Abstract
The meniscal roots, or insertional ligaments, firmly attach the menisci to tibial plateau. These strong attachments anchor the menisci and allow for the generation of hoop stress in the tissue. The meniscal roots have a ligament-like structure that transitions into the fibrocartilagenous structure of the meniscal body. The purpose of this study was to carry out a complete analysis of the structure and tissue organization from the body of the meniscus through the transition region and into the insertional roots. Serial sections were obtained from the meniscal roots into the meniscal body in fixed juvenile bovine menisci. Sections were stained for collagen and proteoglycans (PG) using fast green and safranin-o staining protocols. Unstained sections were imaged used a backlit stereo microscope. Optical projection tomography (OPT) was employed to evaluate the three-dimensional collagen architecture of the root–meniscus transition in lapine menisci. Tie-fibres were observed in the sections of the ligaments furthest from the bovine meniscal body. Blood vessels were observed to be surrounded by these tie-fibres and a PG-rich region within the ligaments. Near the tibial insertion, the roots contained large ligament-like collagen fascicles. In sections approaching the meniscus, there was an increase in tie-fibre size and density. Small tie-fibres extended into the ligament from the epiligamentous structure in the outermost sections of the meniscal roots, while large tie-fibre bundles were apparent at the meniscus transition. The staining pattern indicates that the root may continue into the outer portion of the meniscus where it then blends with the more fibrocartilage-like inner portions of the tissue. In unstained sections it was observed that the femoral side of the epiligamentous structure surrounding the root becomes more fibrous and thickens in the inferior inner portion of the posterior medial root. This thickening changes the shape of the root to more closely resemble the meniscus wedge shape. These observations support the concept of root continuity with the outer portion of the meniscus, thereby connecting with the hoop-like structure of the peripheral meniscus. OPT identified continuous collagen organization from the root into the meniscal body in longitudinal sections. In the radial direction, the morphology of the root continues into the meniscal body consistent with the serially sectioned bovine menisci. Blood vessels were prevalent on the periphery of the root. These blood vessels then arborized to cover the anterior femoral surface of the meniscus. This is the first study of the structural transition between the insertional ligaments (roots) and the fibrocartilagenous body of the menisci. These new structural details are important to understanding the meniscal load-bearing mechanism in the knee.
Keywords: Knee, Meniscus, Meniscal Roots, Optical Projection Tomography
Introduction
The insertional ligaments, or roots, of the menisci are integral to load bearing in the menisci. These roots insert centrally on the tibial plateau and act to resist lateral extrusion of the menisci from the joint (Lerer et al. 2004). Grossly the roots appear ligament-like with large longitudinally oriented collagen bundles. Injury to these insertional ligaments results in rapid degeneration in the knee (Gale et al. 1999). Medial meniscal release, which involves the transection of the anterior or posterior menisco-tibial ligaments, is a surgical technique used to induce osteoarthritis in animal models (Pozzi et al. 2006; Glasson et al. 2007). This injury model reduces the ability of the menisci to generate hoop stresses, thus resulting in lateral extrusion and reduced load-bearing capability. The rapid onset of cartilage damage following meniscal release identifies the importance of the menisci in the overall function of the joint (Glasson et al. 2007).
Recent work on the structure of the meniscus has revealed a more complex fibre architecture than was previously described (Andrews et al. 2013, 2014). These studies identified the hierarchical nature of the tie-fibre structure and the complex woven fibre organization in regions that were previously thought to have fibres oriented purely circumferentially. The structural transition from the fibrocartilagenous meniscal body into the ligament-like meniscal roots allows for the transmission of the complex loading of the menisci into the tibial plateau. The transition of the meniscal roots into the bony insertion at the tibial surface has been studied extensively, including both the structure and mechanical properties in this region (Villegas et al. 2008; Hauch et al. 2009). However, to our knowledge there have been no studies on the transition from the meniscal roots into the fibrocartilagenous body of the menisci.
The development of successful meniscal substitutes must include an area that integrates and functions like the meniscal root in order to insure the correct transmission of load and the resultant knee kinetics. The engineering of this region is informed by a clear understanding of root architecture. Thus, the purpose of this study was to carry out an analysis of the structure and tissue organization from the body of the meniscus through the transition region and into the insertional roots.
Materials and methods
Bovine menisci (n = 2) were obtained from a local abattoir, harvested within 48 h of slaughter. The menisci were dissected with careful attention paid to retaining the four insertional ligaments (anterior and posterior for both medial and lateral menisci). Menisci were then fixed in 100% methanol at −20 °C for 72 h. The insertional ligaments were then cut serially in cross-section (sections ∼1 mm thick), starting from the bony insertion until the body of the meniscus was reached. Sections were washed in phosphate-buffered saline for 1 h, and stained with fast green (0.02% w/v; Sigma, St Louis, MO, USA) and safranin o (0.1% w/v; Sigma) for collagen and proteoglycan (PG), respectively. These whole-mount sections were imaged using a stereo-microscope and digital camera (Zeiss Stemi SV8 microscope with moticam 5.0 M pixel camera; Motic, Richmond, BC, Canada). Several sections were also imaged prior to staining using a backlit stereo microscope, as this technique yields effective visualization of the tie-fibre structure.
Optical projection tomography (OPT)
Optical projection tomography is an imaging technique capable of imaging the collagen and elastin structure of meniscal samples on the meso-scale (∼1–10 mm) at a micro-scale resolution (5–10 μm; Andrews et al. 2013). Hence, to understand the 3D collagen organization in this transitional region further, rabbit menisci were obtained and imaged using OPT. The small size of rabbit menisci allowed for imaging of the entire transitional region in one sample using OPT. While rabbit menisci are significantly smaller than bovine menisci, the general shape and morphology is quite similar to bovine menisci (Proffen et al. 2012). Rabbit menisci (n = 2) were obtained using a secondary tissue use protocol in accordance with the Conjoint Health Research Ethics Board at the University of Calgary. The menisci were dissected and immediately fixed in 100% methanol at 4 °C for 48 h. After fixation, the samples were prepared by cutting 3–4-mm sections of tissue from the insertional root and the transitional regions both medial and lateral menisci. Specimens were scanned using fluorescence OPT (Sharpe et al. 2002) on a Bioptonics 3001M OPT scanner (Bioptonics Microscopy, Edinburgh, Scotland). Each tissue sample was embedded in 1.5% low-melting-point agarose (Life Technologies, Burlington, ON, Canada). The agarose blocks were trimmed and glued to mounts, and dehydrated through three washes of 100% methanol (Fisher, Ottawa, ON, Canada) over 24 h. Specimens were then cleared for 24 h in BABB [1 part benzyl alcohol : (Fisher) : 2 parts benzyl benzoate (Sigma)]. Native autofluorescence was imaged using the GFP-1 channel (exciter 425 nm/40 nm; emitter LP475 nm) at a resolution of 8–10 μm. Raw images were reconstructed into grey-scale slices using nrecon (Skyscan NV, Kontich, Belgium). imagej (NIH open source software) was used to create 3D images from the reconstructed slices.
Results
Sections taken near the tibial insertion were ligament-like morphologically, containing large collagen fascicles. In sections approaching the meniscus, there was an increase in tie-fibre size and density (Figs4). Small tie-fibres extended into the ligament from the epiligamentous structure in the outermost sections of the meniscal roots, while large tie-fibre bundles were apparent at the meniscus transition (Fig.5). The staining pattern of the tissue indicates that the root may continue into the outer portion of the meniscus where it then blends with the more fibrocartilage-like inner portions of the tissue (Figs1, 3 and 4). In the transition region of the meniscus, the outer portion has a ligament-like staining pattern. The proportion of safranin o staining is significantly less in the outer meniscus transition when compared with the inner portion. In both anterior roots and the posterior medial root, this staining pattern is evident (Figs1, 3 and 4). In these three insertions the inferior, inner portion of the meniscus grows in size and increases in PG staining as the tissue transitions into the meniscal body. The posterior lateral root does not demonstrate the same pattern as the other roots. This tissue gradually changes shape from a ligamentous morphology into the wedge shape of the meniscus (Fig.2). However, increasing tie-fibre density and PG staining was also observed in this tissue. Unstained sections were also imaged using a backlit stereo microscope. In these sections it was observed that the femoral side of the epiligamentous structure surrounding the root becomes more fibrous and thickens in the inferior inner portion of the posterior medial root. This thickening changes the shape of the root to more closely resemble the meniscus wedge shape (Fig.5). These observations support the concept of root continuity with the outer portion of the meniscus, thereby connecting with the hoop-like structure of the peripheral meniscus.
Figure 4.
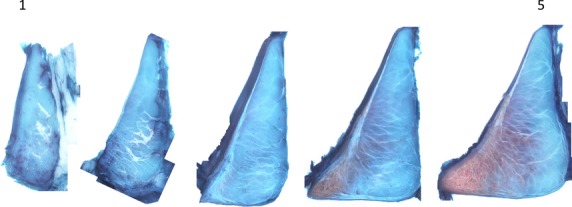
Serial sections of the posterior medial root into the meniscal body. In sections approaching the meniscus, there is a thickening of the epiligamentous of the root to an epimeniscal structure on the femoral and tibial surfaces. This thickening is associated with increased tie-fibre density and branching from the epimeniscal structure. There is also a marked increase in PG staining on the inferior inner portion of the transition into the meniscal body.
Figure 5.
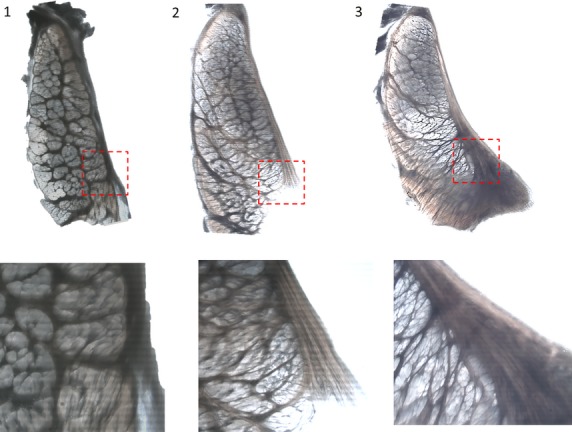
Backlit images taken on a stereo-microscope of unstained, transverse sections of the meniscal root as it transitions into the meniscus. Sections move from the root into the meniscal body (1–3). The outer structure (left side) of the tissue structure appears to change very little from the root into the meniscus. The epiligament on the femoral side of the tissue appears to thicken and increase in branching into the meniscus approaching the meniscus (breakout images 1–3).
Figure 1.
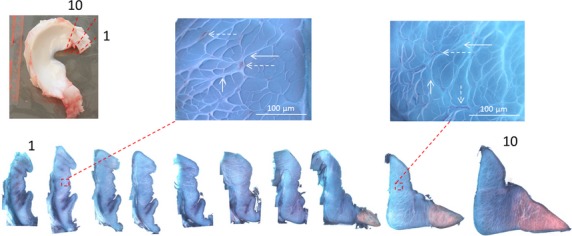
Top left: photo of a medial meniscus (dashed red lines: orientation of sections). Bottom: serial sections of the anterior insertion showing the transition in shape and staining pattern from the ligament to the meniscus. Breakout images: identify blood vessels (dashed arrows) and tie-fibres (solid arrows) in sections.
Figure 3.
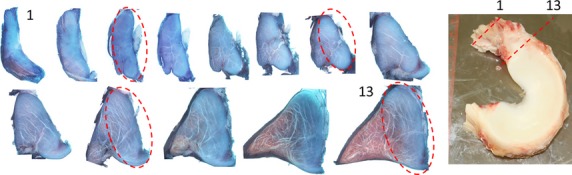
Right: photo of a lateral meniscus. Left: serial sections of the anterior insertion. Note the increase in PG staining as sections approach the meniscus. Portions of the ligament appear to be preserved as it approaches the meniscus (dashed ellipses).
Figure 2.
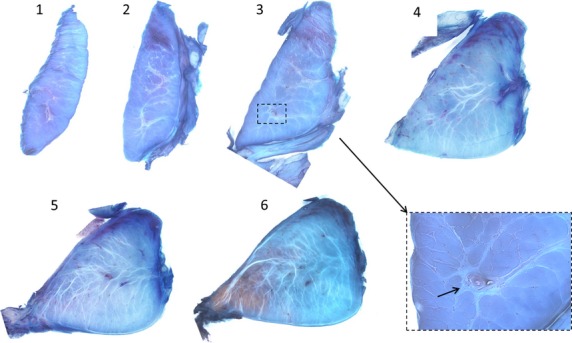
Serial sections from the posterior lateral root into the meniscal body. There is an increase in the number and density of the tie-fibres in the meniscus compared with the root. Tie-fibres were observed in the root. The breakout image shows a blood vessel in the meniscal root surrounded by tie-fibres (solid arrow).
Serial sections of the insertional ligaments identified common structural features between the insertional ligaments and meniscus. Tie-fibres were observed in the sections of the ligaments furthest from the bovine meniscal body (1–2 cm; Fig.2). Diffuse vascularization was observed in the sections (Fig.1). Blood vessels (on the order of 10–100 μm) were situated in the peri-fascicular space and were predominantly oriented along the length of the ligaments. Blood vessels were observed to be surrounded by tie-fibres and a PG-rich region within the ligaments (Figs1 and 2).
Optical projection tomography was capable of imaging the entire structure of the transition in the lapine meniscus (Fig.6). The collagen structure of the root is continuous with the outer portion of the meniscus in the transition region in the lapine menisci. Collagen bundle direction appeared continuous from the root into the meniscal body in longitudinal sections (Fig.6). In the radial direction, the morphology of the root continues into the meniscal body consistent with the serially sectioned bovine menisci. Blood vessels were prevalent on the periphery of the root (Fig.6). These blood vessels then arborized to cover the anterior femoral surface of the meniscus (Fig.6).
Figure 6.
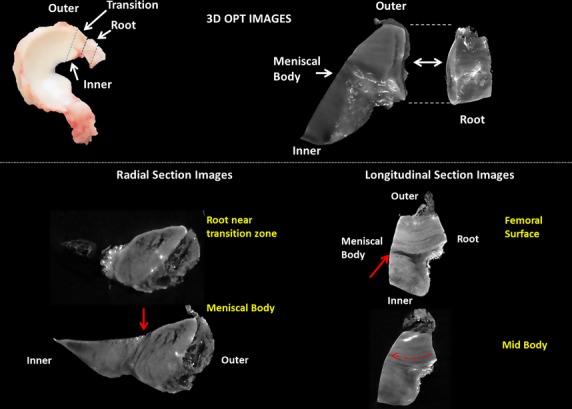
Schematic identifying the regions of the meniscus imaged using optical projection tomography (OPT; top left). 3D reconstruction of the meniscal body and meniscal root (top right). Brightly fluorescing region on the surface of the ligament and meniscal body identify blood vessels. (Bottom) Radial and longitudinal sections identifying the discrete structural changes from the outer meniscus to the inner body of the meniscus. Solid red arrows denote the discrete change from the outer collagen organization to the inner organization. A collagen-sparse area can be seen at the junction.
Discussion
Serial sections of the transition from the meniscal root into the meniscal body were stained with fast green (collagen) and safranin-o [glycosaminoglycan (GAG) component of PG]. Understanding the global distribution of these molecules is useful for understanding the load experienced by the tissue in that region (Benjamin & Ralphs, 1998). Increased GAG staining is related to increased compressive stress in the tissue. In the transition region, the inner portion of the meniscus stains positively for PG and large bundles of complexly woven collagen fibres. The increase in GAG staining is consistent with previous work that has demonstrated increased GAG in regions of compression in tendon (Benjamin & Ralphs, 1998). This organization likely indicates a discrete change from predominantly tensile load bearing in the root and outer portion of the transition, to a combined compression and shear in the inner portion. It is also apparent that the meniscal body has much greater shear stiffness than the meniscal roots, as evidenced by its resistance to changes to shape. The mechanical difference between these structures may be due to the increased thickness of the structure surrounding the menisci (analogous to the epiligament) and increased tie-fibre density in the meniscal body. Further, the increase in PG content, seen in serial sections, would develop pre-stress in the structure through osmotic swelling, resulting in increased shear and compressive stiffness. This compositional and structural transition is in accordance with Pauwel's (1960) theory of causal histogenesis (Benjamin & Ralphs, 1998). As the shape of the meniscus transitions to support compressive load between the femur and tibia there is a concomitant change in the stress state and consequently the structure as the tissue transitions. The compositional transition from the root to the meniscus also supported the structural continuity between the roots and outer portions of the menisci when imaged using a backlit stereo microscope. The natural polarization of the collagen fibres allows light to pass through fibres parallel to the light direction while fibres oblique to the light direction diffuse the light. Finally, the 3D findings from OPT using a lapine model showed a similar structural pattern as was hypothesized from 2D sections in bovine menisci. Collagen bundles could clearly be seen passing from the root into the outer portion of the meniscal body. The discrete differences between the outer and inner portions of the tissue seen in OPT images correspond well with the structural and compositional changes seen in serially stained sections.
The insertional ligaments (roots) of the menisci contain structural similarities with the main body of the menisci. Tie-fibres, originating from the epiligament, were observed in the roots. These fibres were observed to surround fascicles as well as blood vessels and an associated PG-rich region. This perivascular region is consistent with the region recently described in the main body of the menisci (Andrews et al. 2014). This common structural feature may indicate a common loading environment in the outer meniscus and the insertional ligaments. It appears that the insertional ligament likely persists into the meniscal body and blends with the fibrocartilage of the meniscus.
The inner portion of the meniscus comprises more than 50% of the radial width of the sections. This finding may indicate that the tensile load-bearing mechanism is predominantly borne by the outer edge of the meniscus through insertional ligaments. It has been demonstrated in human menisci that experimentally inducing a radial cut of up to 60% of the width of the tissue does not significantly change the contact pressure on the tibial plateau (Bedi et al. 2012). Taken together with these structural findings, it may indicate the hoop stresses are predominantly generated in the outer 40% or less of the menisci. These stresses may then be passed directly into the meniscal roots, which are structurally continuous with the meniscal body for proper functioning of the menisci. This supposition necessitates further study on the complex loading in the inner 60% of the menisci and how it integrates so effectively with the outer hoop and meniscal roots.
Conclusions
This is the first study of the structural transition between the insertional ligaments (roots) and the fibrocartilagenous body of the menisci. These new structural details are important to understanding the meniscal load-bearing mechanism. As this structure is integral to normal meniscal function, it will be an important benchmark in the successful development of tissue-engineered menisci in the future.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgments
The authors gratefully acknowledge the funding support of the Joint Transplantation Program at the University of Calgary, and May Chung for her outstanding technical assistance.
References
- Andrews SH. Ronsky JL. Rattner JB, et al., editors. An evaluation of meniscal collagenous structure using optical projection tomography. BMC Med Imaging. 2013;13:21. doi: 10.1186/1471-2342-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews SH. Rattner JB. Abusara Z, et al. Tie-fibre structure and organization in the knee menisci. J Anat. 2014;224:531–537. doi: 10.1111/joa.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi A. Kelly N. Baad M, et al., editors. Dynamic contact mechanics of radial tears of the lateral meniscus: implications for treatment. Arthroscopy. 2012;28:372–381. doi: 10.1016/j.arthro.2011.08.287. [DOI] [PubMed] [Google Scholar]
- Benjamin M. Ralphs JR. Fibrocartilage in tendons and ligaments–an adaptation to compressive load. J Anat. 1998;193(Pt 4):481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale D. Chaisson C. Totterman S, et al., editors. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage. 1999;7:526–532. doi: 10.1053/joca.1999.0256. [DOI] [PubMed] [Google Scholar]
- Glasson SS. Blanchet TJ. Morris EA, editor. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Hauch KN. Oyen ML. Odegard GM, et al., editors. Nanoindentation of the insertional zones of human meniscal attachments into underlying bone. J Mech Behav Biomed Mater. 2009;2:339–347. doi: 10.1016/j.jmbbm.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerer DB. Umans HR. Hu MX, et al., editors. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33:569–574. doi: 10.1007/s00256-004-0761-2. [DOI] [PubMed] [Google Scholar]
- Pozzi A. Kowaleski MP. Apelt D, et al., editors. Effect of medial meniscal release on tibial translation after tibial plateau leveling osteotomy. Vet Surg. 2006;35:486–494. doi: 10.1111/j.1532-950X.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- Proffen BL. McElfresh M. Fleming BC, et al., editors. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19:493–499. doi: 10.1016/j.knee.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe J. Ahlgren U. Perry P, et al., editors. Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science. 2002;296:541–5. doi: 10.1126/science.1068206. [DOI] [PubMed] [Google Scholar]
- Villegas D. Hansen T. Liu D, et al., editors. A quantitative study of the microstructure and biochemistry of the medial meniscal horn attachments. Ann Biomed Eng. 2008;36:123–131. doi: 10.1007/s10439-007-9403-x. [DOI] [PubMed] [Google Scholar]


