, when the right-sided subcardinal vein merged cranially with the sinusoidal venous system of the liver to form the so-called hepatosubcardinal junction (Fig.5B, asterisk) (Huntington & McClure, 1920; McClure & Butler, 1925; Butler, 1927). In addition, small venous branches from the adrenal glands were observed to drain into the subcardinal system (Fig.5C).
At the caudal region of the mesonephroi, where the subcardinal venous plexus was well-developed, a connection between the dorsolateral caudal cardinal and ventromedial subcardinal veins expanded in diameter on both sides during CS18 to become the main conduits of venous blood from the caudal body region toward the heart (Figs4D, 5C,C' and S5). These conduits, which occupied an almost transverse position, were located at vertebral level L3 (Figs3A, 4D and 5C).
On the left side, however, this conduit and the associated caudal portion of the left caudal cardinal vein soon regressed and had lost continuity with the left subcardinal vein by CS20 (Fig.5D). The left-sided subcardinal vein itself persisted as the draining vein of the left definitive kidney and adrenal gland (Fig.5D). No further developmental modifications of the architecture of the inferior caval vein were observed up to 10 weeks' development except for its transformation into a smooth longitudinal channel (Figs5E and S6).
The development of the azygos veins
The cranial part of both caudal cardinal veins still accompanied the aorta dorsolaterally up to Th12 at CS18, but the disappearance of the intersegmental veins at CS17 and the subsequent interruption of the continuity of the caudal (lumbar) and cranial (thoracic) parts of the caudal cardinal veins showed that the main venous return had been taken over by the inferior caval vein. This resulted in a decrease of vessel diameter of the remaining cranial part of the caudal cardinal veins (Fig.6D). Furthermore, the position of the caudal cardinal veins relative to the sympathetic trunks, which had progressively changed from lateral to ventral between CS15 and CS17 (Fig.6A–C,G,G',H,H'), moved further medially at CS18 (Fig.6D,I,I'). Concurrently with the disappearance of the cranial part of the left-sided cranial cardinal vein between CS21 and CS22 and the formation of the brachiocephalic vein, the cranial part of left-sided caudal cardinal vein became smaller in diameter, was interrupted at several sites, and formed intercardinal anastomoses with the right-sided cranial part of the caudal cardinal vein (Fig.6E,F). This description shows that the cranial part of the caudal cardinal veins had transformed into the right-sided azygos and left-sided hemi-azygos veins by the end of the 7th week of development. No additional modifications in the architecture of the azygos system were observed up to 10 weeks. Noteworthy, we did not observe a connection between the azygos system (Th12) and the remaining parts of the caudal cardinal veins in the caudal region (‘lumbar’ veins) in any of the embryos (Fig.6D–F).
Discussion
Key findings of our study (Fig.2 shows a pictorial abstract) were that the caudal cardinal veins extended caudally between CS11 and CS15 and that the subcardinal veins began to develop as a venous plexus sprouting from the caudal cardinal veins when the caudal expansion of the caudal cardinal veins neared completion at CS15. Between CS15 and CS18, the caudal cardinal veins adapted their course from lateral to medial relative to the laterally expanding lungs, adrenal glands, definitive kidneys, umbilical arteries and sympathetic trunks. At CS18, the caudal cardinal veins themselves became interrupted in the part that overlay the regressing mesonephroi and corresponded to the future diaphragm (Th12-L3). This description shows that the infrarenal part of the inferior caval vein originated from the (right) caudal cardinal vein, whereas the renal part originated from subcardinal veins. The azygos venous system developed from the cranial part of the caudal cardinal veins.
Based on the gradual expansion of the caudal cardinal veins and the gradual changes in the topography of its parts, we see no need to invoke supracardinal, sacrocardinal or medial and lateral sympathetic line veins to explain infrahepatic inferior caval and azygos vein development and, therefore, reject the hypothesis that the caudal venous system is made up from several independently developing components.
Although interindividual variation in the topography of the venous system is well established, we observed remarkably little variation in venous development between embryos of comparable stage. We hypothesize that this apparent discrepancy is due to the fact that variations or malformations often represent ‘frozen stages’ of normal development or, especially in the case of veins, adaptations to changes in blood flow. Embryos which were old enough that they could have incurred a delayed or deviant development, represented a minority in the specimens we studied. Even with a high prevalence, the chance of studying an embryo with a variation would, therefore, be small.
The azygos veins develop from the caudal cardinal veins
Most authors agree upon the development of the azygos venous system from supracardinal or ‘medial sympathetic line veins’ (Huntington & McClure, 1920; McClure & Butler, 1925; Butler, 1927, 1950; Gladstone, 1929; Grünwald, 1938; Cornillie et al. 2008a,b). The supracardinal veins would originate from anastomoses between the intersegmental veins that formed a longitudinal channel dorsomedial to the caudal cardinal veins and that drained into the caudal cardinal veins at the level of Th1 (Huntington & McClure, 1920; McClure & Butler, 1925). After CS18, separate thoracic and lumbar parts would persist. As Butler and colleagues only described the position of the supracardinal veins relative to the caudal cardinal veins and did not take into account their position relative to the sympathetic trunks, Gladstone (1929) invoked medial and lateral sympathetic line veins to explain why the lumbar part of the supracardinal veins was found lateral to the sympathetic trunks and medial in the thoracic part (Fig.1B,D). Both models require regression of the thoracic part of the caudal cardinal veins. We did not observe the regression of a venous system in the thoracic region. Instead, we observed a gradual lateral-to-medial shift in position of the cranial (thoracic) part of the caudal cardinal veins relative to the sympathetic trunks between CS16 and CS18, probably resulting from the expansion of the lungs, as already suggested by Auer (1946). The cranial connection of the left caudal cardinal vein with the common cardinal vein only disappeared after extensive intercardinal anastomoses, including the formation of the brachiocephalic vein, had developed at CS23.
Supracardinal veins do not replace the caudal cardinal veins
Although Butler and colleagues supported a caudal cardinal origin of the infrarenal part of the inferior caval vein in mammals with poorly developed mesonephroi, such as rodents, they claimed that efficient blood drainage of the dorsal body wall and blood supply to the mesonephroi could not be accommodated by the caudal cardinal veins and therefore a new system, the supracardinal (McClure & Butler, 1925; Butler, 1927) or lateral sympathetic line veins (Gladstone, 1929), had to develop (Fig.1B,D). During CS14–CS20, when the body axis straightened, the definitive kidneys expanded away from the umbilical arteries in a cranial (Gruenwald, 1943; Auer, 1946) but also lateral direction, so that the relative position of the caudal cardinal veins with respect to the definitive kidneys changed from dorsal to medial. This gradual change in position of the caudal cardinal veins, which was also described by Auer (1946) and Cornillie et al. (2008a,b), therefore does not require postulation of any new venous system.
The infrarenal portion of the inferior caval and iliac veins does not arise from a sacrocardinal system
Gladstone (1929) first described the extension of caudal cardinal veins into the caudal-most body region while bending round the definitive kidneys and umbilical arteries. Grünwald (1938) and later Fasel & Ludwig (1988) postulated that this ‘C’-shaped course of the caudal extensions of the caudal cardinal veins resulted from the development of a new pair of veins, the ‘sacrocardinal’ veins, because the caudal cardinal veins would not be able to pass the umbilical arteries dorsolaterally at CS15 or to migrate from lateral to medial relative to the definitive kidneys between CS17 and CS18 (Fig.1C). As hypothesized earlier (Gest & Carron, 2003), the umbilical arteries, like the mesonephric arteries, most likely represent lateral branches from the aorta, so that the caudal cardinal veins are always dorsal relative to these structures. Furthermore, Cornillie et al. (2008a,b) recently described in pig embryos that the caudal cardinal veins extended over the umbilical arteries towards the caudal body region. As stated in the paragraph above, the lateral-to-medial movement of the infrarenal part of the caudal cardinal veins relative to the definitive kidneys occurred gradually and could be attributed to a dorsolateral expansion of the underlying organs. Furthermore, we did not find any trace of a newly developing venous plexus on the medial side of the definitive kidneys between CS15 and CS18.
Are the caudal cardinal and subcardinal veins two separate entities?
Hochstetter (1893) described that the caudal cardinal veins split up into medial and lateral divisions (Fig.1A), while Lewis (1902), and most authors since, coined the term ‘subcardinal’ vein to refer to the ventromedial branch. The subcardinal veins drain the portal venous blood from the caudal cardinal veins that perfuses the mesonephric tubules and the arterial blood that perfuses the mesonephric glomeruli (Vollmerhaus et al. 2004). We showed that the caudal cardinal veins formed a venous plexus in and around the mesonephroi between CS15 and CS18, and that this plexus then formed large longitudinal veins at the ventromedial side of the mesonephroi. It, therefore, appears to be a matter of interpretation whether the subcardinal veins are said to develop from the caudal cardinal veins, as described by Hochstetter (1893), and by Shore (1901) and Kampmeier (1920) for amphibians, or as an independent system. Although we agree with Hochstetter and colleagues with respect to the lineage question, we have opted to retain the term ‘subcardinal’ because the caudal cardinal and subcardinal veins differ functionally in that they supply and drain the organs of the urogenital ridge, respectively. In this respect, the cardinal and subcardinal veins resemble the portal and hepatic veins of the liver, which have a similar origin but different names because they differ in topography and function, being the conduits of afferent and efferent hepatic blood, respectively.
Abnormal expansion or persistence of the caudal cardinal veins causes malformations
Malformations that are commonly seen and that are of clinical importance for paediatric cardiologists and radiologists are the absence of the intrahepatic inferior caval vein (suprarenal anomaly), a retro- or circumaortic renal vein (renal anomaly), a retrocaval ureter, or a double- or left-sided inferior caval vein (infrarenal anomalies) (Chuang et al. 1974; Minniti et al. 2002; Malaki et al. 2012). Suprarenal, renal and infrarenal variants or anomalies can be explained as persisting parts of the caudal cardinal veins that normally regress, development and persistence of extra left-right anastomoses, and persistence of more than one connection between the caudal cardinal and subcardinal veins, respectively.
Suprarenal anomalies appear to arise as a failure of the formation of the hepatosubcardinal junction at CS16. Left isomerism, a heterotaxia syndrome, is often characterized by the absence of the hepatosubcardinal junction, presumably due to the absence of right-sided structures, including the right vitelline vein. As the main blood return cannot pass through the liver, one or both caudal cardinal veins remain the main drainage vessels for the caudal part of the body and do not become interrupted at the level of the diaphragm, but persist as ‘azygos continuation’ in >80% of cases (Sharma et al. 1987). Being aware of this anomaly is important when catheterizing a heart or differentiating a right-sided paratracheal or mediastinal mass from the outflow of an enlarged azygos vein into the superior caval vein (Mathews et al. 1999; Bass et al. 2000; Shindo et al. 2000).
Normally, the left renal vein arises from intersubcardinal anastomoses at the ventral side of the aorta. However, a renal vein that courses dorsal to the aorta is also commonly observed. This anomaly probably originates from the development of an extra intercardinal anastomosis. Intercardinal anastomoses normally develop between the cranial cardinal veins (brachiocephalic vein) and the caudal cardinal veins in the thorax (between hemi-azygos and azygos veins) and abdomen (confluence iliac veins into the inferior caval vein). If an intercardinal rather than an intersubcardinal anastomosis persists, a retroaortic renal vein will be found, whereas the persistence of both an intercardinal and intersubcardinal anastomosis will give rise to a circumaortic renal vein. Such extra collateral channels or positional changes are relevant during kidney transplantation (Srivastava et al. 2005).
A double (infrarenal) inferior caval vein originates from a persistent caudal part of the left-sided caudal cardinal vein. This anomaly is associated with venous hypertension and deep-vein thrombosis in the lower limbs due to inadequate venous return (Malaki et al. 2012). A left- rather than a right-sided inferior caval vein is a mirror image in which the left caudal cardinal vein persists and the right one regresses, and is often misdiagnosed as lymphadenopathy (Bass et al. 2000).
Normally, a single cardinal-subcardinal anastomosis persists infrarenally at vertebral level L3 after CS18. A more caudal transition of the caudal cardinal into the subcardinal vein than usually found causes a retrocaval ureter. If the initial junctional plexus between the caudal cardinal and subcardinal veins encompassed the ureter, a peri-ureteric venous ring may form. Such abnormally located veins are often observed in patients with aberrant position of the ureter and can cause recurrent urinary tract infections and (right) ureteral obstruction (Bass et al. 2000; Perimenis et al. 2002).
Conclusion
We reinvestigated the development of the infrahepatic part of the inferior caval and azygos venous systems in human to distinguish concepts that account for developmental changes in the appearance of the caudal venous system. Our findings, which are summarized in Fig.2, clearly support a module in which the infrahepatic part of the inferior caval vein develops from contributions of the caudal cardinal veins or from the subcardinal veins, i.e. a plexus sprouting from the caudal cardinal veins. The azygos venous system represents the cranial part of the caudal cardinal veins. The caudal cardinal and subcardinal veins remodel continuously due to adaptive changes in their position in response to organ growth.
Acknowledgments
We thank Drs Maurice van den Hoff (AMC) and Marco de Ruiter (LUMC) for allowing us to use their institutional series of human embryos. Additional embryos came from the Virtual Human Embryo project (Dr John Cork; Cell Biology & Anatomy, LSU Health Sciences Center, New Orleans; http://virtualhumanembryo.lsuhsc.edu), who made digitized sections available to us. Further, special thanks go to Jaco Hagoort (AMC) for his help in implementing the 3D-PDFs and to Els Terwindt and Greet Mommen (MU) for their technical assistance. The financial support of ‘Stichting Rijp’ is gratefully acknowledged.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Quality check of image transformation from amira 3d to cinema 4d.
Interactive 3D-PDFs of the caudal venous system in human embryos of CS14, CS15, CS16, CS18 and 10 weeks of development, respectively.
Human embryos that were reconstructed and used in the present study.
References
- Auer J. Migration processes during ontogeny with reference to the venous development in the dorsal body wall. J Anat. 1946;80:61–74. [PubMed] [Google Scholar]
- Bass JE, Redwine MD, Kramer LA, et al. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics. 2000;20:639–652. doi: 10.1148/radiographics.20.3.g00ma09639. [DOI] [PubMed] [Google Scholar]
- Bremer JL. The interrelations of the mesonephros, kidney and placenta in different classes of animals. Am J Anat. 1916;19:179–209. [Google Scholar]
- Butler EG. The relative role played by embryonic veins in the development of the mammalian vena cava posterior. Am J Anat. 1927;39:267–353. [Google Scholar]
- Butler H. The development of the azygos veins in the albino rat. J Anat. 1950;84:83–94. [PMC free article] [PubMed] [Google Scholar]
- Butler H, Juurlink BHJ. An Atlas for Staging Mammalian and Chick Embryos. Boca Rotan, FL: CRC Press; 1987. [Google Scholar]
- Carretero A, Ditrich H, Navarro M, et al. Afferent portal venous system in the mesonephros and metanephros of chick embryos: development and degeneration. Anat Rec. 1997;247:63–70. doi: 10.1002/(SICI)1097-0185(199701)247:1<63::AID-AR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Chuang VP, Mena CE, Hoskins PA. Congenital anomalies of the inferior vena cava. Review of embryogenesis and presentation of a simplified classification. Br J Radiol. 1974;47:206–213. doi: 10.1259/0007-1285-47-556-206. [DOI] [PubMed] [Google Scholar]
- Cornillie P, Simoens P. Prenatal development of the caudal vena cava in mammals: review of the different theories with special reference to the dog. Anat Histol Embryol. 2005;34:364–372. doi: 10.1111/j.1439-0264.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- Cornillie P, Van Den Broeck W, Simoens P. Three-dimensional reconstruction of the remodeling of the systemic vasculature in early pig embryos. Microsc Res Tech. 2008a;71:105–111. doi: 10.1002/jemt.20531. [DOI] [PubMed] [Google Scholar]
- Cornillie P, Van Den Broeck W, Simoens P. Origin of the infrarenal part of the caudal vena cava in the pig. Anat Histol Embryol. 2008b;37:387–393. doi: 10.1111/j.1439-0264.2008.00868.x. [DOI] [PubMed] [Google Scholar]
- Fasel J, Ludwig KS. Eine partiell verdoppelte Vena cava inferior: klinische, makroscopische und embryologische Anatomie. Gegenbaurs Morphol Jahrb. 1988;134:143–153. [PubMed] [Google Scholar]
- Gest TR, Carron MA. Embryonic origin of the caudal mesenteric artery in the mouse. Anat Rec. 2003;271A:192–201. doi: 10.1002/ar.a.10022. [DOI] [PubMed] [Google Scholar]
- Gladstone R. Development of the inferior vena cava in the light of recent research, with especial reference to certain abnormatilities, and current descriptions of the ascending lumbar and azygos veins. J Anat. 1929;64:70–93. [PMC free article] [PubMed] [Google Scholar]
- Gruenwald P. The normal changes in the position of the embryonic kidney. Anat Rec. 1943;85:163–176. [Google Scholar]
- Grünwald P. Die Entwickelung der Vena cava caudalis beim Menschen. Z Microsk-Anat Forschung. 1938;43:275–331. [Google Scholar]
- Hochstetter F. “Beiträge zur Entwicklungsgeschichte des Venensystems der Amnioten.” Sauger. Gegenbaurs Morphol Jahrb. 1893;20:543–648. [Google Scholar]
- Huntington GS, McClure CFW. The development of the veins in the domestic cat with special reference, 1) to the share taken by the supracardinal veins in the development of the postcava and azygos veins and 2) to the interpretation of the variant conditions of the postcava and its tributaries, as found in the adult. Anat Rec. 1920;20:1–30. [Google Scholar]
- Jackson CM. On the developmental topography of the thoracic and abdominal viscera. Anat Rec. 1909;3:361–396. [Google Scholar]
- Kampmeier OF. Changes of the systemic venous plan during development and the relation of the lymph hearts to them in Anura. Anat Rec. 1920;19:82–96. [Google Scholar]
- Lewis FT. The development of the vena cava inferior. Am J Anat. 1902;1:229–244. [Google Scholar]
- Malaki M, Willis AP, Jones RG. Congenital anomalies of the inferior vena cava. Clin Radiol. 2012;67:165–171. doi: 10.1016/j.crad.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Mathews R, Smith PA, Fishman EK, et al. Anomalies of the inferior vena cava and renal veins: embryologic and surgical considerations. Urology. 1999;53:873–880. doi: 10.1016/s0090-4295(99)00007-2. [DOI] [PubMed] [Google Scholar]
- McClure CFW, Butler EG. The development of the posterior vena cava inferior in man. Am J Anat. 1925;35:331–383. [Google Scholar]
- Minniti S, Visentini S, Procacci C. Congenital anomalies of the venae cavae: embryological origin, imaging features and report of three new variants. Eur Radiol. 2002;12:2040–2055. doi: 10.1007/s00330-001-1241-x. [DOI] [PubMed] [Google Scholar]
- Muller F, O'Rahilly R. Segmentation in staged human embryos: the occipitocervical region revisited. J Anat. 2003;203:297–315. doi: 10.1046/j.1469-7580.2003.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rahilly R, Muller F. Developmental Stages of Human Embryos, Including a Revision of Streeter's ‘Horizons'and a Survey of the Carnegie Collection. Washington, DC: Carnegie Institute of Washington; 1987. [Google Scholar]
- O'Rahilly R, Muller F. Somites, spinal ganglia, and centra. Enumeration and interrelationships in staged human embryos, and implications for neural tube defects. Cells Tissues Organs. 2003;173:75–92. doi: 10.1159/000068948. [DOI] [PubMed] [Google Scholar]
- O'Rahilly R, Muller F. Developmental stages in human embryos: revised and new measurements. Cells Tissues Organs. 2010;192:73–84. doi: 10.1159/000289817. [DOI] [PubMed] [Google Scholar]
- Perimenis P, Gyftopoulos K, Athanasopoulos A, et al. Retrocaval ureter and associated abnormalities. Int Urol Nephrol. 2002;33:19–22. doi: 10.1023/a:1014436432109. [DOI] [PubMed] [Google Scholar]
- Pooh RK, Shiota K, Kurjak A. Imaging of the human embryo with magnetic resonance imaging microscopy and high-resolution transvaginal 3-dimensional sonography: human embryology in the 21st century. Am J Obstet Gynecol. 2011;204:77. doi: 10.1016/j.ajog.2010.07.028. e71–16. [DOI] [PubMed] [Google Scholar]
- Reagan FP, Ronbinson A. The later development of the inferior vena cava in man and in carnivora. Proceedings of the Anatomical Society of Great Britian and Ireland. J Anat. 1927;61:482–484. [Google Scholar]
- Sharma SHIV, Devine W, Anderson RH, et al. Identification and analysis of the left atrial isomerism. Am J Cardiol. 1987;60:1157–1160. doi: 10.1016/0002-9149(87)90410-3. [DOI] [PubMed] [Google Scholar]
- Shindo S, Kubota K, Kojima A, et al. Anomalies of inferior vena cava and left renal vein: risks in aortic surgery. Ann Vasc Surg. 2000;14:393–396. doi: 10.1007/s100169910071. [DOI] [PubMed] [Google Scholar]
- Shore TW. The development of the renal-portals and fate of the posterior cardinal veins in the frog. J Anat Physiol. 1901;36(Pt 1):20–46. [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Singh KJ, Suri A, et al. Inferior vena cava in urology: importance of developmental abnormalities in clinical practice. ScientificWorldJournal. 2005;5:558–563. doi: 10.1100/tsw.2005.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedemann K, Egerer G. Vascularization and glomerular ultrastructure in the pig mesonephros. Cell Tissue Res. 1984;238:165–175. doi: 10.1007/BF00215158. [DOI] [PubMed] [Google Scholar]
- Vollmerhaus B, Reese S, Roos H. The blood vessels of the mesonephros of domestic cattle (Bos taurus), a corrosion cast study. Anat Histol Embryol. 2004;33:200–207. doi: 10.1111/j.1439-0264.2004.00537.x. [DOI] [PubMed] [Google Scholar]
- Yamada S, Samtani RR, Lee ES. Developmental atlas of the early first trimester human embryo. Dev Dyn. 2010;239:1585–1595. doi: 10.1002/dvdy.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality check of image transformation from amira 3d to cinema 4d.
Interactive 3D-PDFs of the caudal venous system in human embryos of CS14, CS15, CS16, CS18 and 10 weeks of development, respectively.
Human embryos that were reconstructed and used in the present study.