Abstract
Objective
Examine, in a field study, circadian phase changes associated with two different light–dark exposures patterns, one that was congruent with a phase advanced sleep schedule and the other that was incongruent with an advanced schedule.
Methods
Twenty-one adults (mean age ± SD = 22.5 ± 3.9 years; 11 women) participated in the 12 day study. After a five day baseline period, participants were all given individualized, fixed, 90 minute advanced sleep schedules for one week. Participants were randomly assigned to one of two groups, an advance group with a light–dark exposure prescription designed to advance circadian phase or a delay group with light–dark exposure prescription designed to delay circadian phase. The advance group received two morning hours of short-wavelength (blue) light (λmax ≈ 476 ± 1 nm, full-width-half-maximum ≈ 20 nm) exposure and three evening hours of light restriction (orange-filtered light, λ < 525 nm = 0). The delay group received blue light for three hours in the evening and light restriction for two hours in the morning. Participants led their normal lives while wearing a calibrated wrist-worn light exposure and activity monitor.
Results
After seven days on the 90 minute advanced sleep schedule, circadian phase advanced 132 ± 19 minutes for the advance group and delayed 59 ± 7.5 minutes for the delay group.
Conclusions
Controlling the light–dark exposure pattern shifts circadian phase in the expected direction irrespective of the fixed advanced sleep schedule.
1. Introduction
The master clock in the suprachiasmatic nuclei (SCN) orchestrates circadian rhythms at every level of physiology, from overt behavior to single cells. Retinal light exposures affect the phase relationship between the external clock time and the endogenous master clock time. Short-wavelength (blue) light is most effective for stimulating the master clock [1,2]. Empirically, the spectral sensitivity of the human circadian system, as measured by nocturnal melatonin suppression and by phase shifting, peaks at approximately 460 nm [1–4]. The magnitude and the direction of phase adjustments in the SCN resulting from retinal light exposure are characterized by the Phase Response Curve (PRC) [5–8]. The PRC can be characterized as a 24 hour cycle function with both a phase advance and a phase delay region. A well-defined PRC can be used to predict the best time of light delivery for the treatment of circadian misalignment, such as advanced sleep phase disorder (ASPD) or delayed sleep phase disorder (DSPD). In most humans, light applied during early evening and the first half of the night should delay the phase of the master clock, whereas light delivered during the late night and the early morning should advance its phase [7, 9].
The effectiveness of light sources with increased short-wavelength radiation for treating different circadian sleep disorders in the field has been investigated. Two field studies exposing subjects to narrow-band, 470 nm peaking light in the morning were undertaken in attempts to phase advance circadian phase in adolescents [10] and in “night owl” young adults [11]. In the adolescent study, all participants in a between-subjects design had delayed bedtimes (one and a half hours) and wake times (three hours) on weekends relative to weekdays. All participants delayed circadian phase from Friday to Sunday [as measured by changes in the time of dim light melatonin onset (DLMO)], by approximately the same amount irrespective of whether or not they were exposed to a 470 nm light for one hour upon awakening on weekends. The authors suggest that morning light treatment presented after delayed wakeup times during weekends, had no benefit for advancing circadian phase in adolescents. However, light exposures at other times of the day were not monitored, so it is not known whether, for example, uncontrolled exposure to evening light canceled out or reduced the effect of the morning light treatment.
In the “night owl” study, there was no difference in circadian phase advance, as measured by a change in the time of DLMO between one group of participants who experienced only an advanced sleep schedule (one and a half to two hours a day for six consecutive days) and another group of participants who received one hour exposure to a 470 nm light upon awakening in addition to an advanced sleep schedule. Unlike the adolescent study, light exposures during the light treatment and over the course of the waking period were continuously monitored in both groups. Although circadian light exposures in the first three hours after awakening were significantly greater in the group exposed to the morning blue light (verifying compliance to the experimental protocol), the total circadian light exposures while awake did not differ between groups. These data suggest that the entire daily light exposure profiles need to be considered when attempting to predict circadian phase and thereby, to correct circadian sleep disorders in real-life applications.
The present field study was designed to more systematically examine the relationship between an advanced sleep schedule and prescribed light treatments. All participants were placed on a fixed, advanced sleep schedule where both morning and evening light exposures were controlled. Half the participants received a schedule-reinforcing advance regime of light and dark while the other half received a schedule-contradicting delay regime of light and dark. It was hypothesized that the light–dark exposure patterns determine circadian phase, not the sleep schedule.
2. Methods
2.1. Participants
Twenty-one participants (11 women) were recruited by word-of-mouth and email. Potential participants were asked to fill out the Munich Chronotype Questionnaire (MCTQ) [12]. Those who normally woke up between 0630 and 0800, and went to sleep between 2300 and 0130, and had regular sleep patterns were accepted into the study. (Bedtimes and wake times reported by participants during recruitment are shown in Table 1) All participants reported that they had no major health problems and that they did not take pharmaceuticals, except for women taking birth control pills. Participants ranged in age from 18 to 30 years old [mean age ± standard deviation (SD): 22.5 ± 3.9]. Each participant chosen for the study had to demonstrate an ability to use instant messaging and to respond quickly to prompts from the experimenter with his or her own personal mobile device. All participants were provided written informed consent approved by Rensselaer's Institute Review Board and were paid for their participation in the study.
Table 1.
Participant characteristics obtained during subject recruitment.
| Participant characteristics | Advance group (n = 10) | Delay group (n = 11) | ||
|---|---|---|---|---|
|
|
|
|||
| Mean (SD) | Range | Mean (SD) | Range | |
| Age (years) | 22 (3) | 18–30 | 23 (4) | 18–32 |
| MCTQ-reported weekday wake times (h) | 0755 (0009) | 0730–0800 | 0716 (0028) | 0630–0800 |
| MCTQ-reported weekday bedtimes (h) | 2357 (0036) | 2315–0130 | 2327 (0027) | 2300–0030 |
| MCTQ-reported weekend wake times (h) | 0716 (0028) | 0730–1130 | 0849 (0044) | 0730–1000 |
| MCTQ-reported weekend bedtimes (h) | 2327 (0027) | 2315–0200 | 0012 (0030) | 2345–0130 |
2.2. Study Overview
Every participant completed the 12 day mixed-design study, during which they were asked to wear a wrist-worn Daysimeter-D [13] at all times while awake and asleep, except during showering and swimming. Participants kept their normal schedule during the first five days. At the end of this baseline period, participants reported to the laboratory for collection of evening saliva samples. Melatonin concentrations were used to assess circadian phase, as measured by the time of their DLMO. Participants were then randomly assigned to the advance group (n = 10) or the delay group (n = 11) for the next seven days. All participants were given an advanced sleep schedule; those in the advance group received a light prescription designed to advance their circadian phase in concert with the advanced sleep schedule, while those in the delay group received a light prescription designed to delay their circadian phase in opposition to the advanced sleep schedule. At the end of the intervention week, participants again reported to the laboratory for evening saliva sample collection for a second circadian phase assessment.
2.3. Apparatus
2.3.1. Communication between participants and experimenter
Google Chat, an instant messaging system that maintains a message log, was used throughout the study to issue instructions to participants and to record their responses. The two-way Google Chat messaging was conducted with a mobile device (cell phone, smartphone, tablet computer) that the participants carried with them at all times.
2.3.2. Light and activity measurements
Throughout the study participants wore a Daysimeter-D on the wrist at all times, including during sleep, except when showering or swimming. Participants were asked to avoid covering the devices with their coats and sleeves. This device continuously measures and records personal light exposure and activity levels [13]. It is calibrated in terms of photopic illuminance (lux), “circadian illuminance” (CLA), and circadian stimulus (CS). Values of CLA are spectrally weighted illuminance values according to the model of phototransduction by Rea et al. [3,14] and scaled so that 1000 lux of CIE Illuminant A (incandescent light source at 2856 K) is equivalent to 1000 units of CLA. CS values are transformed CLA values ranging from 0 to 0.7. CS values are proportional to levels of nocturnal melatonin suppression, from 0% at threshold to 70% at saturation, during the midpoint of nocturnal melatonin production after one hour of light exposure for a 2.3 mm diameter pupil [15]. Because CS is defined in terms of the circadian system's input–output relationship, it is considered a better measure of the effectiveness of light for stimulating the circadian system than either lux or CLA. Activity, using an Activity Index (AI) was also continuously recorded [16].
2.3.3. Eyewear
Participants in both groups wore two types of eyewear during prescribed time periods, orange-tinted glasses and blue-light goggles.
Nearly all optical radiation below 525 nm is filtered by the orange-tinted glasses (“Orange Glasses,” UV Process Supply, Chicago IL, # I005-017). These glasses have been used in previous studies to filter out retinal exposure to circadian active light (e.g., [17]). Each pair of glasses was fitted with a Daysimeter-D. The experimenter used the activity data from these devices to verify compliance with the study protocol.
The blue-light goggles were custom-made by mounting four light emitting diodes (LEDs) to safety goggle lenses (λmax ≈ 476 ± 1 nm, full-width-half-maximum (FWHM) ≈ 20 nm). Two LEDs were mounted to both lenses; one was located above and one was located below the center of each lens. To minimize discomfort glare and risk for blue-light hazard [18], polycarbonate translucent theatrical filter material diffused the light emitted by the LEDs (Roscolux #116, Rosco, Stamford, CT). Each set of goggles was calibrated in the laboratory using a spectrometer (Oriel Instaspec IV spectrometer; Oriel Instruments; Stratford, CT) with an UV–VIS optical fiber ending in a Lambertian diffuser. Left and right goggle lens irradiances were measured separately and the current from a remote 9V battery was adjusted with a control circuit until the mean corneal illuminance (left and right lenses) reached 40 lux (40 μW/cm2). Each pair of goggles was fitted with a Daysimeter-D to monitor compliance.
2.4. Outcome measures
2.4.1. Personal light exposures
Personal light exposure data from the Daysimeter-D devices were obtained for data analysis; all three measures of personal light exposure, photopic illuminance (lux), CLA, and CS exposure levels, were examined.
2.4.2. Circadian phase assessments
Circadian phase assessments were based upon melatonin concentrations from evening saliva samples obtained using the Salivette system (Sarstedt, Nürmbrecht, Germany). To prevent contamination, participants were not allowed to eat or drink between saliva sample times. After centrifuged, saliva samples were frozen at −20°C until assayed for melatonin levels. Saliva samples were later assayed by radioimmunoassay using a commercially available kit from Labor Diagnostika Nord (Nordhorn, Germany). The limit of detection was 0.9 pg/mL and the intra- and inter-assay coefficients of variability were determined to be 11.4% and 12.7%, respectively. All saliva samples from one evening were assayed in the same batch.
Saliva collection began two hours prior to the predicted time of DLMO [19] and continued every half hour until two hours after the predicted time of DLMO. DLMO threshold for each melatonin profile from participants was calculated by taking the average of the three lowest points plus twice the standard deviation of these points [20]. The DLMO time for each profile was the time (as determined by linear interpolation) that the fitted curve exceeded and remained above the DLMO threshold. Phase shifting was determined by subtracting DLMO in the baseline assessment period from DLMO obtained at the end of the intervention week. A negative difference would mean the participant exhibited a circadian phase delay after the intervention relative to the baseline period, whereas a positive difference would mean that the participant exhibited a circadian phase advance after the intervention week relative to the baseline period.
2.4.3. Subjective sleepiness
Every four hours (while awake) participants completed the Karolinska Sleepiness Scale (KSS) survey [21] sent by the experimenter using the instant messaging system.
2.5. Procedures
2.5.1. Baseline period
To begin the study participants reported to the laboratory for instructions for using instant messaging during the study period and to receive a wrist-worn Daysimeter-D. Bedtimes and wake times were not fixed during the five day baseline period, but participants were asked to send a message to the experimenter when they awoke each morning and when they went to sleep each night. They were instructed to complete the KSS survey sent by the experimenter every four waking hours through the end of the study. Participants were also asked to complete the KSS just before going to bed. Instant messages were sent at 0800, 1200, 1600, and 2000 and participants were requested to reply within 15 minutes unless circumstances prohibited it (e.g., during driving; in a classroom). If no response was received within 15 minutes, another message was sent. The experimenter continued sending messages every 15 minutes until the participant responded.
The fifth evening of the baseline period, all participants returned to the laboratory for approximately four hours to complete saliva sample collection. Using the algorithm by Martin and Eastman [19], timing for the saliva collection was estimated from the average reported (MCTQ) and the observed (over the prior five days) bedtimes/wake times of the participants. During saliva sample collection participants remained in a room illuminated with dim red (λmax = 630 nm, FWHM = 22 nm) light (<2.5 lux at the cornea). They were allowed to perform normal computer work, read, and play games. Electronic devices (computers, tablets, phones, and portable media players) were dimmed to the lowest possible brightness and were covered with an orange filter (Roscolux # 21 Golden Amber, Rosco, Stamford, CT, USA); spectral transmittance of these orange filters was not greater than 2% from 380 to 550 nm.
Before leaving the laboratory, participants were each given a plastic bag containing the orange-tinted glasses, the blue-light goggles, two fresh 9V batteries (one for replacement if necessary), and a set of instructions. The instructions included a schedule for bedtimes and wake times and when to wear each type of eyewear. Participants were reminded that Daysimeter-D devices were attached to the eyewear to confirm compliance with the prescribed light and activity schedules. Participants were randomly assigned to the advance group or the delay group without their knowledge.
2.5.2. Intervention week
During the intervention week (the subsequent seven consecutive days) the sleep schedules for everyone in the advance group and in the delay group were advanced by one and a half hours relative to their self-reported sleep schedules during the baseline period.
Both lighting interventions consisted of wearing prescribed eyewear during two intervals each day, a two hour interval immediately following the assigned wake time, and a three hour interval proceeding the assigned bedtime. Participants in the advance group were required to wear the blue-light goggles upon awakening and the orange-tinted glasses prior to bedtime; those in the delay group were required to wear the orange-tinted glasses during the morning interval and the blue-light goggles during the evening interval.
3. Statistical analyses
DLMO, KSS and the three types of light exposure measures, photopic illuminance (lux), CLA and CS, were analyzed using PASWStatistics 18.0 software (SPSS, Chicago, IL, USA).
The light exposure data for each measure were divided into two intervals, the first two hours upon awakening (phase advance portion of the PRC) and the last three hours before bedtime (phase delay portion of the PRC). It should be noted that light data from one participant in the advance group were lost. One-between (advance group versus delay group) and two-within [intervals (morning versus evening) and weeks (baseline period versus intervention week)] mixed analyses of variance (ANOVAs) were conducted, one for each of the three light exposure measures.
For DLMO and KSS, mixed two-factor ANOVAs were used to test the effects of weeks (baseline period versus intervention week) and groups (advance group versus delay group). Two-tailed Student's t-tests were used to further compare the main effects and interactions. One sample, two-tailed t-tests were also used to determine if the DLMO phase shifts for both groups were significantly different from zero.
4. Results
4.1. Light exposures
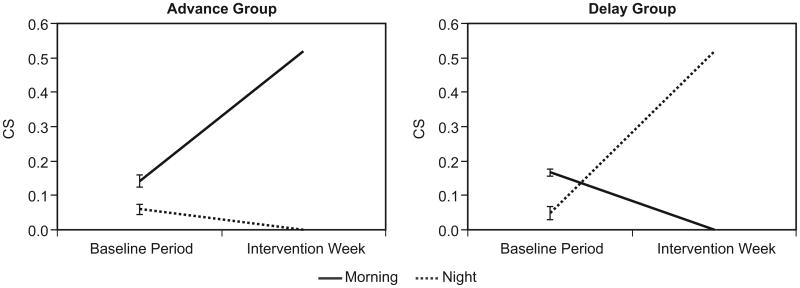
The statistical outcomes were essentially identical for all three light exposure measures, CS, log CLA, and log lux. Mean light exposure values, using any measure, were statistically identical for both groups (always, p > 0.05). For all three light exposure measures, intervals, weeks and the interaction between these two variables were highly significant (always, p < 0.0001). Fig. 1 shows the patterns of CS light exposures for the two groups. Table 2 lists CS, log CLA, and log lux values during the baseline and intervention periods, for both groups.
Fig. 1.
CS light exposures, advance and delay groups. Absence of error bars for the intervention week data reflects the use of the blue-light goggles and the orange-tinted glasses.
Table 2.
CS, log CLA, and log lux values, baseline and intervention periods, for the advance group and the delay group.
| Light levels | Baseline period | Intervention period | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| CS | log CLA | log lux | CS | log CLA | log lux | |
| First 2 hours after waking (mean (SD)) | ||||||
| Advance group (n = 9) | 0.1417 (0.0554) | 1.71 (0.31) | 1.72 (0.32) | 0.52 (0) | 2.84 (0.16) | 1.94 (0.09) |
| Delay group (n = 11) | 0.1664 (0.0545) | 1.80 (0.27) | 1.80 (0.25) | 0 (0) | 0.07 (0.06) | 0.08 (0.06) |
| Last 3 hours before sleep (mean (SD)) | ||||||
| Advance group (n = 9) | 0.0598 (0.0417) | 1.21 (0.32) | 1.26 (0.30) | 0 (0) | 0.01 (0.00) | 0.01 (0.00) |
| Delay group (n = 11) | 0.0487 (0.0340) | 1.15 (0.37) | 1.23 (0.35) | 0.52 (0) | 2.80 (0.15) | 1.86 (0.12) |
4.2. Circadian phase (DLMO)
The ANOVA revealed a significant main effect of weeks (F1,1 = 13.8; p = 0.001) and a significant interaction between weeks and groups (F1,19 = 94.3; p < 0.0001). There was also a significant main effect of groups (F1,1 = 59.2; p < 0.0001). Two-tailed Student's t-tests revealed that DLMO times at the end of the baseline period were not statistically different between groups (t19 = 0.93, p = 0.36), but DLMO times following the intervention week were significantly different (t19 = 13.50, p < 0.001).
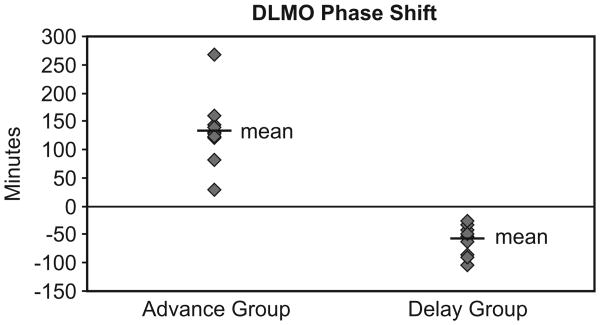
The mean ± standard error of the mean (SEM) phase shift for the advance group was +132 ± 19 minutes and for the delay group it was −59 ± 7.5 minutes. The one sample t-test revealed that both, the delay group and the advance group exhibited phase shifts that were significantly greater than zero (t10 = 7.9; p < 0.0001 and t9 = 6.9; p < 0.0001, respectively).
DLMO times following the baseline period and following the intervention, phase shifts from the baseline period to intervention week, reported bedtimes, wake times, and sleep times for both groups are listed in Table 3. Fig. 2 illustrates phase shifts for the 11 participants in the delay group and the 10 participants in the advance group.
Table 3.
Bedtimes, wake times, DLMO times, and phase angles between DLMO and bedtimes.
| Sleep times, salivary DLMO, phase angle | Advance group (n = 10) | Delay group (n = 11) | ||
|---|---|---|---|---|
|
|
|
|||
| Mean (SD) | Range | Mean (SD) | Range | |
| Reported bedtimes | ||||
| Baseline bedtime (h, min) | 0011 (0036) | 2245–0105 | 0001 (0042) | 2217–0153 |
| Intervention bedtime (h, min) | 2234 (0034) | 2113–2324 | 2223 (0024) | 2133–2304 |
| Reported wake times | ||||
| Baseline wake time (h, min) | 0802 (0040) | 0534–0934 | 0725 (0040) | 0602–0917 |
| Intervention wake time (h, min) | 0635 (0029) | 0537–0925 | 0548 (0025) | 0438–0641 |
| Reported sleep times | ||||
| Baseline total sleep time (min) | 458 (60) | 327–579 | 424 (67) | 288–570 |
| Intervention total sleep time (min) | 477 (40) | 392–556 | 442 (34) | 339–520 |
| Difference in total sleep time (min) | 18 (24) | –7–80 | 18 (33) | –10–119 |
| DLMO | ||||
| Baseline DLMO time (h, min) | 2106 (0044) | 2018–2248 | 2048 (0023) | 2000–2138 |
| Intervention DLMO time (h, min) | 1850 (0027) | 1815–1949 | 2147 (0029) | 2108–2248 |
| DLMO shift (min; delay: negative) | 132 (19) | 268–29 | –59 (7.5) | –25–105 |
| Phase angle between DLMO and bedtime | ||||
| Night 1, baseline (h, min) | 0303 (0037) | 0156–0347 | 0300 (0052) | 0153–0508 |
| Night 2, after intervention wk (h, min) | 0345 (0053) | 0211–0501 | 0057 (0045) | 0002–0259 |
Fig. 2.
Salivary DLMO Phase Shifts. Diamonds indicate phase shifts in salivary DLMO for participants in the advance (n = 10) and delay (n = 11) groups.
4.3. Subjective sleepiness
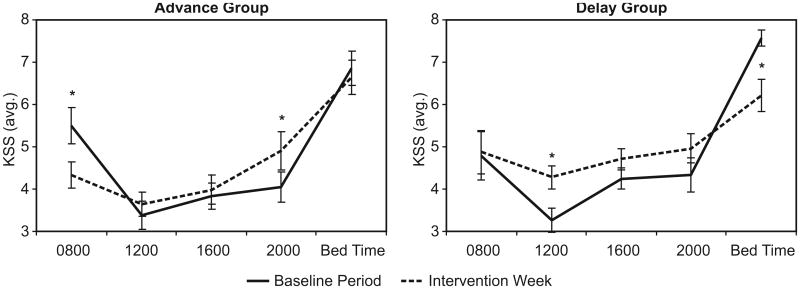
From the ANOVA using KSS scores, there was no significant difference between the advance and delay groups (F1,19 = 2.29; p = 0.5) nor was there a difference between the baseline period and the intervention week (F1,19 = 0.14; p = 0.7). The mean ± SEM KSS scores was 4.7 ± 0.22 for the advance group and 4.9 ± 0.21 for the delay group; the mean ± SEM KSS scores for the baseline period was 4.8 ± 0.15 and for the intervention week was 4.9 ± 0.21. In addition there was no significant interaction between groups and weeks (F4,76 = 0.26; p = 0.6). There was, however, a significant main effect of time (F4,16 = 41.2; p < 0.001), a significant interaction between time and weeks (F4,16 = 10.7; p < 0.001), and a three-way interaction between groups, time and weeks (F4,76 = 2.2; p < 0.01), which is illustrated in Fig. 3.
Fig. 3.
KSS Averages by time-of-day for the advance and delay groups. Asterisks identify significant differences between the baseline period and the intervention week for each group.
As expected and shown in Fig. 3, subjective sleepiness is lowest during the middle of the day, with higher KSS scores early in the morning and just before going to bed at night. The KSS scores during the baseline period were very similar for the two groups, but their responses differed considerably during the intervention week. Using post-hoc, paired two-tailed Student's t-tests, subjective sleepiness for the advance group was significantly lower upon rising (t9 = 2.91, p = 0.02) and was significantly lower for the delay group before going to bed (t10 = 2.97, p = 0.01). The delay group reported significantly more sleepiness at noon (1200) during the intervention week than during the baseline period (t10 = 3.59, p < 0.01) while the advance group reported significantly more sleepiness at 2000 (t9 = 2.31, p = 0.05).
5. Discussion
The present study further elucidates the relationship between a fixed sleep schedule and controlled light–dark exposures in the field. Controlling the light–dark exposure pattern shifts circadian phase in the expected direction. Consistent with the conclusions reached by Danilenko et al. [22] from a controlled laboratory study, the sleep pattern itself has little measurable effect on circadian phase in the present field study.
In their field study, Sharkey et al. [11] showed that a fixed, advanced sleep schedule advanced circadian phase independent of a deliberate morning blue-light exposure intervention. They concluded, based upon the continuously measured daily light exposures, that morning light exposures associated with an earlier wake time was probably sufficient to advance circadian phase, with or without an additional blue-light exposure intervention. Unlike the present study, Sharkey and colleagues did not control evening light exposure. It is interesting to note that participants in the advance group of the present study received similar phase advancing morning blue light exposures as the subjects did in the study by Sharkey and colleagues. In contrast to the light exposures in that study, however, evening light exposure was restricted for participants in the advance group by the orange-tinted glasses intervention in the present study. Subjects in the previous study advanced circadian phase by, on average, one and a half hours, whereas participants in the advance group of the present study advanced circadian phase by, on average, 2.2 hours. Thus, controlling both morning and evening light exposures is important for predicting the magnitude as well as the direction of circadian phase shift.
Although the advanced sleep schedule seemed to have little effect on circadian phase, it is probably important, nevertheless, to coordinate the sleep schedule with the light–dark schedule. Mitchell et al. [23], for example, showed that, in a population of nightshift workers, a shifted sleep schedule with facilitating bright light exposure was more successful in obtaining entrainment than it was when workers were given a conflicting bright light schedule. Despite limited research in this area, a light–dark exposure pattern that reinforces the sleep and eating schedules appears to be more effective than one that does not. Indeed, the subjective sleepiness responses in the present study lend support to this conclusion. The prescribed light–dark exposure pattern for the advance group was in concert with their prescribed sleep schedule. As a result, their subjective sleepiness at 0800 during the intervention week, when they were required to wake up one and a half hour earlier, was reduced relative to the same time during the baseline period. In contrast, participants in the delay group were sleepier at bedtimes during the baseline period than during the intervention week. The present data clearly suggest that it is important for clinical applications of sleep schedules and of the light–dark pattern to work together not just to alter circadian phase, but also to ensure good sleep. Coordinating the phase of the master clock with sleep as well as with the phases of peripheral clocks is also an important consideration for clinical applications. The liver clock, for example, is strongly influenced by food delivery time and coordinating food intake with light exposure might be quite important to maintaining entrainment [24]. More research is certainly needed to examine the potentially complicated and interesting interactions among light, sleep and food intake.
In summary, the present study showed that the light–dark exposure pattern, not the fixed sleep schedules, determines the direction of circadian phase change. This study underscores the point that it is necessary to control the entire daily light–dark exposure pattern to obtain an expected circadian phase change. Controlling morning light exposure alone may not necessarily produce the expected changes in circadian phase [10,11]. By controlling both morning and evening circadian light exposures, changes in circadian phase can be reliably achieved. For clinical applications of circadian phase disorders, coordinating sleep schedules with light–dark exposure patterns probably leads to better and more consistent entrainment.
Supplementary Material
Acknowledgments
This paper is taken in part from a dissertation to be submitted in partial fulfillment for the degree of Architectural Sciences with a Concentration in Lighting in the School of Architecture at Rensselaer Polytechnic Institute.
The work was funded by Office of Naval Research (grant # N00014-11-1-0572), National Institute on Aging (grant # R01AG034157), and Cornelia T. Bailey. The authors wish to thank the outstanding researchers and staff members at the Lighting Research Center, including: Andrew Bierman, Dennis Guyon, Robert Hamner, Terry Klein, Sharon Lesage, Ines Martinovic, Rebekah Mullaney, Barbara Plitnick, Nick Skinner, Aaron Smith, and Bonnie Westlake.
Footnotes
Conflict of Interest: The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: http://dx.doi.org/10.1016/j.sleep.2012.12.011.
References
- 1.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21(16):6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535(Pt 1):261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Rev. 2005;50(2):213–28. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Wright HR, Lack LC. Effect of light wavelength on suppression and phase delay of the melatonin rhythm. Chronobiol Int. 2001;18(5):801–8. doi: 10.1081/cbi-100107515. [DOI] [PubMed] [Google Scholar]
- 5.Honma K, Honma S, Wada T. Phase-dependent shift of free-running human circadian rhythms in response to a single bright pulse. Experientia. 1987;43(11-12):1205–7. doi: 10.1007/BF01945525. [DOI] [PubMed] [Google Scholar]
- 6.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(Pt 3):945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jewett ME, Rimmer DW, Duffy JF, Klerman EB, Kronauer RE, Czeisler CA. Human circadian pacemaker is sensitive to light throughout subjective day without evidence of transients. Am J Physiol. 1997;273(5 Pt 2):R1800–1809. doi: 10.1152/ajpregu.1997.273.5.r1800. [DOI] [PubMed] [Google Scholar]
- 8.Minors DS, Waterhouse JM, Wirz-Justice A. A human phase response curve to light. Neurosci Lett. 1991;133(1):36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- 9.Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(Pt 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiol Int. 2010;27(7):1469–92. doi: 10.3109/07420528.2010.503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharkey KM, Carskadon MC, Figueiro MG, Zhu Y, Rea MS. Effects of an advanced sleep schedule and morning short-wavelength light exposure on circadian phase in young adults with late sleep schedules. Sleep Med. 2011;12(7):685–92. doi: 10.1016/j.sleep.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18(1):80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 13.Figueiro MG, Hamner R, Bierman A, Rea MS. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Light Res Tech. doi: 10.1177/1477153512450453. in press. Epub 22 June 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rea MS, Figueiro MG, Bierman A, Hamner R. Modeling the spectral sensitivity of the human circadian system. Light Res Tech. in press. Epub 14 Dec 2011. [Google Scholar]
- 15.Rea MS, Figueiro MG, Bierman A, Bullough JD. Circadian light. J Circadian Rhythms. 2010;8:2. doi: 10.1186/1740-3391-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bierman A, Klein TR, Rea MS. The Daysimeter: A device for measuring optical radiation as a stimulus for the human circadian system. Meas Sci Technol. 2005;16:2292–2299. [Google Scholar]
- 17.Figueiro MG, Brons JA, Plitnick B, Donlan B, Leslie RP. Measuring circadian light and its impact on adolescents. Light Res Tech. 2011;43(2):201–15. doi: 10.1177/1477153510382853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bullough JD. The blue-light hazard: A review. J Illum Eng Soc. 2000;29(2):6–14. [Google Scholar]
- 19.Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002;19(4):695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- 20.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12(5):457–66. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 21.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52(1-2):29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 22.Danilenko KV, Cajochen C, Wirz-justice A. Is sleep per se a zeitgeber in humans? J Biol Rhythms. 2003;18(2):170–8. doi: 10.1177/0748730403251732. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell PJ, Hoese EK, Liu L, Fogg LF, Eastman CI. Conflicting bright light exposure during night shifts impedes circadian adaptation. J Biol Rhythms. 1997;12(1):5–15. doi: 10.1177/074873049701200103. [DOI] [PubMed] [Google Scholar]
- 24.Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Ann Rev Biochem. 2002;71:307–31. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.