Abstract
Dyslipidemia and insulin resistance are commonly associated with catabolic or lipodystrophic conditions (such as cancer and sepsis) and with pathological states of nutritional overload (such as obesity-related type 2 diabetes). Two common features of these metabolic disorders are adipose tissue dysfunction and elevated levels of tumour necrosis factor-alpha (TNF-α). Herein, we review the multiple actions of this pro-inflammatory adipokine on adipose tissue biology. These include inhibition of carbohydrate metabolism, lipogenesis, adipogenesis and thermogenesis and stimulation of lipolysis. TNF-α can also impact the endocrine functions of adipose tissue. Taken together, TNF-α contributes to metabolic dysregulation by impairing both adipose tissue function and its ability to store excess fuel. The molecular mechanisms that underlie these actions are discussed.
Keywords: Obesity, Type 2 diabetes, Metabolic syndrome, Insulin resistance, Dyslipidemia, TNF signalling, Lipid metabolism, Antiadipogenesis
1. Adipose tissue-derived TNF-α in metabolic disease
Tumour necrosis factor-alpha (TNF-α) is a multi-functional cytokine that can regulate many cellular and biological processes such as immune function, cell differentiation, proliferation, apoptosis and energy metabolism. It is synthesised as a 26-kDa transmembrane monomer (mTNF-α) [1] that undergoes proteolytic cleavage by the TNF-α converting enzyme (TACE) to yield a 17-kDa soluble TNF-α molecule (sTNF-α) [2]. Both sTNF-α and mTNF-α can effect biological and metabolic responses [3,4] suggesting that mTNF-α may mediate paracrine and autocrine signals, leaving sTNF-α to mediate endocrine effects [5]. However, more recent indications are that the endocrine actions of sTNF-α depend on the maintenance of high circulating levels, which are more likely to occur in catabolic disease states such as the cachectic conditions associated with sepsis and cancer. In contrast, in obesity-related type 2 diabetes (T2D) the levels of both mTNF-α and sTNF-α are increased in adipose tissue [6,7] but circulating levels of sTNF-α are lower than in the catabolic diseases. Therefore whether adipose-derived sTNF-α exerts endocrine effects in systemic insulin resistance has been debated. Initially, circulating levels were often found to be low and sometimes not significantly elevated, prompting the conclusion that adipose tissue does not release TNF-α systemically. However, with the improved sensitivity and specificity of detection reagents, circulating sTNF-α now seems well correlated to BMI [8] and impairment in TNF-α processing can improve systemic insulin sensitivity [9,10]. Moreover, the diffuse nature of adipose tissue and its close association with metabolically relevant tissues such as muscle and pancreas suggests that the local but chronic production of adipose tissue-derived sTNF-α may still target non-adipose tissues during obesity. The confirmation that cytokine-producing macrophages are present in adipose tissue of obese diabetics further supports this notion [11]. Whether this also implicates TNF-α produced by resident macrophages (e.g. Kupffer cells) in the physiological regulation of energy metabolism in other tissues such as liver remains to be established. Importantly, as outlined in this review, adipose tissue-derived TNF-α can also affect systemic energy homeostasis indirectly by regulating both adipose function and expandability. This is pertinent to our understanding of the mechanisms of TNF-α action in obesity-associated metabolic dysregulation.
Evidence that TNF-α can itself impact on cellular metabolism in catabolic disease states dates back to the early 1980s when it was first purified and confirmed to be the long sought-after cancer toxin and also the cachexia-inducing factor, cachectin. More recently, TNF-α was shown to be relevant to adipocyte metabolism. In vitro studies initially suggested that TNF-α affects glucose homeostasis in adipocytes [12], promotes lipolysis in cultured adipocytes [13] and potently inhibits adipocyte differentiation and lipogenesis [14,15]. However, the first demonstration that TNF-α may be relevant to metabolic diseases associated with overnutrition (such as obesity-related T2D) was made by Hotamisligil and colleagues, who showed that TNF-α is elevated in adipose tissue from obese diabetic rodents and is a mediator of obesity-related insulin resistance and T2D [6].
The most compelling evidence of this subsequently came from studies showing that genetic blockade of TNF-α action can restore insulin sensitivity in vitro and in vivo [16,17]. Thus, TNF-α production appears to contribute to the pathogenesis of rodent obesity-induced insulin resistance. Evidence that this is also true for humans has been less well established and complicated by inconsistent early correlations of circulating sTNF-α with obesity. Additionally, several studies found that short-term treatment with an anti-TNF-α antibody [18,19] or with a sTNFR1-IgG1 chimera [20,21] failed to improve insulin sensitivity in obese type 2 diabetics or subjects with visceral obesity. One problem with interpreting these negative findings comes with the difficulty in determining whether local TNF-α activity (both mTNF-α and sTNF-α) is completely neutralised by such acute treatment. They also contrast with recent reports that find more chronic treatment with anti-TNF-α antibodies improves insulin sensitivity in both lean [22-24] and obese patients [25]. Furthermore, mounting evidence suggests that human obesity promotes a state of chronic, low-grade inflammation that contributes to insulin resistance and T2D. Taken together, TNF-α and/or its mechanisms of action might serve as a therapeutic targets to treat disorders associated with chronic inflammation, impaired glucose tolerance and dyslipidaemia.
1.1. What is the cellular source of adipose tissue-derived sTNF-α?
The vast majority of adipose tissue volume is comprised of lipid-laden adipocytes, and both isolated or differentiated adipocytes can produce TNF-α. Hence, it was initially assumed that adipocytes were the predominant source of the elevated adipose tissue TNF-α in obesity. However, adipose tissue also contains a significant stromovascular fraction (SVF), which contains numerous metabolically relevant cell types. These include preadipocytes, endothelial cells, smooth muscle cells, fibroblasts, leukocytes and macrophages, and more recent studies have demonstrated that these SVF cells can produce substantially more TNF-α than adipocytes [11,26-28]. Indeed, obesity is associated with an increased infiltration of macrophages into adipose tissue [11,27,29,30], and it is likely that these adipose tissue macrophages (ATMs) are predominantly responsible for the elevated production of TNF-α during obesity [11]. Furthermore, recent mouse models have demonstrated that both the recruitment and classical activation of ATMs are required for the development of insulin resistance that is associated with obesity. Intriguingly, a recent study reports that, although macrophage-derived TNF-α can impair insulin sensitivity in TNF-α KO mice, the absence of TNF-α in macrophages only does not protect otherwise wild-type mice from the development of obesity-related insulin resistance [31]. Hence the relative contribution of macrophage and non-macrophage-derived TNF-α to obesity-induced insulin resistance remains incompletely understood.
1.2. What induces TNF-α expression in adipose tissue?
An important question that remains incompletely addressed is the nature of the original trigger that induces TNF-α production in adipose tissue. The regulation of TNF-α gene transcription clearly is cell type-specific, however since the vast majority of TNF-α production comes from classically activated macrophages, much interest is focused on identifying the initial trigger(s) that stimulate recruitment and activation of these immune cells into adipose tissue. One hypothesis is that increased adipocyte death in expanding adipose tissue may induce chemoattractant signals that recruit monocytes. Indeed, obesity in both rodents and humans is associated with increased numbers of apoptotic and necrotic adipocytes in white and brown adipose tissue (WAT and BAT, respectively)[32-34], and one study finds that the majority of macrophages in WAT of obese mice and humans are localised to dead adipocytes [34]. That obesity is associated with increased adipocyte death is consistent with the notion that adipose tissue may have a limited capacity for expansion that is reached during chronic over-nutrition. However, since secreted cytokines can also stimulate further cytokine production from and promote apoptosis of target cells, it is not currently apparent which is cause or consequence.
Nonetheless, it is clear that proper immune function is closely linked to nutritional status [35], and this may be due to the fact that dietary fatty acids (FA) can alter cytokine production [36]. This has prompted the alternative but not mutually exclusive suggestion that dietary components, in particular specific fats, may play a significant role in regulating the inflammatory profile of adipose tissue, at least in diet-induced obesity. Indeed, n–6 FA appear to be pro-inflammatory whereas long-chain, marine-derived n–3 FA elicit anti-atherogenic and anti-inflammatory effects. Intriguingly, calorie restriction and weight loss are associated with decreased cytokine production [37], providing a rational basis for nutritional strategies that target immune modulation.
With respect to TNF-α production specifically, levels of this cytokine are known to be nutritionally regulated but, importantly, can be enhanced by hyperinsulinaemia alone [38]. However, it is currently unclear whether the elevated production of TNF-α in obesity-related diabetes occurs secondarily to hyperinsulinaemia. Furthermore, in vivo cytokine action results from a complex interplay between networks of pro- and anti-inflammatory cytokines. It is therefore likely that a co-ordinate regulation of anti-inflammatory cytokines such as IL-10 may play a role in both TNF-α production and action in vivo.
1.3. Molecular mechanisms of TNF-α action in adipocytes
TNF-α mediates its biological effects on adipose tissue via two distinct cell surface receptors: tumour necrosis factor-alpha receptor 1 (TNFR1) (a 55- or 60-kDa peptide in rodents and humans, respectively); and tumour necrosis factor-alpha receptor 2 (TNFR2) (a 75- or 80-kDa peptide in rodents and humans, respectively). Both receptors are ubiquitously expressed transmembrane glycoproteins that trimerise upon ligand binding. Like TNF-α, both TNFRs can be proteolytically cleaved to release soluble forms (sTNFR). These may be involved in the neutralisation and excretion of TNF-α and thereby may modulate TNF-α activity both temporally and spatially [39]. These soluble receptors may also be more accessible biomarkers for elevated TNF-α activity. The circulating levels of both soluble TNFRs can be nutritionally regulated [8] and have been reported to increase in both obesity and in non-obese adults with pro-atherogenic lipid profiles [40,41].
Although TNFR1 and TNFR2 are highly homologous in their ligand-binding extracellular domains, their intracellular domains exhibit no sequence homology and they activate divergent signalling pathways [42]. Results from numerous investigations suggest that the signals transduced by TNFR1 mediate the majority of effects of TNF-α on adipose tissue function. Human and murine TNF-α are equally effective at impairing insulin signalling in murine adipocytes [43]. This suggests that signals transduced via TNFR1 are sufficient for this effect, because human TNF-α can only activate murine TNFR1 [44]. Furthermore, lack of TNFR1, but not lack of TNFR2, significantly improves insulin sensitivity in ob/ob mice and cultured adipocytes [45,46]. Finally, studies using TNFR1 and/or TNFR2-deficient preadipocytes show that it is TNFR1 that is required for the inhibition of adipogenesis by both soluble and transmembrane TNF-α [4,47]. Thus, TNF-α modulates adipose tissue function predominantly through TNFR1. Nevertheless, other studies suggest that TNF-α may also mediate some effects on adipocytes through TNFR2 [40,48-50]. However, knowledge of the exact role of TNFR2 in TNF-α-mediated adipose tissue dysfunction is limited. In contrast, many of the signalling pathways activated via TNFR1 have been implicated in mediating the actions of TNF-α on adipose tissue (Fig. 1). Herein, we will limit our discussion to the pathways that have been shown to effect aspects of adipocyte biology specifically.
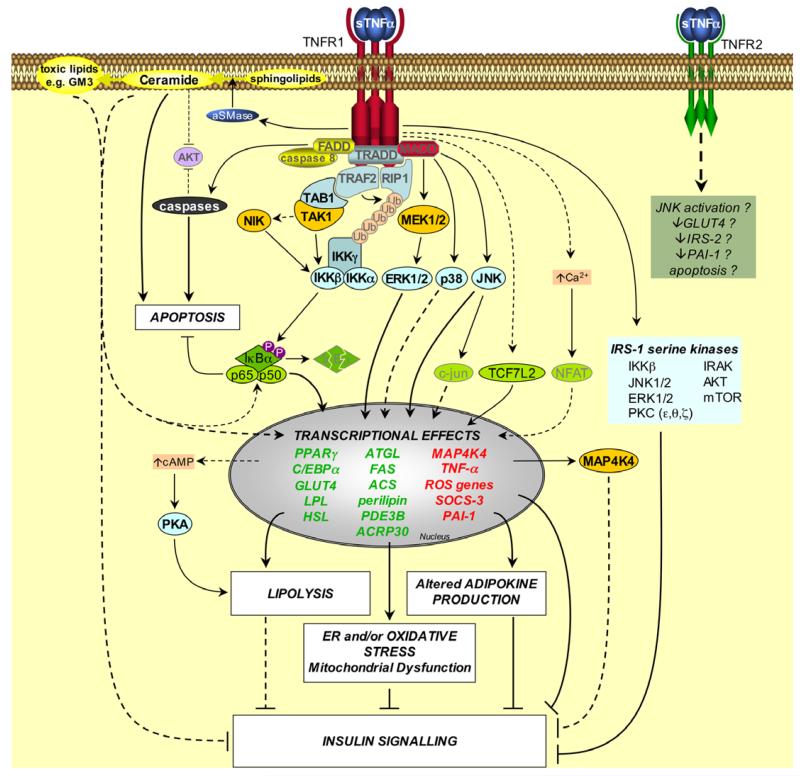
Fig. 1. Signalling pathways induced by TNF-α in adipose tissue.
Upon TNFR1 activation the adapter molecule TRADD (TNFR-associated death domain protein) interacts with the TNFR1-DD. TRADD recruits downstream adapter molecules such as Fas-associated death domain protein (FADD), TNFR-associated factor 2 (TRAF2), receptor-interacting protein 1 (RIP1) and mitogen-activated protein kinase (MAPK)-activating death domain protein (MADD). These adapters then mediate the activation of divergent downstream signalling pathways. Apoptosis: FADD recruits caspases such as caspase 8 to TNFR1, thereby forming the TNFR1 death-inducing signalling complex (DISC). This complex activates downstream caspases, leading to apoptosis. Ceramide production: the TNFR1-DD activates acidic sphingomyelinase (aSMase), which hydrolyses sphingolipids to produce ceramide. Ceramides can be converted into toxic lipids, such as the ganglioside GM3. Ceramides and gangliosides may then mediate transcriptional effects, possibly by activation of NFκB. Ceramides can also stimulate apoptosis and impair insulin signalling, and both ceramide and caspases may inhibit AKT. NFκB activation: TRAF2 and RIP1 mediate NFκB activation by recruiting the IKK (inhibitor of NFκB (IκB) kinase) complex to TNFR1. TRAF2 interacts with IKKβ and IKKα, and also promotes the K63-linked polyubiquitination of RIP1 (Ub, ubiquitin); Ubiquitinated RIP1 binds to IKKγ. TRAF2 also recruits TAK1 (TGFβ-activated kinase 1) to TNFR1 via an interaction with TAB1 (TAK1 binding protein 1), which may result in activation of TAK1. This also brings TAK1 into close proximity of the IKK complex. TAK1 may activate IKKβ either by phosphorylating it directly, or by activating NIK (NFκB-inducing kinase), which then phosphorylates IKKβ. Active IKKβ phosphorylates IκBα on conserved serines, leading to its polyubiquitination and proteasomal degradation. This liberates the bound NFκB dimers (e.g. the prototypical p65/p50 heterodimer), which translocate into the nucleus to mediate transcriptional effects. MAPK activation: TNF-α activates various MAPKs in adipocytes, including ERK1/2, p38 MAPK and JNK, possibly via the adapter MADD. ERK1/2 and JNK can each regulate transcription by suppressing the activity of PPARγ. JNK may also exert transcriptional effects via c-jun. JNK, ERK1/2, IKKβ and other kinases have also been implicated in TNF-α-induced serine phosphorylation of IRS-1, which suppresses insulin signalling. Other effects: TNF-α can activate PKA (protein kinase A) by increasing cAMP levels, possibly via transcriptional effects. TNF-α can activate the transcription factor NFAT via Ca2+, however this has not been established in adipocytes. In preadipocytes TNF-α can stimulate transcription by TCF7L2 (transcription factor 7-like 2). The transcriptional effects of TNF-α promote ER stress, oxidative stress and mitochondrial dysfunction, lipolysis and altered adipokine expression, thereby compromising insulin signalling and adipocyte lipid metabolism. TNFR2 may also mediate some effects of TNF-α in adipose tissue, albeit by unknown mechanisms. Factors that have not been directly established in TNF-α-induced signals in adipocytes are faded. Dashed lines indicate effects that are mediated by indirect or unestablished mechanisms. Question marks indicate effects that remain to be fully established. Genes written in red or green are upregulated or downregulated by TNF-α, respectively.
Neither TNFR has intrinsic catalytic activity. Instead, they can transmit signals by recruiting intracellular adapter proteins, which interact with distinct domains of the cytoplasmic portions of the receptors to activate specific downstream signals. For example, several adapter complexes are recruited to the trimeric death domain of TNFR1 (TNFR1-DD), which has been implicated in mediating many of the effects of TNF-α on adipocyte biology [47,51] (Fig. 1). As its name indicates the TNFR1-DD is responsible for the cytotoxic signals induced by TNF-α. However, as with many TNF-α actions, these can be cell type-specific; although preadipocytes are more sensitive to TNF-α-induced cell death, TNF-α can also induce apoptosis in adipocytes [52,53]. This is likely to be mediated by TNFR1, at least in brown adipocytes [53].
The TNFR1-DD can also activate cell survival and pro-inflammatory signalling pathways that lead to the activation of nuclear factor-kappa B (NFκB) and of mitogen-activated protein kinase (MAPK) cascades, such as those involving extracellular signal-regulated protein kinase (ERK), p38 MAPK and c-Jun N-terminal kinase (JNK) (Fig. 1). These signalling pathways have been reported to be activated by TNF-α in adipose tissue and remain good candidates for mediating metabolic dysregulation [54-58]. However, TNFR1 and its DD can activate a number of additional signalling pathways with metabolic relevance to adipocyte biology, such as acidic sphingomyelinase (aSMase), nuclear factor of activated T-cells (NFAT), protein kinase A (PKA), CREB, protein kinase C (PKC), PI3K and calcium release (Fig. 1). However, the receptor-proximal events that lead to these remain to be completely elucidated, as do the mechanisms that facilitate sustained TNF-α-induced signalling.
2. TNF-α and adipocyte insulin resistance
Adipose tissue is a key regulator of systemic carbohydrate metabolism and may play an important role in glucose sensing. This is supported by the fact that mice engineered to selectively lack the glucose transporter GLUT4 in their adipose tissue develop insulin resistance in liver and skeletal muscle, resulting in systemic glucose intolerance and hyperinsulinaemia [59]. It is now well established that TNF-α can induce a state of insulin resistance in adipocytes [60]. Indeed, the mechanisms underlying this effect have been the subject of intense research. Early reports identified that TNFR1-induced signals are required and sufficient to impair insulin action [43,46,48], and a recent report suggests that these can be narrowed down to those induced by the TNFR1-DD [51]. However, several downstream mechanisms have been proposed by which TNF-α might cause insulin resistance in adipose tissue. These are discussed next.
2.1. Transcriptional mechanisms of TNF-α-induced insulin resistance
TNF-α suppresses the expression of many proteins that are required for insulin-stimulated glucose uptake in adipocytes, such as the insulin receptor (IR), insulin receptor substrate-1 (IRS-1) and GLUT4 [61-63]. Although mechanisms such as accelerated proteasomal degradation (e.g. IRS-1) and impaired translation may be important, more is known about how TNF alters their expression at the transcriptional level.
Amongst the first transcription factors shown to be targeted by TNF-α signalling in adipocytes was the ‘adipogenic master regulator’, peroxisome proliferator-activated receptor gamma (PPARγ) [64-66]. TNF-α can target PPARγ by inhibiting the expression of PPARγ mRNA and also through suppression of its transcriptional activity. The latter can occur by promoting serine phosphorylation of key regulatory residues in the N-terminal domain of PPARγ (e.g. S112 in murine PPARγ2 or S84 in human PPARγ1) [67,68]. This phosphorylation event can be mediated by JNK and extracellular signal-regulated kinase (ERK1/2) [67,68] and has been implicated in TNF-α-induced suppression of PPARγ in hepatic stellate cells [69]. Furthermore, in vitro over expression of PPARγ S112A in 3T3-L1 adipocytes prevents the TNF-α-mediated downregulation of IRS-1 [70], and mice with a homozygous S112A mutation in PPARγ remain insulin-sensitive on a high fat diet [71]. Hence, TNF-α may suppress PPARγ activity in adipocytes by promoting its phosphorylation. TNF-α may also inhibit PPARγ activity through activation of the classic TNF-α-induced transcription factor, NFκB. Specifically, p65/RelA has been suggested to directly bind to PPARγ in complex with its co-activator, PGC1, thereby preventing binding to PPARγ response elements [57]. This is consistent with the observation that p65 can suppresses PPARγ activity in the absence of any NFκB DNA-binding sites [66].
Suppression of PPARγ mRNA levels is also observed following treatment with TNF-α. How this is achieved remains incompletely understood. One mechanism stems from the suggestion that PPARγ expression is itself upregulated by PPARγ activity. Hence, in TNF-α-treated adipocytes, downregulation of PPARγ activity is likely to also result in reduced mRNA levels. This would also be true for another adipogenic transcription factor and PPARγ target gene, CCAAT/enhancer binding protein (C/EBPα). TNF-α also suppresses C/EBPα mRNA expression. Since the GLUT4 promoter contains response elements for, and is regulated by, both PPARγ and C/EBPα [54], it is likely that TNF-α can suppress GLUT4 expression via a PPARγ and C/EBPα-dependent mechanism. Whether this is true for all genes that are downregulated in insulin-resistant adipose tissue is unclear.
Recently, the protein kinase mitogen activated protein kinase kinase kinase kinase 4 (MAP4K4), an activator of the JNK signalling pathway, has also been implicated in TNF-α-induced suppression of PPARγ and GLUT4 mRNA in adipocytes [72]. TNF-α upregulates MAP4K4 expression in mature adipocytes in a TNFR1-dependent manner, and knockdown of MAP4K4 attenuates TNF-α-induced suppression of GLUT4 expression [72,73]. Hence MAP4K4 may contribute to the suppression of PPARγ and GLUT4 by TNF-α (Fig. 1). However, how MAP4K4 activation leads to the suppression of PPARγ is currently unknown, as is the role (if any) of MAP4K4 in the pathogenesis of metabolic complications in vivo.
The transcriptional events required for TNF-α-induced insulin resistance may also involve the transcriptional activity of NFκB itself, and hence NFκB target gene expression. However, this has yet to be formally demonstrated with respect to TNF-α-induced insulin resistance in adipocytes. Despite this, there is ample evidence to support the requirement for the upstream signals that lead to NFκB activation in TNF-α-induced insulin resistance in vitro and in vivo [63,74-76]. Furthermore, several potential NFκB target genes that are upregulated by TNF-α in adipocytes have been implicated in adipocyte insulin resistance. One example is suppressor of cytokine signalling-3 (SOCS-3), which inhibits IR-dependent IRS-1 tyrosine phosphorylation and thereby suppresses insulin-stimulated glucose uptake [77-79] (Fig. 2). Overexpression of SOCS-3 in adipose tissue causes local insulin resistance [80], whereas knockdown of SOCS-3 in adipocytes attenuates the TNF-α-mediated suppression of tyrosine phosphorylation of IRS-1 and IRS-2 [79]. Hence, the upregulation of SOCS-3 may contribute to TNF-α-mediated suppression of IR-proximal signalling (Fig. 2).
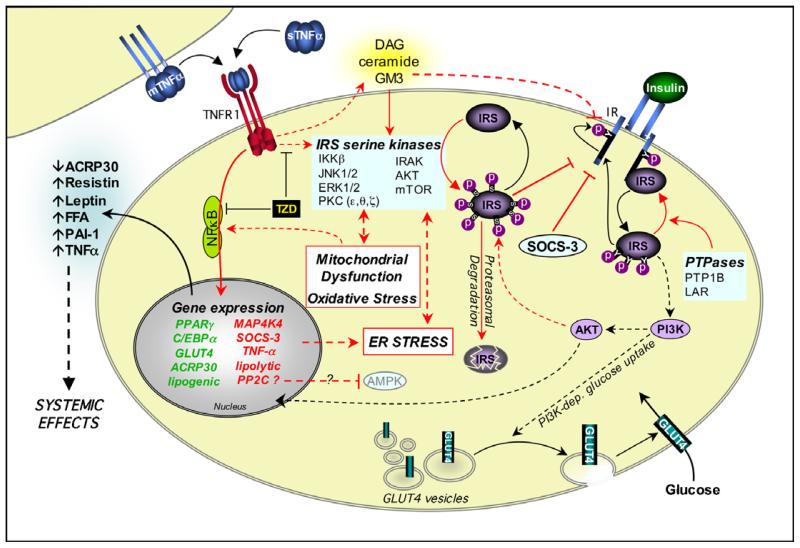
Fig. 2. Mechanisms of TNF-α-induced insulin resistance in adipose tissue.
Insulin mediates metabolic effects by binding to the insulin receptor (IR). The IR has intrinsic tyrosine kinase activity, and insulin binding promotes autophosphorylation of the IR on key intracellular tyrosines. This allows the recruitment of insulin receptor substrates (IRS) such as IRS-1. IR-bound IRS proteins are themselves phosphorylated on tyrosine residues, allowing the activation of signalling pathways such as phosphatidylinositol 3-kinase (PI3K) and AKT. These exert downstream effects such as translocation of GLUT4 from intracellular storage vesicles to the plasma membrane. Protein tyrosine phosphatases (PTPases) can abrogate IR-proximal signalling. TNF-α also impairs IR-proximal signalling through the activation of IRS serine kinases and the production of toxic lipids, as described in Figure 1. TNF-α further compromises insulin action through transcriptional effects, possibly mediated via NFκB activation. These cause downregulation of components required for insulin responsiveness and upregulation of factors that may further impair both local and systemic insulin sensitivity, such as SOCS-3. Transcriptional effects may also promote cellular stresses that can impair insulin signalling. Thiazolidinediones (TZD) impair TNF-α-induced insulin resistance. Black arrows indicate effects that promote insulin signalling. Red arrows indicate effects that antagonise insulin signalling. Effects on gene expression are indicated as described in Fig. 1.
Finally, TNF-α may induce insulin resistance through the upregulation of PP2C gene expression, resulting in suppression of AMP-activated protein kinase (AMPK) activity. This has recently been implicated in skeletal muscle insulin resistance [81]. Whether this occurs in adipose tissue remains to be determined. However, given that metformin may be able to reverse some actions of TNF-α in adipose tissue [82], this seems an intriguing possibility (Fig. 2).
2.2. Crosstalk between TNF-α and proximal insulin signalling
TNF-α can also compromise IR/IRS-1 signalling independently of transcriptional regulation [83]. This is thought to occur by direct signalling events that mediate a crosstalk between TNFR1-induced pathways and proximal insulin signals (Figs. 1 and 2). Indeed, TNF-α inhibits insulin-stimulated tyrosine phosphorylation of both the IR and IRS proteins. The latter has been best studied and occurs by induction of serine phosphorylation of IRS-1 [43,84,85]. Over 20 IRS-1 serine kinases and an even greater number of putative serine phosphorylation sites have been implicated in the negative regulation of insulin signalling. In vitro studies have established that some sites can be phosphorylated by multiple serine kinases and numerous sites can be phosphorylated by the same kinase [85]. Whether these kinases are also relevant in vivo has begun to be substantiated by investigations of their genetic ablation, at least for JNK, PKCθ and IκB kinase beta (IKKβ) (liver-specific), in rodent models of obesity and insulin resistance. The use of site-specific antibodies has also been instrumental in dissecting the involvement of site-specific kinase activities in stimulus-induced phosphorylation. However, very few of these serine phosphorylation sites have been studied with respect to TNF-α action in adipocytes. One site that has been well characterised is serine 307 in rIRS1 (corresponding to S312 in human IRS-1). Phosphorylation of this residue impairs IR-IRS interactions and promotes IRS-1 degradation (Fig. 2) [86-88]. Studies with pharmacological inhibitors and/or activators have implicated numerous upstream kinases in IRS-S307 phosphorylation, including ERK1/2 [83,86], JNK [87,89], IKKβ [90], and mTOR [91]. Although each of these can be activated by TNF-α, not all of these have been demonstrated to directly interact with endogenous IRS-1 in adipocytes and the sequential nature of the signalling cascade(s) remains to be delineated. Furthermore additional kinases such as PKC isoforms have also been implicated in TNF-α-induced insulin resistance in non-adipose cells and these may also be involved in Ser307 phosphorylation in adipocytes.
In addition to impairing protein-protein interactions, TNF-α-induced serine phosphorylation can also disrupt protein–phospholipid interactions. Recently, our laboratory and others have independently identified S24 of IRS-1 as a substrate site for PKC and IRAK [92,93]. This single modification is sufficient to impair lipid binding, intracellular localisation of the IRS1-PH domain and reduce IR-IRS1 interactions and signalling. This ultimately reduces insulin-stimulated signalling and glucose uptake. TNF-α, IL-1 or the diacylglycerol mimetic, phorbol 12-myristate 13-acetate (PMA), can all induce phosphorylation of S24. However, unlike S307 or S612, this site is not phosphorylated following chronic insulin treatment or by C-2 ceramides [93]. Hence, this represents a modification of IRS-1 that is regulated by a limited range of pathological stimuli and that may not play a role in physiological negative feedback regulation of insulin signalling.
In addition to altering cellular serine kinase activity, TNF-α action can alter membrane lipid composition and increase the availability of reactive lipids such as diacylglycerols and ceramides (Figs. 1 and 2). These can lead to insulin resistance through several means, including activating serine kinase signalling cascades and altered membrane fluidity, which impairs receptor internalisation, cycling and function. TNF-α-induced generation of ceramides has previously been linked to insulin resistance via IRS-1 serine kinases [43,94-97] (Fig. 2) and ceramide biosynthesis has recently been suggested to be required for rodent obesity-induced insulin resistance in vivo [98]. Furthermore, ceramides can be metabolised to glycosphingolipids and gangliosides such as GM3, which have also been shown to alter IR tyrosine kinase signal transduction [99] (Fig. 2). Indeed, we have shown that TNF-α raises GM3 levels in adipocytes and both obesity- and TNF-α-induced insulin resistance can be reversed by inhibiting the activity of glucosylceramide synthase, a key enzyme in the synthesis of glycosphingolipids and gangliosides [100]. Hence, TNF-α-induced insulin resistance can be mediated through TNFR1-dependent signals that activate SMase and thereby increase the levels of ceramides and gangliosides (Figs. 1 and 2).
2.3. Role of TNF-α in obesity-associated ER stress, oxidative stress and mitochondrial dysfunction
Obesity-related insulin resistance has recently been linked to oxidative stress and organelle dysfunction, i.e. mitochondrial dysfunction and endoplasmic reticulum (ER) stress [35]. These are all features that can also be induced by TNF-α and which have been linked to activation of the JNK or NFκB pathways (Figs. 1 and 2). Indeed, it has been suggested that production of reactive oxygen species (ROS) contributes to both TNF-α-induced NFκB activation [101] and TNF-α-induced insulin resistance [102] (Fig. 2). Additionally, oxidative stress may promote increased production of TNF-α under adverse metabolic conditions [103]. Interestingly, in adipose tissue TNF-α upregulates the expression of many genes that encode proteins involved in responses to ER stress or oxidative stress [62] (Figs. 1 and 2). Furthermore, TNF-α downregulates the expression of genes that encode components of the electron transport chain [104] and may thereby induce mitochondrial dysfunction. Indeed, TNF-α has recently been shown to inhibit FA oxidation in differentiated human adipocytes [104].
3. TNF-α and adipocyte endocrinology
Adipose tissue is now also recognised as an endocrine organ that secretes many products that can impact on numerous aspects of whole organism biology, including energy homeostasis, immune function and reproduction [105]. Several of these adipose tissue-derived secreted proteins, termed ‘adipokines’, are also involved in obesity-related metabolic complications. Interestingly, TNF-α action on adipose tissue can alter the production of many adipokines and this is relevant to the systemic effects of TNF-α on insulin sensitivity and whole body energy homeostasis (Fig. 2).
3.1. Insulin-sensitising adipokines
The principle insulin-sensitising adipokines are adiponectin and leptin [105]. Adiponectin enhances insulin sensitivity and improves the serum lipid profile through AMPK activation and increased FA oxidation [106,107]. Conversely, disruption of adiponectin expression causes insulin resistance [108]. TNF-α may therefore induce systemic insulin resistance and dyslipidaemia by suppressing the production of adiponectin [63,109-111]; indeed, circulating levels of adiponectin inversely correlate with plasma levels of TNF-α [112,113]. The mechanism by which this is mediated is likely to involve suppression of PPARγ [114], C/EBPβ [115] and/or through JNK activation[58,88].
The regulation of leptin production by TNF-α is less clear. Instead of reducing the production of this insulin-sensitiser, TNF-α promotes leptin release from adipocytes, [116,117] and circulating TNF-α levels positively correlate with serum leptin concentrations in obese patients with T2D [118,119]. In the absence of TNF-α, obesity-induced hyperleptinaemia is significantly reduced. However, the mechanism by which this is mediated is currently unclear as the transcriptional upregulation is context-dependent [116,120,121].
Visfatin (also called PBEF/Nampt) has recently been identified as a putative adipokine that is secreted by visceral fat and that stimulates insulin signalling [122]. However, the debate regarding its role as an adipokine continues [123], and studies investigating the effects of TNF-α on visfatin expression have yielded conflicting results; whereas TNF-α can suppress expression of visfatin in 3T3-L1 adipocytes [124], one recent study suggests that TNF-α markedly upregulates visfatin expression in human adipose tissue [125]. Hence the exact role of visfatin and its regulation by TNF-α requires further investigation.
3.2. Pro-inflammatory adipokines and resistin
In non-obese healthy subjects, the cytokine-mediated interplay between the immune system and adipose tissue maybe the means of releasing surplus fuel for utilisation by activated immune cells during infection and/or inflammation. TNF-α can regulate the production of other pro-inflammatory cytokines (e.g. IL-6 and IL-1) and thereby further mediate and/ or amplify its effects on peripheral organs.
Resistin was originally implicated in the pathogenesis of obesity-associated insulin resistance in mice [126], whereas such a role in humans is still debated. This is due in part to species-specific differences in its cellular source [127]. Nonetheless, in humans, resistin has many features of a pro-inflammatory cytokine and may play a role in inflammatory diseases. Some studies suggest that regulatory interactions exist between TNF-α and resistin, but these may also be cell type-specific. TNF-α suppresses expression of resistin in 3T3-L1 adipocytes[128], whereas it promotes expression of resistin from human peripheral blood mononuclear cells (PBMC) [129]. The latter may be a major source of resistin in humans. Conversely, human resistin promotes the expression of TNF-α and other cytokines by various cell types via an NFκB-dependent pathway [129,130]. Therefore, in the context of the metabolic syndrome, it is possible that increased expression of resistin could contribute to the elevated levels of adipose tissue TNF-α during obesity. This possibility has yet to be investigated.
3.3. PAI-1
Although plasminogen activator inhibitor-1 (PAI-1) has traditionally been linked to the pathogenesis of atherosclerosis, recent evidence suggests that it is also involved in the development of obesity and insulin resistance [131]. TNF-α upregulates PAI-1 expression in adipocytes and adipose tissue via a pathway that involves activation of p42/p44, PKC, p38, PI3-K, NFκB and ROS production [50,63,132-135] (Fig. 2). Interestingly, TNFR2-derived signals may actually attenuate the induction of PAI-1 expression by TNF-α [50] (Fig. 1). Nevertheless, TNF-α is likely to contribute to obesity-associated increases in PAI-1 and may thereby promote the cardiovascular complications of metabolic syndrome.
4. TNF-α and adipocyte lipid metabolism
The most distinguishing aspect of adipocytes is their ability to store (through lipogenesis) and release (through lipolysis and/or thermogenesis) surplus energy appropriately. Hence proper adipocyte function plays a pivotal role in the regulation of whole-body lipid metabolism. These functions can be regulated by numerous extracellular stimuli, such as insulin, cortisol, catecholamines, growth hormone, testosterone, free fatty acids (FFA) and cytokines [136]. TNF-α action on adipocytes can directly alter lipid metabolism through inhibition of FFA uptake and lipogenesis and stimulation of FFA release via lipolysis. In this way, adipose tissue-derived TNF-α can contribute to the development of dyslipidaemia and resultant metabolic complications.
4.1. Inhibition of fatty acid uptake and lipogenesis by TNF-α
In adipocytes excess energy is stored in the form of lipid droplets rich in neutral triacylglycerols (TAG). TAG synthesis (or lipogenesis) results from the esterification of FA-derived Acyl CoA and Gycerol-3-P. FA are derived from three sources: (a) from the circulation, (b) from lipolysis of intracellular TAG or (c) de novo FA synthesis from glucose. Similarly, Glycerol-3-P can be sourced from glycerol, glucose, and amino acids (Fig. 3A). Hence, the overall metabolic flux of lipids into TAG is dependent on the availability of relevant substrates and the regulation of several enzymatic pathways, which may include those that are not classically thought of as lipogenic.
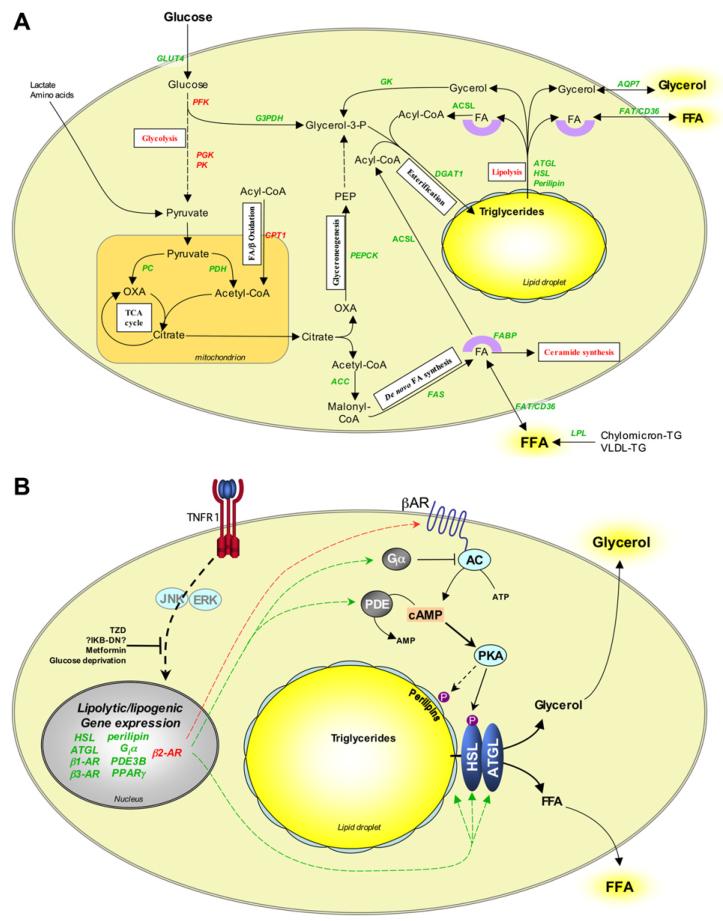
Fig. 3. Effects of TNF-α on adipocyte lipid metabolism and lipolysis.
(A) Pathways involved in adipocyte lipogenesis and their modulation by TNF-α. Adipocytes take up glucose, glycerol and FFA from serum and convert these to triglycerides via numerous biochemical pathways. White boxes indicate the nature of the pathways involved. TNF-α modulates the expression of many of the enzymes and other proteins that regulate these pathways, thereby compromising adipocyte triglyceride storage. (B) Mechanisms of TNF-α-induced lipolysis in adipocytes. During lipolysis triglycerides are hydrolysed into FFA and glycerol. Stimulus-induced lipolysis is mediated by hormone-sensitive lipase (HSL), whereas basal lipolysis may be regulated by adipose triglyceride lipase (ATGL). Lipolysis is further regulated by the perilipins, a family of phosphoproteins that are localised at the surface of lipid droplets in adipocytes. Perilipins normally inhibit lipolysis by preventing access of lipases to the lipid droplets. PKA stimulates lipolysis by phosphorylating HSL and the perilipins. This activates HSL and enables it to access substrates in the lipid droplets. Downregulation of cAMP by phosphodiesterases (PDE) or stimulation of Giα-coupled receptors abrogates PKA activation and thereby inhibits lipolysis. TNF-α stimulates lipolysis via a glucose-dependent mechanism that likely involves transcriptional effects. These may be mediated via JNK, ERK1/2 and NFκB, resulting in upregulation of cAMP and downregulation of perilipins. Some of these effects, such as downregulation of Giα subtypes, are specific to rodent adipocytes. Arrows and gene names in red and green indicate upregulation and downregulation, respectively. PEPCK, phosphoenolpyruvate carboxykinase; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; FABP, fatty acid-binding protein; FAT, fatty acid translocase; ACS, aceyl-CoA synthetase long chain; DGAT1, diacylglycerol acyltransferase 1; AQP7, aquaporin 7; GK, glycerol kinase; G3PDH, glyceraldehyde-3-phosphate dehydrogenase; PFK, phosphofructokinase; PGK, phosphoglycerate kinase; PK, pyruvate kinase; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase; TCA, tri-carboxylic acid; CPT1 carnitine palmitoyltransferase 1; OXA, oxaloacetate; PEP, phosphoenol pyruvate; TG, triglyceride; VLDL, very low-density lipoprotein.
By inducing adipocyte insulin resistance, TNF-α can impair glucose uptake into adipocytes. TNF-α also inhibits the uptake of FFA. Although the mechanism of FFA uptake is being debated, TNF-α does downregulate the expression of FA transport protein (FATP) and translocase (FAT) in adipose tissue[137], as well as the FA-binding protein FABP4/aP2. FFA uptake into adipocytes is also facilitated by the extracellular expression and activity of lipoprotein lipase (LPL) [136]. TNF-α has been shown to downregulate LPL expression in both murine adipose tissue and in 3T3-L1 adipocytes [62,66], likely via a TNFR1-dependent pathway [138]. However, studies investigating the effect of TNF-α on LPL expression in human adipose tissue have reported conflicting results [139,140].
In addition to suppressing gene expression of key proteins of FA uptake, TNF-α reduces the transcript levels and expression of many proteins involved in glyceroneogenesis, de novo FA synthesis and esterification (Fig. 3A). This leads to impaired triglyceride storage in adipose tissue. Notably, most of these genes are regulated by PPARγ activity. Thus TNF-α may mediate these effects primarily through the inhibition of PPARγ activity and expression.
4.2. TNF-α-induced lipolysis
The primary mechanism by which surplus fuel is made available from adipocytes is through stimulated lipolysis. This process liberates FA and glycerol from stored lipid and is physiologically activated by hormones such as catecholamines that stimulate cAMP production and PKA-dependent phosphorylation of hormone-sensitive lipase (HSL) and perilipins (Fig. 3B). TNF-α also potently stimulates lipolysis and this may contribute to hyperlipidaemic conditions. The increased availability of FFA may be shuttled into de novo ceramide production in adipose tissue (Fig. 3A) but can also cause lipotoxicity-induced insulin resistance in more distal organs.
Mechanistically, TNF-α can stimulate lipolysis in the absence of insulin, suggesting that TNF-α does not simply antagonise insulin’s anti-lipolytic actions. In addition, extracellular glucose is required for TNF-α-mediated adipocyte lipolysis[141], suggesting that a nutritional status or substrate availability is required (e.g. provision of ATP). However, the mechanism by which TNF-α induces adipocyte lipolysis has yet to be completely elucidated. Nevertheless, some information is available. Activation of a TNFR1-dependent pathway is both necessary and sufficient [13,46,138,142] and the downstream signals involve activation of ERK1/2, JNK, AMPK, IKK and PKA, since inhibitors of these kinases can prevent TNF-α-induced lipolysis [55,143,144]. Furthermore, a recent study suggests that, in adipocytes, TNF-α signals via a trimeric G protein, Gαq/11, and an adapter protein, β-arrestin-1, to mediate ERK activation and lipolysis [145]. However, TNF-α-induced activation of ERK1/2, JNK and IKK in adipocytes is rapid and transient [54,55], whereas detection of TNF-α-induced lipolysis takes >6 h. This suggests that more distal TNF-α-induced events remain to be identified, which are likely to be controlled by transcriptional regulation (Fig. 3B). This is consistent with the observations that TNF-α-induced lipolysis is sensitive to inhibition by TZD and NFκB inhibitors [146]. Furthermore, TNF-α treatment decreases the expression of many genes involved in preventing lipolysis, such as Giα, PDE3B, and perilipin [55,143,146-148] (Fig. 3B). Again, TNF-α-mediated suppression of PPARγ is likely to play an important role since inhibition of PPARγ results in decreased perilipin expression [149], and adenoviral expression of PPARγ S112A in 3T3-L1 adipocytes attenuates TNF-α-induced decreases in TG content [70]. Nevertheless, a less-intuitive aspect that remains unresolved is that TNF-α also decreases the expression of key lipolytic genes e.g. HSL, ATGL, β1-AR and β3-AR [62,150]. In contrast, very few reports have identified upregulation of lipolytic genes (e.g. β2-AR upregulation in murine adipocytes) [151] (Fig. 3B).
5. TNF-α and adipocyte differentiation
In addition to its effects on lipid metabolism in mature adipocytes, TNF-α may impair the lipid storage capacity of adipose tissue by suppressing the recruitment and differentiation of new adipocytes from precursor cells. The transcriptional regulation of adipocyte differentiation has been extensively studied, at least in vitro (for reviews see, [152,153]). In brief, adipogenesis is driven by a transcriptional cascade, which is characterised by the early, transient expression of C/EBPβ and C/EBPδ. This precedes the induction of C/EBPα and PPARγ, the master regulators of adipogenesis. TNF-α inhibits adipogenesis by preventing the induction of PPARγ and C/EBPα expression [47], thereby also preventing the induction of genes that are responsible for the mature adipocyte phenotype (e.g. aP2, Glut4, ATGL) [62].
5.1. Transcriptional targets of TNF-α in preadipocytes
Although TNF-α does not prevent the early expression of C/EBPβ or C/EBPδ, it is likely that it inhibits the PPARγ-mediated expression of PPARγ and C/EBPα, as has been discussed above (see Section 2.1). This is proposed to be mediated via the TAK1/TAB1/NIK kinase cascade to activate the p65/RelA subunit of NFκB, which acts to sequester and prevent PGC/PPARγ complex function [57]. Currently, it is not clear whether this is the primary target of TNF-α in preadipocytes, as these cells tend to have very low levels of PPARγ protein (compared to mature adipocytes) and basal PPARγ transcript levels are not downregulated during TNF-α-induced anti-adipogenesis [47]. Nonetheless, TZD treatment can antagonise TNF-α-induced anti-adipogenesis [154], confirming that PPARγ function is an important factor. Additionally, MAP4K4 has been proposed to be a negative regulator of PPARγ and adipogenesis and its expression can be induced by TNF-α (at least in adipocytes) [72]; however its role and mechanism of action in TNF-α-induced anti-adipogenesis in preadipocytes remains to be defined.
Another negative regulator of adipogenesis that acts early and suppresses PPARγ and C/EBPα expression is Wnt10b[155]. This is an important physiological factor involved in lineage commitment and determination. The canonical Wnt signalling pathway regulates the expression of Wnt target genes via stabilisation of β-catenin, a co-activator of the T-cell factor (TCF) family of transcription factors. Activation of this pathway has been shown to inhibit PPARγ expression and lipid accumulation both in vitro and in vivo. Conversely, PPARγ can negatively regulate canonical Wnt signalling [156]. Recently, we noticed that TNF-α-induced suppression of PPARγ and C/EBPα coincides with enhanced expression of several reported mediators of anti-adipogenesis that are also targets of the Wnt/β-catenin/TCF4 pathway. These include c-myc, cyclin D1, and PPARdelta [157-160]. By abrogating β-catenin/TCF signalling in 3T3-L1 cells, either via stable knockdown of β-catenin or by overexpressing dominant-negative TCF4 (dnTCF4), we demonstrated that TNF-α-induced anti-adipogenesis is dependent on β-catenin/TCF4 activity [47]. Therefore both TNF-α and canonical Wnt signalling pathways seem to converge to inhibit adipogenesis at the level of TCF4-dependent transcription. Intriguingly, TCF4 is also called transcription factor 7 like-2 (TCF7L2), and recent studies have identified a strong association between at least 2 SNPS in the TCF7L2 gene and type 2 diabetes [161,162]. These risk-conferring genotypes are also strongly associated with impaired β-cell function, development and possibly survival [163,164], while their adipose tissue expression may be associated with susceptibility of obese individuals to developing diabetes [165].
In addition to transcriptional events, TNFR-proximal events required for TNF-α-induced anti-adipogenesis have been elucidated. Here, both secreted and transmembrane TNF-α can inhibit adipogenesis via TNFR1 and a functional TNFR1-DD [4,47,166]. We have also shown that TNF-α-induced maintenance of β-catenin during anti-adipogenesis requires a functional TNFR1-DD [47]. A number of signals downstream of this domain have been linked to suppression of C/EBPα and PPARγ. These include activation of ERK, JNK, IKKβ and ceramide synthesis via aSMase [56,167], each of which has been suggested to exert anti-adipogenic effects[57,68,88,167,168]. However, this contrasts with other reports suggesting that inhibition of JNK activation does not reverse TNF-α-mediated suppression of PPARγ expression [56,72]. Furthermore, ERK activation may stimulate PPARγ expression during the early stages of adipogenesis, possibly by enhancing C/EBPβ activity [169,170], and NFκB activity reportedly increases during adipogenesis without TNF-α [171]. In contrast, exogenous ceramides do appear to mimic TNF-α actions to inhibit adipogenesis by blocking the transcriptional activity of C/EBPβ, resulting in suppression of C/EBPα and PPARγ mRNA [172]. However, the identities of the mediators in ceramide-induced anti-adipogenesis remain unknown.
The dissection of upstream signals has been complicated by the fact that, unlike adipocytes, preadipocytes can be particularly sensitive to TNF-α-induced cell death during anti-adipogenesis. This is often enhanced when preadipocytes are pre-treated with inhibitors of pro-survival signals, including NFκB. Indeed, ceramides are well known inducers of apoptosis and this is entirely consistent with TNF-α-induced toxic signals emanating from the TNFR1-DD. However pharmacological attempts to separate apoptotic signals from anti-adipogenic signals have been notoriously difficult. Nonetheless, we and others have demonstrated that picomolar concentrations of TNF-α can inhibit adipogenesis without inducing cell death [47,56]. Furthermore, in our recent study we observed that knock down of β-catenin in 3T3-L1 preadipocytes enhanced their sensitivity to TNF-α-induced cell death. Taken together we speculate that the signals induced by TNFR1-DD that lead to anti-adipogenesis in vitro are intricately involved in cell survival as well as differentiation. Future investigations should shed light on the nature of these events and whether this is relevant to adipose tissue plasticity and/or expansion in vivo.
6. TNF-α, brown adipose tissue and thermogenesis
Whereas white adipose tissue (WAT) is primarily responsible for storage and release of surplus fuel and generation of endocrine signals, brown adipose tissue (BAT) specialises in energy expenditure via beta-oxidation that is coupled to thermogenesis [173]. The thermogenic capacity of BAT is an important factor that contributes to the development of obesity-associated metabolic dysregulation. Indeed, obesity is associated with reductions in thermogenically active BAT [32,53,174] and transgenic mice lacking BAT become obese [173]. Conversely, increased UCP-1 expression in BAT is associated with protection against metabolic syndrome in mice [175]. Numerous studies suggest that TNF-α can modulate the thermogenic capacity of BAT. However, these studies have yielded conflicting results. For example, some reports suggest that administration of TNF-α increases BAT thermogenic activity, possibly by upregulating UCP expression [176,177]. This is consistent with the role of TNF-α in mediating increased energy expenditure in catabolic states (e.g during cachexia). However, TNF-α may mediate these effects by targeting the central nervous system, rather than BAT per se [178]. In contrast, some studies suggest that TNF-α impairs BAT thermogenesis via downregulation of UCP-1 and β3-AR expression [53,151,179,180]. These discrepancies may result from differences in the experimental models used. Nevertheless, compelling evidence for the role of TNF-α in obesity-associated alterations in BAT thermogenesis comes from studies in obese mice that lack TNF-α function. Indeed, the obesity-associated decreases in UCP-1 and β3-AR expression are attenuated in these mice [53]. Accordingly, the lack of TNFRs significantly improves the thermoadaptive capacity of obese animals [53].
TNF-α may also compromise BAT thermogenesis by promoting BAT atrophy or by impairing BAT differentiation. Obesity is associated with increased apoptosis of brown adipocytes [32], and several studies demonstrate that TNF-α can induce apoptosis of brown adipocytes [32,53,181]. Similarly, TNF-α may prevent the differentiation of brown adipocytes[180,182]. Several studies suggest that adrenergic stimulation promotes the transdifferentiation of white adipocytes into brown adipocytes (reviewed in [173]). Indeed, this phenomenon is significantly impaired in mice lacking the β3-AR [183]. Hence, by downregulating β3-AR expression in WAT, TNF-α might indirectly attenuate the transdifferentiation of white adipocytes into brown adipocytes and thereby impair thermogenesis.
7. Summary
Although the oncolytic and cachectic properties of TNF-α have been studied for over a century, the last decade alone has unveiled many new aspects of TNF-α action on adipocyte biology. In addition to its role in inducing insulin resistance in adipose tissue, its local actions can impact on whole body insulin sensitivity through increased FFA and altered adipokine production. Furthermore, TNF-α can also significantly alter the lipid storage and oxidative capacity of adipose tissues. Hence, adipose tissue-derived TNF-α can contribute to the metabolic complications associated with obesity by altering both adipose tissue function and expandability.
This multi-faceted nature of TNF-α is entirely in keeping with its multifunctional classification and so it should come as no surprise that this potent cytokine is capable of stimulating a variety of signalling pathways in a context-dependent manner. While the exact components of these signals remain to be elucidated, it is becoming increasingly clear that a complex network of signals emanating from the TNFR1 death domain converge with other signalling pathways to influence cell fate determination and metabolism. It is likely that these signals will vary significantly depending upon the amount and duration of exposure, as well nutritional status and the extracellular milieu. However, as new ‘omic’ technologies become available, it should be possible to investigate the influence of these additional factors and better our understanding at a more global, systems level.
Acknowledgements
We apologise for the inevitable omission of many relevant references due to space limitations. Research in our lab is funded by a Medical Research Council studentship (W.C.), a Biotechnology and Biological Sciences Research Council (BBSRC) David Phillips Fellowship (J.K.S.) and grants from Diabetes UK, BBSRC and European Union Sixth Framework Programme.
Abbreviations
- TNF-α
tumour necrosis factor-alpha
- sTNF-α
soluble tumour necrosis factor-alpha
- mTNF-α
transmembrane tumour necrosis factor-alpha
- T2D
type 2 diabetes
- TNFR1
tumour necrosis factor-alpha receptor 1
- TNFR2
tumour necrosis factor-alpha receptor 2
- WAT
white adipose tissue
- BAT
brown adipose tissue
- PPARγ
peroxisome proliferator-activated receptor gamma
- C/EBPα
CCAAT/enhancer binding protein
- LPL
lipoprotein lipase
- HSL
hormone sensitive lipase
- NFκB
nuclear factor-kappa B
- IκB
inhibitor of NFκB
- IKKβ
IκB kinase beta
- JNK
c-jun N-terminal kinase
- ERK1/2
extracellular signal-regulated kinase
- AMPK
AMP-activated protein kinase
References
- [1].Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- [2].Black RA, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- [3].Perez C, Albert I, DeFay K, Zachariades N, Gooding L, Kriegler M. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell. 1990;63:251–258. doi: 10.1016/0092-8674(90)90158-b. [DOI] [PubMed] [Google Scholar]
- [4].Xu H, Sethi JK, Hotamisligil GS. Transmembrane tumor necrosis factor (TNF)-alpha inhibits adipocyte differentiation by selectively activating TNF receptor 1. J. Biol. Chem. 1999;274:26287–26295. doi: 10.1074/jbc.274.37.26287. [DOI] [PubMed] [Google Scholar]
- [5].Grell M. Tumor necrosis factor (TNF) receptors in cellular signaling of soluble and membrane-expressed TNF. J. Inflamm. 1995;47:8–17. [PubMed] [Google Scholar]
- [6].Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- [7].Xu H, Uysal KT, Becherer JD, Arner P, Hotamisligil GS. Altered tumor necrosis factor-alpha (TNF-alpha) processing in adipocytes and increased expression of transmembrane TNF-alpha in obesity. Diabetes. 2002;51:1876–1883. doi: 10.2337/diabetes.51.6.1876. [DOI] [PubMed] [Google Scholar]
- [8].Zahorska-Markiewicz B, Janowska J, Olszanecka-Glinianowicz M, Zurakowski A. Serum concentrations of TNF-alpha and soluble TNF-alpha receptors in obesity. Int. J. Obes. Relat. Metab. Disord. 2000;24:1392–1395. doi: 10.1038/sj.ijo.0801398. [DOI] [PubMed] [Google Scholar]
- [9].Togashi N, Ura N, Higashiura K, Murakami H, Shimamoto K. Effect of TNF-alpha-converting enzyme inhibitor on insulin resistance in fructose-fed rats. Hypertension. 2002;39:578–580. doi: 10.1161/hy0202.103290. [DOI] [PubMed] [Google Scholar]
- [10].Serino M, et al. Mice heterozygous for tumor necrosis factor-alpha converting enzyme are protected from obesity-induced insulin resistance and diabetes. Diabetes. 2007;56:2541–2546. doi: 10.2337/db07-0360. [DOI] [PubMed] [Google Scholar]
- [11].Weisberg S, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stephens JM, Pekala PH. Transcriptional repression of the GLUT4 and C/EBP genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. J. Biol. Chem. 1991;266:21839–21845. [PubMed] [Google Scholar]
- [13].Kawakami M, Murase T, Ogawa H, Ishibashi S, Mori N, Takaku F, Shibata S. Human recombinant TNF suppresses lipoprotein lipase activity and stimulates lipolysis in 3T3-L1 cells. J. Biochem. (Tokyo) 1987;101:331–338. doi: 10.1093/oxfordjournals.jbchem.a121917. [DOI] [PubMed] [Google Scholar]
- [14].Beutler B, Greenwald D, Hulmes JD, Chang M, Pan YC, Mathison J, Ulevitch R, Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316:552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- [15].Torti FM, Dieckmann B, Beutler B, Cerami A, Ringold GM. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985;229:867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- [16].Ventre J, Doebber T, Wu M, MacNaul K, Stevens K, Pasparakis M, Kollias G, Moller DE. Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes. 1997;46:1526–1531. doi: 10.2337/diab.46.9.1526. [DOI] [PubMed] [Google Scholar]
- [17].Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-[alpha] function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- [18].Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes. 1996;45:881–885. doi: 10.2337/diab.45.7.881. [DOI] [PubMed] [Google Scholar]
- [19].Di Rocco P, Manco M, Rosa G, Greco AV, Mingrone G. Lowered tumor necrosis factor receptors, but not increased insulin sensitivity, with infliximab. Obes. Res. 2004;12:734–739. doi: 10.1038/oby.2004.86. [DOI] [PubMed] [Google Scholar]
- [20].Paquot N, Castillo MJ, Lefebvre PJ, Scheen AJ. No increased insulin sensitivity after a single intravenous administration of a recombinant human tumor necrosis factor receptor: Fc fusion protein in obese insulin-resistant patients. J. Clin. Endocrinol. Metab. 2000;85:1316–1319. doi: 10.1210/jcem.85.3.6417. [DOI] [PubMed] [Google Scholar]
- [21].Dominguez H, et al. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J. Vasc. Res. 2005;42:517–525. doi: 10.1159/000088261. [DOI] [PubMed] [Google Scholar]
- [22].Kiortsis DN, Mavridis AK, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann. Rheum. Dis. 2005;64:765–766. doi: 10.1136/ard.2004.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tam LS, Tomlinson B, Chu TT, Li TK, Li EK. Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clin. Rheumatol. 2007;26:1495–1498. doi: 10.1007/s10067-007-0539-8. [DOI] [PubMed] [Google Scholar]
- [24].Huvers FC, Popa C, Netea MG, van den Hoogen FH, Tack CJ. Improved insulin sensitivity by anti-TNF{alpha} antibody treatment in patients with rheumatic diseases. Ann. Rheum. Dis. 2007;66:558–559. doi: 10.1136/ard.2006.062323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yazdani-Biuki B, Stelzl H, Brezinschek HP, Hermann J, Mueller T, Krippl P, Graninger W, Wascher TC. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-alpha antibody infliximab. Eur. J. Clin. Invest. 2004;34:641–642. doi: 10.1111/j.1365-2362.2004.01390.x. [DOI] [PubMed] [Google Scholar]
- [26].Ross S, Erickson RL, Gerin I, DeRose PM, Bajnok L, Longo KA, Misek DE, Kuick R, Hanash SM, Atkins KB, Andresen SM, Nebb HI, Madsen L, Kristiansen K, MacDougald OA. Microarray analyses during adipogenesis: understanding the effects of Wnt signaling on adipogenesis and the roles of liver X receptor alpha in adipocyte metabolism. Mol. Cell Biol. 2002;22:5989–5999. doi: 10.1128/MCB.22.16.5989-5999.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fain JN, Bahouth SW, Madan AK. TNFalpha release by the nonfat cells of human adipose tissue. Int. J. Obes. Relat. Metab. Disord. 2004;28:616–622. doi: 10.1038/sj.ijo.0802594. [DOI] [PubMed] [Google Scholar]
- [29].Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Coenen KR, Gruen ML, Chait A, Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56:564–573. doi: 10.2337/db06-1375. [DOI] [PubMed] [Google Scholar]
- [31].De Taeye BM, Novitskaya T, McGuinness OP, Gleaves L, Medda M, Covington JW, Vaughan DE. Macrophage TNF-alpha contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2007;293:E713–E725. doi: 10.1152/ajpendo.00194.2007. [DOI] [PubMed] [Google Scholar]
- [32].Nisoli E, Briscini L, Tonello C, De Giuli-Morghen C, Carruba MO. Tumor necrosis factor-alpha induces apoptosis in rat brown adipocytes. Cell Death Differ. 1997;4:771–778. doi: 10.1038/sj.cdd.4400292. [DOI] [PubMed] [Google Scholar]
- [33].Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, Defuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling and obesity complications. Diabetes. 2007 doi: 10.2337/db07-0767. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [34].Cinti S, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- [35].Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- [36].Kelley DS. Modulation of human immune and inflammatory responses by dietary fatty acids. Nutrition. 2001;17:669–673. doi: 10.1016/s0899-9007(01)00576-7. [DOI] [PubMed] [Google Scholar]
- [37].Ugochukwu NH, Figgers CL. Caloric restriction inhibits up-regulation of inflammatory cytokines and TNF-alpha, and activates IL-10 and haptoglobin in the plasma of streptozotocin-induced diabetic rats. J. Nutr. Biochem. 2007;18:120–126. doi: 10.1016/j.jnutbio.2006.03.008. [DOI] [PubMed] [Google Scholar]
- [38].McTernan PG, et al. Insulin and rosiglitazone regulation of lipolysis and lipogenesis in human adipose tissue in vitro. Diabetes. 2002;51:1493–1498. doi: 10.2337/diabetes.51.5.1493. [DOI] [PubMed] [Google Scholar]
- [39].Gatanaga T, et al. Purification and characterization of an inhibitor (soluble tumor necrosis factor receptor) for tumor necrosis factor and lymphotoxin obtained from the serum ultrafiltrates of human cancer patients. Proc. Natl. Acad. Sci. USA. 1990;87:8781–8784. doi: 10.1073/pnas.87.22.8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hotamisligil GS, Arner P, Atkinson RL, Spiegelman BM. Differential regulation of the p80 tumor necrosis factor receptor in human obesity and insulin resistance. Diabetes. 1997;46:451–455. doi: 10.2337/diab.46.3.451. [DOI] [PubMed] [Google Scholar]
- [41].Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am. J. Physiol. 1999;277:E971–E975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- [42].MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14:477–492. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- [43].Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM. Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J. Biol. Chem. 1996;271:13018–13022. doi: 10.1074/jbc.271.22.13018. [DOI] [PubMed] [Google Scholar]
- [44].Lewis M, Tartaglia LA, Lee A, Bennett GL, Rice GC, Wong GH, Chen EY, Goeddel DV. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc. Natl. Acad. Sci. USA. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Uysal KT, Wiesbrock SM, Hotamisligil GS. Functional analysis of tumor necrosis factor (TNF) receptors in TNF-alpha-mediated insulin resistance in genetic obesity. Endocrinology. 1998;139:4832–4838. doi: 10.1210/endo.139.12.6337. [DOI] [PubMed] [Google Scholar]
- [46].Sethi JK, Xu H, Uysal KT, Wiesbrock SM, Scheja L, Hotamisligil GS. Characterisation of receptor-specific TNFalpha functions in adipocyte cell lines lacking type 1 and 2 TNF receptors. FEBS Lett. 2000;469:77–82. doi: 10.1016/s0014-5793(00)01250-3. [DOI] [PubMed] [Google Scholar]
- [47].Cawthorn WP, Heyd F, Hegyi K, Sethi JK. Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ. 2007;14:1361–1373. doi: 10.1038/sj.cdd.4402127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Liu LS, Spelleken M, Rohrig K, Hauner H, Eckel J. Tumor necrosis factor-alpha acutely inhibits insulin signaling in human adipocytes: implication of the p80 tumor necrosis factor receptor. Diabetes. 1998;47:515–522. doi: 10.2337/diabetes.47.4.515. [DOI] [PubMed] [Google Scholar]
- [49].Good M, Newell FM, Haupt LM, Whitehead JP, Hutley LJ, Prins JB. TNF and TNF receptor expression and insulin sensitivity in human omental and subcutaneous adipose tissue-influence of BMI and adipose distribution. Diab. Vasc. Dis. Res. 2006;3:26–33. doi: 10.3132/dvdr.2006.003. [DOI] [PubMed] [Google Scholar]
- [50].Pandey M, Tuncman G, Hotamisligil GS, Samad F. Divergent roles for p55 and p75 TNF-alpha receptors in the induction of plasminogen activator inhibitor-1. Am. J. Pathol. 2003;162:933–941. doi: 10.1016/s0002-9440(10)63888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Csehi SB, Mathieu S, Seifert U, Lange A, Zweyer M, Wernig A, Adam D. Tumor necrosis factor (TNF) interferes with insulin signaling through the p55 TNF receptor death domain. Biochem. Biophys. Res. Commun. 2005;329:397–405. doi: 10.1016/j.bbrc.2005.01.140. [DOI] [PubMed] [Google Scholar]
- [52].Prins JB, Niesler CU, Winterford CM, Bright NA, Siddle K, O’Rahilly S, Walker NI, Cameron DP. Tumor necrosis factor-alpha induces apoptosis of human adipose cells. Diabetes. 1997;46:1939–1944. doi: 10.2337/diab.46.12.1939. [DOI] [PubMed] [Google Scholar]
- [53].Nisoli E, et al. Tumor necrosis factor alpha mediates apoptosis of brown adipocytes and defective brown adipocytefunction in obesity. Proc. Natl. Acad. Sci. USA. 2000;97:8033–8038. doi: 10.1073/pnas.97.14.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jain RG, Phelps KD, Pekala PH. Tumor necrosis factor-alpha initiated signal transduction in 3T3-L1 adipocytes. J. Cell Physiol. 1999;179:58–66. doi: 10.1002/(SICI)1097-4652(199904)179:1<58::AID-JCP8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- [55].Ryden M, Dicker A, van Harmelen V, Hauner H, Brunnberg M, Perbeck L, Lonnqvist F, Arner P. Mapping of early signaling events in tumor necrosis factor-alpha-mediated lipolysis in human fat cells. J. Biol. Chem. 2002;277:1085–1091. doi: 10.1074/jbc.M109498200. [DOI] [PubMed] [Google Scholar]
- [56].Chae GN, Kwak SJ. NF-kappaB is involved in the TNF-alpha induced inhibition of the differentiation of 3T3-L1 cells by reducing PPARgamma expression. Exp. Mol. Med. 2003;35:431–437. doi: 10.1038/emm.2003.56. [DOI] [PubMed] [Google Scholar]
- [57].Suzawa M, et al. Cytokines suppress adipogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat. Cell Biol. 2003;5:224–230. doi: 10.1038/ncb942. [DOI] [PubMed] [Google Scholar]
- [58].Kim KY, Kim JK, Jeon JH, Yoon SR, Choi I, Yang Y. c-Jun N-terminal kinase is involved in the suppression of adiponectin expression by TNF-alpha in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2005;327:460–467. doi: 10.1016/j.bbrc.2004.12.026. [DOI] [PubMed] [Google Scholar]
- [59].Abel ED, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- [60].Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14:447–455. doi: 10.1016/s1359-6101(03)00052-2. [DOI] [PubMed] [Google Scholar]
- [61].Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J. Biol. Chem. 1997;272:971–976. doi: 10.1074/jbc.272.2.971. [DOI] [PubMed] [Google Scholar]
- [62].Ruan H, Miles PD, Ladd CM, Ross K, Golub TR, Olefsky JM, Lodish HF. Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha: implications for insulin resistance. Diabetes. 2002;51:3176–3188. doi: 10.2337/diabetes.51.11.3176. [DOI] [PubMed] [Google Scholar]
- [63].Ruan H, Hacohen N, Golub TR, Van Parijs L, Lodish HF. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. 2002;51:1319–1336. doi: 10.2337/diabetes.51.5.1319. [DOI] [PubMed] [Google Scholar]
- [64].Miles PD, Romeo OM, Higo K, Cohen A, Rafaat K, Olefsky JM. TNF-alpha-induced insulin resistance in vivo and its prevention by troglitazone. Diabetes. 1997;46:1678–1683. doi: 10.2337/diab.46.11.1678. [DOI] [PubMed] [Google Scholar]
- [65].Peraldi P, Xu M, Spiegelman BM. Thiazolidinediones block tumor necrosis factor-alpha-induced inhibition of insulin signaling. J. Clin. Invest. 1997;100:1863–1869. doi: 10.1172/JCI119715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ruan H, Pownall HJ, Lodish HF. Troglitazone antagonizes tumor necrosis factor-alpha-induced reprogramming of adipocyte gene expression by inhibiting the transcriptional regulatory functions of NF-kappaB. J. Biol. Chem. 2003;278:28181–28192. doi: 10.1074/jbc.M303141200. [DOI] [PubMed] [Google Scholar]
- [67].Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- [68].Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J. Biol. Chem. 1997;272:5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- [69].Sung CK, She H, Xiong S, Tsukamoto H. Tumor necrosis factor-alpha inhibits peroxisome proliferator-activated receptor gamma activity at a posttranslational level in hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G722–G729. doi: 10.1152/ajpgi.00411.2003. [DOI] [PubMed] [Google Scholar]
- [70].Iwata M, et al. Pioglitazone ameliorates tumor necrosis factor-alpha-induced insulin resistance by a mechanism independent of adipogenic activity of peroxisome proliferator–activated receptor-gamma. Diabetes. 2001;50:1083–1092. doi: 10.2337/diabetes.50.5.1083. [DOI] [PubMed] [Google Scholar]
- [71].Rangwala SM, Rhoades B, Shapiro JS, Rich AS, Kim JK, Shulman GI, Kaestner KH, Lazar MA. Genetic modulation of PPARgamma phosphorylation regulates insulin sensitivity. Dev. Cell. 2003;5:657–663. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- [72].Tang X, et al. An RNA interference-based screen identifies MAP4K4/NIK as a negative regulator of PPARgamma, adipogenesis, and insulin-responsive hexose transport. Proc. Natl. Acad. Sci. USA. 2006;103:2087–2092. doi: 10.1073/pnas.0507660103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Tesz GJ, Guilherme A, Guntur KV, Hubbard AC, Tang X, Chawla A, Czech MP. Tumor necrosis factor alpha (TNFalpha) stimulates Map4k4 expression through TNF-alpha receptor 1 signaling to c-Jun and activating transcription factor 2. J. Biol. Chem. 2007;282:19302–19312. doi: 10.1074/jbc.M700665200. [DOI] [PubMed] [Google Scholar]
- [74].Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- [75].Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- [77].Peraldi P, Filloux C, Emanuelli B, Hilton DJ, Van Obberghen E. Insulin induces suppressor of cytokine signaling-3 tyrosine phosphorylation through janus-activated kinase. J. Biol. Chem. 2001;276:24614–24620. doi: 10.1074/jbc.M102209200. [DOI] [PubMed] [Google Scholar]
- [78].Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J. Biol. Chem. 2001;276:47944–47949. doi: 10.1074/jbc.M104602200. [DOI] [PubMed] [Google Scholar]
- [79].Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol. Cell Biol. 2004;24:5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Shi H, Cave B, Inouye K, Bjorbaek C, Flier JS. Overexpression of suppressor of cytokine signaling 3 in adipose tissue causes local but not systemic insulin resistance. Diabetes. 2006;55:699–707. doi: 10.2337/diabetes.55.03.06.db05-0841. [DOI] [PubMed] [Google Scholar]
- [81].Steinberg GR, et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006;4:465–474. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- [82].Ren T, He J, Jiang H, Zu L, Pu S, Guo X, Xu G. Metformin reduces lipolysis in primary rat adipocytes stimulated by tumor necrosis factor-alpha or isoproterenol. J. Mol. Endocrinol. 2006;37:175–183. doi: 10.1677/jme.1.02061. [DOI] [PubMed] [Google Scholar]
- [83].Engelman JA, Berg AH, Lewis RY, Lisanti MP, Scherer PE. Tumor necrosis factor alpha-mediated insulin resistance, but not dedifferentiation, is abrogated by MEK1/2 inhibitors in 3T3-L1 adipocytes. Mol. Endocrinol. 2000;14:1557–1569. doi: 10.1210/mend.14.10.0542. [DOI] [PubMed] [Google Scholar]
- [84].Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- [85].Zick Y. Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci. STKE. 2005:pe4. doi: 10.1126/stke.2682005pe4. [DOI] [PubMed] [Google Scholar]
- [86].Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J. Clin. Invest. 2001;107:181–189. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gao Z, Zuberi A, Quon MJ, Dong Z, Ye J. Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J. Biol. Chem. 2003;278:24944–24950. doi: 10.1074/jbc.M300423200. [DOI] [PubMed] [Google Scholar]
- [88].Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- [89].Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J. Biol. Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- [90].Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J. Biol. Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- [91].Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc. Natl. Acad. Sci. USA. 2001;98:4640–4645. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kim JA, et al. Phosphorylation of Ser24 in the pleckstrin homology domain of insulin receptor substrate-1 by Mouse Pelle-like kinase/interleukin-1 receptor-associated kinase: cross-talk between inflammatory signaling and insulin signaling that may contribute to insulin resistance. J. Biol. Chem. 2005;280:23173–23183. doi: 10.1074/jbc.M501439200. [DOI] [PubMed] [Google Scholar]
- [93].Nawaratne R, Gray A, Jorgensen CH, Downes CP, Siddle K, Sethi JK. Regulation of insulin receptor substrate 1 pleckstrin homology domain by protein kinase C: role of serine 24 phosphorylation. Mol. Endocrinol. 2006;20:1838–1852. doi: 10.1210/me.2005-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Grigsby RJ, Dobrowsky RT. Inhibition of ceramide production reverses TNF-induced insulin resistance. Biochem. Biophys. Res. Commun. 2001;287:1121–1124. doi: 10.1006/bbrc.2001.5694. [DOI] [PubMed] [Google Scholar]
- [95].Fernandez-Veledo S, Hernandez R, Teruel T, Mas JA, Ros M, Lorenzo M. Ceramide mediates TNF-alpha-induced insulin resistance on GLUT4 gene expression in brown adipocytes. Arch. Physiol. Biochem. 2006;112:13–22. doi: 10.1080/13813450500508137. [DOI] [PubMed] [Google Scholar]
- [96].Long SD, Pekala PH. Lipid mediators of insulin resistance: ceramide signalling down-regulates GLUT4 gene transcription in 3T3-L1 adipocytes. Biochem. J. 1996;319(Pt 1):179–184. doi: 10.1042/bj3190179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50:2563–2571. doi: 10.2337/diabetes.50.11.2563. [DOI] [PubMed] [Google Scholar]
- [98].Holland WL, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- [99].Tagami S, et al. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J. Biol. Chem. 2002;277:3085–3092. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]
- [100].Aerts JM, et al. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 2007;56:1341–1349. doi: 10.2337/db06-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- [102].Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- [103].Esposito K, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- [104].Dahlman I, Forsgren M, Sjogren A, Nordstrom EA, Kaaman M, Naslund E, Attersand A, Arner P. Downregulation of electron transport chain genes in visceral adipose tissue in type 2 diabetes independent of obesity and possibly involving tumor necrosis factor-alpha. Diabetes. 2006;55:1792–1799. doi: 10.2337/db05-1421. [DOI] [PubMed] [Google Scholar]
- [105].Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J. Lipid Res. 2007;48:1253–1262. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Yamauchi T, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- [107].Yamauchi T, et al. Globular adiponectin protected ob/ ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J. Biol. Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- [108].Kubota N, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- [109].Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- [110].Degawa-Yamauchi M, et al. Regulation of adiponectin expression in human adipocytes: effects of adiposity, glucocorticoids, and tumor necrosis factor alpha. Obes. Res. 2005;13:662–669. doi: 10.1038/oby.2005.74. [DOI] [PubMed] [Google Scholar]
- [111].Kappes A, Loffler G. Influences of ionomycin, dibutyryl-cycloAMP and tumour necrosis factor-alpha on intracellular amount and secretion of apM1 in differentiating primary human preadipocytes. Horm. Metab. Res. 2000;32:548–554. doi: 10.1055/s-2007-978684. [DOI] [PubMed] [Google Scholar]
- [112].Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am. J. Physiol. Endocrinol. Metab. 2003;285:E527–E533. doi: 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- [113].Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- [114].Matsuzawa Y. Adiponectin: identification, physiology and clinical relevance in metabolic and vascular disease. Atheroscler. Suppl. 2005;6:7–14. doi: 10.1016/j.atherosclerosissup.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [115].Kita A, et al. Identification of the promoter region required for human adiponectin gene transcription: Association with CCAAT/enhancer binding protein-beta and tumor necrosis factor-alpha. Biochem. Biophys. Res. Commun. 2005;331:484–490. doi: 10.1016/j.bbrc.2005.03.205. [DOI] [PubMed] [Google Scholar]
- [116].Kirchgessner TG, Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J. Clin. Invest. 1997;100:2777–2782. doi: 10.1172/JCI119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Finck BN, Johnson RW. Tumor necrosis factor (TNF)-alpha induces leptin production through the p55 TNF receptor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R537–R543. doi: 10.1152/ajpregu.2000.278.2.R537. [DOI] [PubMed] [Google Scholar]
- [118].Katsuki A, et al. Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1998;83:859–862. doi: 10.1210/jcem.83.3.4618. [DOI] [PubMed] [Google Scholar]
- [119].Pfeiffer A, Janott J, Mohlig M, Ristow M, Rochlitz H, Busch K, Schatz H, Schifferdecker E. Circulating tumor necrosis factor alpha is elevated in male but not in female patients with type II diabetes mellitus. Horm. Metab. Res. 1997;29:111–114. doi: 10.1055/s-2007-979001. [DOI] [PubMed] [Google Scholar]
- [120].Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J. Clin. Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Trujillo ME, Lee MJ, Sullivan S, Feng J, Schneider SH, Greenberg AS, Fried SK. Tumor necrosis factor alpha and glucocorticoid synergistically increase leptin production in human adipose tissue: role for p38 mitogen-activated protein kinase. J. Clin. Endocrinol. Metab. 2006;91:1484–1490. doi: 10.1210/jc.2005-1901. [DOI] [PubMed] [Google Scholar]
- [122].Fukuhara A, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- [123].Sethi JK. Is PBEF/Visfatin/Nampt an authentic adipokine relevant to the metabolic syndrome? Curr. Hypertens. Rep. 2007;9:33–38. doi: 10.1007/s11906-007-0007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M, Fasshauer M. Hormonal regulation of the novel adipocytokine visfatin in 3T3-L1 adipocytes. J. Endocrinol. 2005;185:R1–R8. doi: 10.1677/joe.1.06211. [DOI] [PubMed] [Google Scholar]
- [125].Hector J, et al. TNF-alpha alters visfatin and adiponectin levels in human fat. Horm. Metab. Res. 2007;39:250–255. doi: 10.1055/s-2007-973075. [DOI] [PubMed] [Google Scholar]
- [126].Steppan CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- [127].Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- [128].Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Tumor necrosis factor alpha is a negative regulator of resistin gene expression and secretion in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2001;288:1027–1031. doi: 10.1006/bbrc.2001.5874. [DOI] [PubMed] [Google Scholar]
- [129].Kaser S, Kaser A, Sandhofer A, Ebenbichler CF, Tilg H, Patsch JR. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem. Biophys. Res. Commun. 2003;309:286–290. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- [130].Nagaev I, Bokarewa M, Tarkowski A, Smith U. Human Resistin Is a Systemic Immune-Derived Proinflammatory Cytokine Targeting both Leukocytes and Adipocytes. PLoS ONE. 2006;1:e31. doi: 10.1371/journal.pone.0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ma LJ, et al. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 2004;53:336–346. doi: 10.2337/diabetes.53.2.336. [DOI] [PubMed] [Google Scholar]
- [132].Araki S, Dobashi K, Kubo K, Yamamoto Y, Asayama K, Shirahata A. N-acetylcysteine attenuates TNF-alpha induced changes in secretion of interleukin-6, plasminogen activator inhibitor-1 and adiponectin from 3T3-L1 adipocytes. Life Sci. 2006;79:2405–2412. doi: 10.1016/j.lfs.2006.08.004. [DOI] [PubMed] [Google Scholar]
- [133].Pandey M, Loskutoff DJ, Samad F. Molecular mechanisms of tumor necrosis factor-alpha-mediated plasminogen activator inhibitor-1 expression in adipocytes. Faseb J. 2005;19:1317–1319. doi: 10.1096/fj.04-3459fje. [DOI] [PubMed] [Google Scholar]
- [134].Samad F, Yamamoto K, Loskutoff DJ. Distribution and regulation of plasminogen activator inhibitor-1 in murine adipose tissue in vivo. Induction by tumor necrosis factor-alpha and lipopolysaccharide. J. Clin. Invest. 1996;97:37–46. doi: 10.1172/JCI118404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Samad F, Uysal KT, Wiesbrock SM, Pandey M, Hotamisligil GS, Loskutoff DJ. Tumor necrosis factor alpha is a key component in the obesity-linked elevation of plasminogen activator inhibitor 1. Proc. Natl. Acad. Sci. USA. 1999;96:6902–6907. doi: 10.1073/pnas.96.12.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ramsay TG. Fat cells. Endocrinol. Metab. Clin. North Am. 1996;25:847–870. doi: 10.1016/s0889-8529(05)70358-3. [DOI] [PubMed] [Google Scholar]
- [137].Memon RA, Feingold KR, Moser AH, Fuller J, Grunfeld C. Regulation of fatty acid transport protein and fatty acid translocase mRNA levels by endotoxin and cytokines. Am. J. Physiol. 1998;274:E210–E217. doi: 10.1152/ajpendo.1998.274.2.E210. [DOI] [PubMed] [Google Scholar]
- [138].Lopez-Soriano J, Llovera M, Carbo N, Garcia-Martinez C, Lopez-Soriano FJ, Argiles JM. Lipid metabolism in tumour-bearing mice: studies with knockout mice for tumour necrosis factor receptor 1 protein. Mol. Cell Endocrinol. 1997;132:93–99. doi: 10.1016/s0303-7207(97)00125-1. [DOI] [PubMed] [Google Scholar]
- [139].Fried SK, Zechner R. Cachectin/tumor necrosis factor decreases human adipose tissue lipoprotein lipase mRNA levels, synthesis, and activity. J. Lipid Res. 1989;30:1917–1923. [PubMed] [Google Scholar]
- [140].Kern PA. Recombinant human tumor necrosis factor does not inhibit lipoprotein lipase in primary cultures of isolated human adipocytes. J. Lipid Res. 1988;29:909–914. [PubMed] [Google Scholar]
- [141].Green A, Rumberger JM, Stuart CA, Ruhoff MS. Stimulation of lipolysis by tumor necrosis factor-alpha in 3T3-L1 adipocytes is glucose dependent: implications for long-term regulation of lipolysis. Diabetes. 2004;53:74–81. doi: 10.2337/diabetes.53.1.74. [DOI] [PubMed] [Google Scholar]
- [142].Green A, Dobias SB, Walters DJ, Brasier AR. Tumor necrosis factor increases the rate of lipolysis in primary cultures of adipocytes without altering levels of hormone-sensitive lipase. Endocrinology. 1994;134:2581–2588. doi: 10.1210/endo.134.6.8194485. [DOI] [PubMed] [Google Scholar]
- [143].Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes. 2002;51:2929–2935. doi: 10.2337/diabetes.51.10.2929. [DOI] [PubMed] [Google Scholar]
- [144].Zu L, Jiang H, He J, Xu C, Pu S, Liu M, Xu G. Salicylate Blocks Lipolytic Actions of Tumor Necrosis Factor-TNF-{alpha} in Primary Rat Adipocytes. Mol. Pharmacol. 2007 doi: 10.1124/mol.107.039479. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [145].Kawamata Y, Imamura T, Babendure JL, Lu JC, Yoshizaki T, Olefsky JM. Tumor necrosis factor receptor-1 can function through a G alpha q/11-beta-arrestin-1 signaling complex. J. Biol. Chem. 2007;282:28549–28556. doi: 10.1074/jbc.M705869200. [DOI] [PubMed] [Google Scholar]
- [146].Souza SC, Yamamoto MT, Franciosa MD, Lien P, Greenberg AS. BRL 49653 blocks the lipolytic actions of tumor necrosis factor-alpha: a potential new insulin-sensitizing mechanism for thiazolidinediones. Diabetes. 1998;47:691–695. doi: 10.2337/diabetes.47.4.691. [DOI] [PubMed] [Google Scholar]
- [147].Souza S, Palmer HJ, Kang YH, Yamamoto MT, Muliro KV, Paulson KE, Greenberg AS. TNF-alpha induction of lipolysis is mediated through activation of the extracellular signal related kinase pathway in 3T3-L1 adipocytes. J. Chem. Biol. 2003;89:1077–1086. doi: 10.1002/jcb.10565. [DOI] [PubMed] [Google Scholar]
- [148].Ryden M, Arvidsson E, Blomqvist L, Perbeck L, Dicker A, Arner P. Targets for TNF-alpha-induced lipolysis in human adipocytes. Biochem. Biophys. Res. Commun. 2004;318:168–175. doi: 10.1016/j.bbrc.2004.04.010. [DOI] [PubMed] [Google Scholar]
- [149].Tamori Y, Masugi J, Nishino N, Kasuga M. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes. 2002;51:2045–2055. doi: 10.2337/diabetes.51.7.2045. [DOI] [PubMed] [Google Scholar]
- [150].Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M, Fasshauer M. Isoproterenol, TNFalpha, and insulin downregulate adipose triglyceride lipase in 3T3-L1 adipocytes. Mol. Cell Endocrinol. 2005;240:43–49. doi: 10.1016/j.mce.2005.06.002. [DOI] [PubMed] [Google Scholar]
- [151].Hadri KE, Courtalon A, Gauthereau X, Chambaut-Guerin AM, Pairault J, Feve B. Differential regulation by tumor necrosis factor-alpha of beta1-, beta2-, and beta3-adrenoreceptor gene expression in 3T3-F442A adipocytes. J. Biol. Chem. 1997;272:24514–24521. doi: 10.1074/jbc.272.39.24514. [DOI] [PubMed] [Google Scholar]
- [152].Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- [153].Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ohsumi J, Sakakibara S, Yamaguchi J, Miyadai K, Yoshioka S, Fujiwara T, Horikoshi H, Serizawa N. Troglitazone prevents the inhibitory effects of inflammatory cytokines on insulin-induced adipocyte differentiation in 3T3-L1 cells. Endocrinology. 1994;135:2279–2282. doi: 10.1210/endo.135.5.7956951. [DOI] [PubMed] [Google Scholar]
- [155].Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- [156].Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem. J. 2003;376:607–613. doi: 10.1042/BJ20030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Freytag S, Geddes TJ. Reciprocal regulation of adipogenesis by Myc and C/EBPalpha. Science. 1992;256:379–382. doi: 10.1126/science.256.5055.379. [DOI] [PubMed] [Google Scholar]
- [158].Shi Y, Hon M, Evans RM. The peroxisome proliferator-activated receptor delta, an integrator of transcriptional repression and nuclear receptor signaling. PNAS. 2002;99:2613–2618. doi: 10.1073/pnas.052707099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Fu M, et al. Cyclin D1 inhibits peroxisome proliferator-activated receptor gamma-mediated adipogenesis through histone deacetylase recruitment. J. Biol. Chem. 2005;280:16934–16941. doi: 10.1074/jbc.M500403200. [DOI] [PubMed] [Google Scholar]
- [160].Ninomiya-Tsuji J, Torti FM, Ringold GM. Tumor necrosis factor-induced c-myc expression in the absence of mitogenesis is associated with inhibition of adipocyte differentiation. Proc. Natl. Acad. Sci. USA. 1993;90:9611–9615. doi: 10.1073/pnas.90.20.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Grant SF, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- [162].Hattersley AT. Prime suspect: the TCF7L2 gene and type 2 diabetes risk. J. Clin. Invest. 2007;117:2077–2079. doi: 10.1172/JCI33077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Cauchi S, et al. Transcription factor TCF7L2 genetic study in the French population: expression in human beta-cells and adipose tissue and strong association with type 2 diabetes. Diabetes. 2006;55:2903–2908. doi: 10.2337/db06-0474. [DOI] [PubMed] [Google Scholar]
- [164].Saxena R, et al. Common single nucleotide polymorphisms in TCF7L2 are reproducibly associated with type 2 diabetes and reduce the insulin response to glucose in nondiabetic individuals. Diabetes. 2006;55:2890–2895. doi: 10.2337/db06-0381. [DOI] [PubMed] [Google Scholar]
- [165].Watanabe RM, Allayee H, Xiang AH, Trigo E, Hartiala J, Lawrence JM, Buchanan TA. Transcription factor 7-like 2 (TCF7L2) is associated with gestational diabetes mellitus and interacts with adiposity to alter insulin secretion in Mexican Americans. Diabetes. 2007;56:1481–1485. doi: 10.2337/db06-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Xu H, Hotamisligil GS. Signaling pathways utilized by tumor necrosis factor receptor 1 in adipocytes to suppress differentiation. FEBS Lett. 2001;506:97–102. doi: 10.1016/s0014-5793(01)02889-7. [DOI] [PubMed] [Google Scholar]
- [167].Font de Mora J, Porras A, Ahn N, Santos E. Mitogen-activated protein kinase activation is not necessary for,but antagonizes, 3T3-L1 adipocytic differentiation. Mol. Cell Biol. 1997;17:6068–6075. doi: 10.1128/mcb.17.10.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Tominaga S, Yamaguchi T, Takahashi S, Hirose F, Osumi T. Negative regulation of adipogenesis from human mesenchymal stem cells by Jun N-terminal kinase. Biochem. Biophys. Res. Commun. 2005;326:499–504. doi: 10.1016/j.bbrc.2004.11.056. [DOI] [PubMed] [Google Scholar]
- [169].Prusty D, Park BH, Davis KE, Farmer SR. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002;277:46225–46232. doi: 10.1074/jbc.M207776200. [DOI] [PubMed] [Google Scholar]
- [170].Park B-H, Qiang L, Farmer SR. Phosphorylation of C/EBP{beta} at a consensus extracellular signal-regulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol. Cell Biol. 2004;24:8671–8680. doi: 10.1128/MCB.24.19.8671-8680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Berg AH, Lin Y, Lisanti MP, Scherer PE. Adipocyte differentiation induces dynamic changes in NF-kappaB expression and activity. Am. J. Physiol. Endocrinol. Metab. 2004;287:E1178–E1188. doi: 10.1152/ajpendo.00002.2004. [DOI] [PubMed] [Google Scholar]
- [172].Sprott KM, Chumley MJ, Hanson JM, Dobrowsky RT. Decreased activity and enhanced nuclear export of CCAAT-enhancer-binding protein beta during inhibition of adipogenesis by ceramide. Biochem. J. 2002;365:181–191. doi: 10.1042/BJ20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Cinti S. The role of brown adipose tissue in human obesity. Nutr. Metab. Cardiovasc. Dis. 2006;16:569–574. doi: 10.1016/j.numecd.2006.07.009. [DOI] [PubMed] [Google Scholar]
- [174].Lowell BB, Flier JS. Brown adipose tissue, beta 3-adrenergic receptors, and obesity. Annu. Rev. Med. 1997;48:307–316. doi: 10.1146/annurev.med.48.1.307. [DOI] [PubMed] [Google Scholar]
- [175].Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc. Natl. Acad. Sci. USA. 2007;104:2366–2371. doi: 10.1073/pnas.0610416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Coombes RC, Rothwell NJ, Shah P, Stock MJ. Changes in thermogenesis and brown fat activity in response to tumour necrosis factor in the rat. Biosci. Rep. 1987;7:791–799. doi: 10.1007/BF01116752. [DOI] [PubMed] [Google Scholar]
- [177].Masaki T, Yoshimatsu H, Chiba S, Hidaka S, Tajima D, Kakuma T, Kurokawa M, Sakata T. Tumor necrosis factor-alpha regulates in vivo expression of the rat UCP family differentially. Biochim. Biophys. Acta. 1999;1436:585–592. doi: 10.1016/s0005-2760(98)00173-8. [DOI] [PubMed] [Google Scholar]
- [178].Plata-Salaman CR. Central nervous system mechanisms contributing to the cachexia-anorexia syndrome. Nutrition. 2000;16:1009–1012. doi: 10.1016/s0899-9007(00)00413-5. [DOI] [PubMed] [Google Scholar]
- [179].Valladares A, Roncero C, Benito M, Porras A. TNF-alpha inhibits UCP-1 expression in brown adipocytes via ERKs. Opposite effect of p38MAPK. FEBS Lett. 2001;493:6–11. doi: 10.1016/s0014-5793(01)02264-5. [DOI] [PubMed] [Google Scholar]
- [180].Porras A, Valladares A, Alvarez AM, Roncero C, Benito M. Differential role of PPAR[gamma] in the regulation of UCP-1 and adipogenesis by TNF-[alpha] in brown adipocytes. FEBS Lett. 2002;520:58–62. doi: 10.1016/s0014-5793(02)02762-x. [DOI] [PubMed] [Google Scholar]
- [181].Porras A, Alvarez AM, Valladares A, Benito M. TNF-alpha induces apoptosis in rat fetal brown adipocytes in primary culture. FEBS Lett. 1997;416:324–328. doi: 10.1016/s0014-5793(97)01204-0. [DOI] [PubMed] [Google Scholar]
- [182].Valverde AM, Teruel T, Navarro P, Benito M, Lorenzo M. Tumor necrosis factor-alpha causes insulin receptor substrate-2-mediated insulin resistance and inhibits insulin-induced adipogenesis in fetal brown adipocytes. Endocrinology. 1998;139:1229–1238. doi: 10.1210/endo.139.3.5854. [DOI] [PubMed] [Google Scholar]
- [183].Jimenez M, Barbatelli G, Allevi R, Cinti S, Seydoux J, Giacobino JP, Muzzin P, Preitner F. Beta 3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. Eur. J. Biochem. 2003;270:699–705. doi: 10.1046/j.1432-1033.2003.03422.x. [DOI] [PubMed] [Google Scholar]